- *Corresponding Author:
- Y. H. Kuan
Department of Pharmacology, School of Medicine, Chung Shan Medical University, Taiwan
E-mail: kuanyh@csmu.edu.tw
| Date of Submission | 22 September 2016 |
| Date of Revision | 05 January 2017 |
| Date of Acceptance | 23 April 2017 |
| Indian J Pharm Sci 2017; 79(3):411-419 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Yi-Chi-Tsung-Ming-Tang is a traditional Chinese medicine formula often prescribed for preventing or treating dizziness, tinnitus, mental fatigue and blurred vision. Recent study has demonstrated that Aβ(1-40)-induced neurotoxicity could be improved by this drug. The aim of present study was to determine the effects and protective mechanism of Yi-Chi-Tsung-Ming-Tang on Aβ(1-40)-induced death of primary cortical neurons. Primary cultures of Sprague-Dawley rat cortical neurons were exposed to Aβ(1-40), after the treatment with Yi-Chi-Tsung-Ming-Tang for 1 h. Methyl-thiazolyl-tetrazolium reduction assays were used to detect cell viability and the expression of acetylcholine receptors, N-methyl-D-aspartate receptors and phosphorylated and non-phosphorylated forms of tau were measured by western blot. Fluorometric assays were applied to detect the generation of reactive oxygen species. Pretreatment of primary cortical neurons with Yi-Chi-Tsung-Ming-Tang significantly inhibited Aβ(1-40)-induced cytotoxicity and reversed Aβ(1-40)-induced β-amyloid accumulation and acetylcholine receptor expression in a concentration-dependent manner. In addition, not only the Aβ(1-40)-reduced expression of N-methyl-D-aspartate receptors 1/2A was reversed but also the Aβ(1-40)-induced reactive oxygen species generation and tau phosphorylation expression were inhibited by Yi-Chi-Tsung-Ming-Tang in a concentration-dependent manner.
Keywords
Yi-Chi-Tsung-Ming-Tang, Aβ, reactive oxygen species, tau, NMDARs
Alzheimer's disease (AD) is an irreversible, progressive neurodegenerative disease characterized by the loss of memory, which results in dementia, cognitive defect, behavioural disturbance and neuropsychiatric symptoms [1]. A report published in 2006 had shown that 26.6 million people were affected by AD and approximately $156 billion were spent annually on caring for AD patients worldwide. By the year of 2050, the prevalence of AD is expected to quadruple and shall place a considerable burden about health cost on society [2]. Up to now, the drug treatment for AD mainly evolved around two mechanisms, which involved the inhibitors of acetyl cholinesterase and the stimulators of N-methyl-D-aspartate receptors (NMDAR). Unfortunately, these drugs do not much improve the symptoms caused by AD [3].
The neuropathological hallmarks of AD contain amyloid-beta (Aβ) aggregation, cerebral amyloid angiopathy, neurofibrillary tangles formation, neuron death, and synaptic loss [4]. Aβ is the peptide fragment derived from an integral membrane protein, the amyloid precursor protein (APP), by secreatases processing. Aβ(1-40) is one of the Aβ isoforms and the most abundant variant in vivo [5,6]. Aβ and its derived oligomeric species can bind to NMDAR thus facilitating the endocytosis and activation of NMDAR. In addition, Aβ and its derived oligomers induce hyper phosphorylation of tau, which results in selfaggregation and microtubule destabilization. These molecule-associated pathogens, including up-regulated reactive oxygen species (ROS) generation and downregulated acetylcholine receptor (AchR) expression, induce excitotoxicity in hippocampal and cortical neurons [7,8].
Yi-Chi-Tsung-Ming-Tang (YCTMT) is a traditional Chinese medicine formula and presented by the Chinese physician, Dong-Yuan Li, in Jin Dynasty (AD 1200s). In traditional medicine, YCTMT has been used to prevent symptoms such as dizziness, tinnitus, deafness, mental fatigue, and blurred vision [9-11]. In a previous study, we have demonstrated that YCTMT has a therapeutic effect on AD induced by Aβ(1-40) in vivo [12]. In the present, we aimed to study the molecular mechanism and protective effects of YCTMT on Aβ(1-40)-induced cytotoxicity in cultured primary cortical neurons.
Materials and Methods
YCTMT is composed of Astragalus membranaceus (Fisch.) Bunge, Ginseng quinquefolium (L.) Alph. Wood, Pueraria lobata (Willd.) Ohwi, Paeonia lactiflora Pall, Phellodendron chinense C.K.Schneid, Vitex rotundifolia L.f., Cimicifuga foetida L. and Glycyrrhiza uralensis Fisch. in a ratio of 5:5:5:1:1:1.5:3:5 (dry weight). All components were purchased from a Chinese herbal shop in Taichung, Taiwan and were certified at the Department of Chinese Pharmaceutical Sciences and Chinese Medicine Resources, China Medical University, Taichung, Taiwan. Voucher specimens (No. 2011YCTMT-A~H) have been deposited at the China Medical University. Aβ(1-40) was purchased from Tocris Bioscience (Ellisville, MO, USA) and dissolved in a vehicle containing 35% acetonitrile and 0.1% trifluoroacetic acid. Glycyrrhizin was purchased from Tokyo Chemical Industry Co. (Tokyo, Japan). Polyvinylidene fluoride (PVDF) membrane filter was obtained from Millipore Corp. (Bedford, MA, USA). Other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
High performance liquid chromatography (HPLC) analysis of YCTMT:
Hot water extract of YCTMT was prepared by dissolving it in methanol (5.0 mg/ml). Sample and standard solutions of glycyrrhizin were filtered through a filter cartridge (pore size of 0.22 μm) prior to analysis. The chemical content quantification was performed on a Shimadzu HPLC system equipped with Shimadzu LC-20AT pump, Shimadzu SIL-20 auto sampler, and Shimadzu SPD-M20A detector. The HPLC profile was performed on a RP-18 column (Cosmosil 5C18-AR-II, 4.6×250 mm, 5 μm) at a flow rate of 1.0 ml/min, detected at UV 254 nm. The injection volume was 10 μl. The mobile phase was composed of 0.1% TFA water solution (A) and methanol (B). The solvent gradient was as follows: 0-60 min from 5% B to 55% B. By diluting the stock solution, a series of standard solutions (glycyrrhizin) were prepared with concentrations of 2, 1, 0.5, 0.25, 0.1, 0.05, and 0.025 mg/ml used to calculate the concentration of examined compounds.
Liquid chromatography tandem-mass spectrometry (LC-MS/MS) analysis of YCTMT:
LC-MS/MS experiments were performed on a Dionex Ultimate 3000 HPLC system (Dionex, Germany) coupled with an ESI-ion trap MS (HCTultra PTM Discovery, Bruker Daltonics, Germany). A linear gradient on a C18 LC column (Atlantis T3 C18 5 μm 2.1×150 mm) was used to separate the water extract of YCTMT with a flow rate of 0.25 ml/min. The mobile phase A consisted of water containing 0.1% v/v formic acid and mobile phase B containing 99.9% v/v acetonitrile and 0.1% v/v formic acid. A gradient elution was applied from 5% v/v B to 60% B in the first 25 min, and to 90% B over 5 min. It was then held at 90% B for another 4 min at the flow rate of 0.25 ml/min, which was followed by a return to the starting conditions and re-equilibration of the column for 5 min with 5% B v/v prior to the next injection. The ESI source was operated in positive or negative ion mode. Nitrogen was used as a nebulizing (45 psi) and drying gas (10 l/min, 350°). For the MS/MS settings, the most eight intense ions from each MS full scan spectrum were automatically selected as the precursor ion peaks for the following auto MS/MS experiments. Helium was used as the collision gas.
Cortical neuron cultures:
Sprague-Dawley rats were used to obtain primary cultures of cortical neurons. The protocols for animal experiments were performed according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Animal Experimental Committee of China Medical University under permit number: 96-113-C. Cultures of primary cortical neurons were prepared from cerebral cortices of 18 d-old embryonic rats [13]. Briefly, pooled dissected cerebral cortices were digested with papain solution and plated at a density of 8×105 cells/cm2 on poly-D-lysine-coated dishes. One day after seeding, the culture medium was replaced with Neurobasal serum-free medium containing 1% B27 supplement, 0.5 mM l-glutamine, and 1% penicillin/streptomycin. Neurobasal serum-free medium was replaced every three days and 10 μM cytosine arabinoside was added to the medium on the third day. The purity of primary neurons was over 95% and was employed between 12 to 14 d in culture.
Cell viability assays:
T h e 3 - ( 4 , 5 - d i m e t h y l t h i a z o l - 2 - y l ) - 2 , 5 - diphenyltetrazolium bromide tetrazolium (MTT) reduction assay was used to monitor cell viability by measuring the mitochondrial succinic dehydrogenase activity as described previously [14]. Experimental neuron cultures were either treated with 0-20 μM of Aβ(1-40) alone for 24 h or first treated with various concentrations of YCTMT for 1 h followed by 10 μM of Aβ(1-40) for 24 h. As for the positive control group, 1% Triton X-100 was added to the well, followed by 0.5 mg/ml MTT solution. After 24 h incubation, the quantity of formazan was determined by eluting with dimethyl sulfoxide and measured at 550 nm using a microplate reader (MR4000; Dynatech, Chantilly, VA). Eqn. 1: percent cell viability (%) = OD550 value of treated groups/OD550 value of untreated control×100.
Western blot:
Cultures were washed twice with phosphate-buffered saline (PBS) and harvested in RIPA lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 50 mM sodium fluoride, 0.2 mM sodium orthovanadate, 1 μg/ml leupeptin, 1 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride). After centrifugation, the protein concentration in the supernatant was determined by Bradford assay. Protein extracts were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes. After blocking with 5% non-fat milk in PBS for 1 h, the membranes were washed with PBS containing 0.1% Tween-20 (PBST), and incubated at room temperature for 2 h with antibodies including β-amyloid, AchR, NMDAR1, NMDAR2, β-actin, and phosphorylated and non-phosphorylated forms of tau, respectively. Then the membranes were washed again and the horseradish peroxidase-labelled IgG was added at room temperature for 1 h. The blots were developed using enhanced chemiluminescence reagents [15].
Measurement of intracellular ROS generation:
After YCTMT pretreatment, cultured neurons were incubated with 20 μM 2,7-dichlorfluorescein-diacetate (DCFH-DA) for 30 min in the dark, then washed, and the intracellular content of ROS was measured by using the semi-quantitative fluorescence technique [16] with a fluorescence microplate reader (Molecular Devices, CA, USA) at excitation/emission wavelengths of 400/505 nm. Results were expressed as fluorescence intensity.
Statistical analysis:
At least three independent experiments were performed as indicated in the figure legends. All data obtained were analysed using one-way analysis of variance (ANOVA) followed by post-hoc between-group analyses using Dunnett and Scheffé’s test for multigroup comparisons and expressed as mean±standard deviation (SD). The criterion for statistical significance was P<0.05 for all evaluations.
Results and Discussion
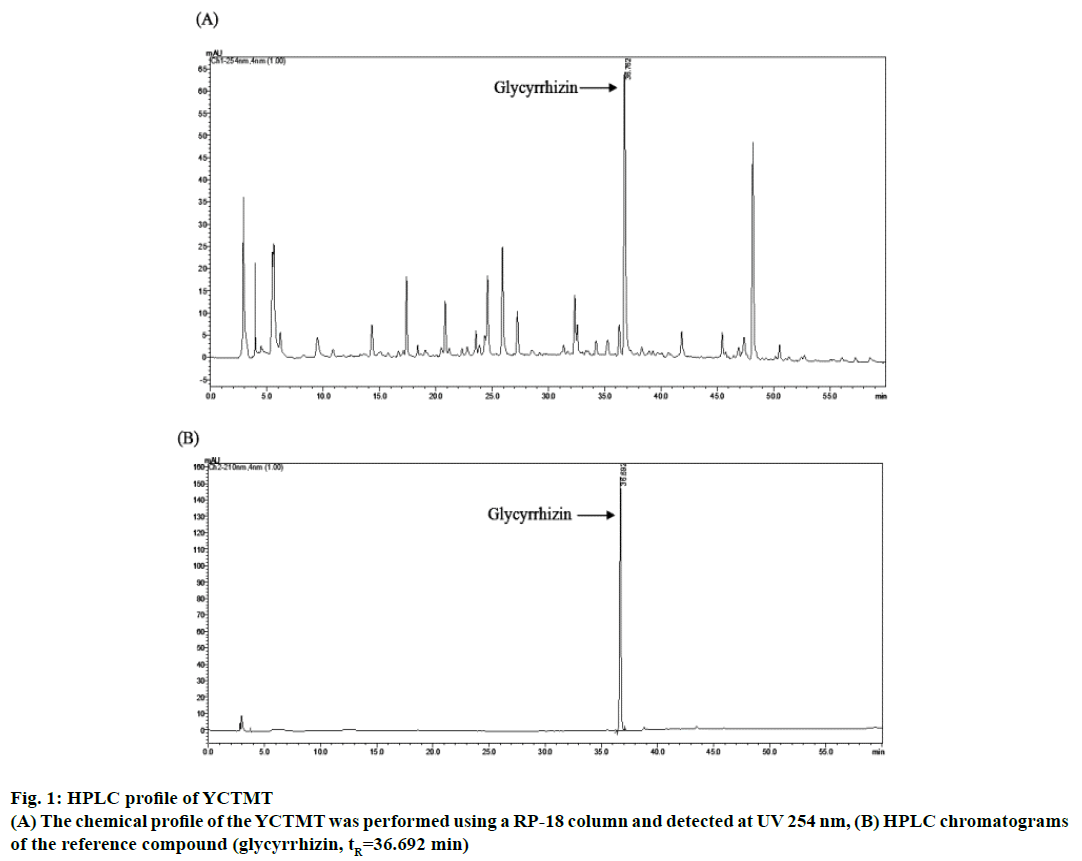
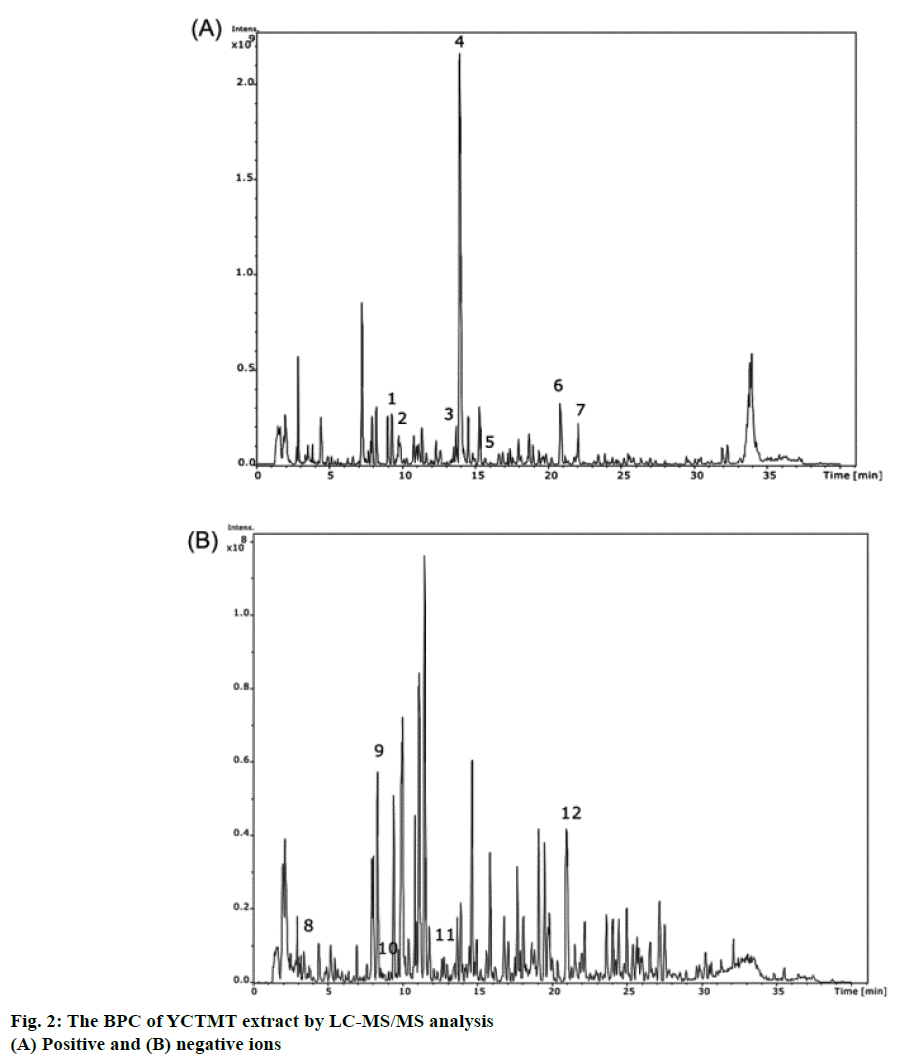
The reference compound of glycyrrhizin was identified in YCTMT by HPLC, which acted as an indicator compound for quality check of extraction procedure of each batch (Figure 1). The content of glycyrrhizin was calculated to be 0.78 mg/ml of YCTMT by HPLC quantification. The components of YCTMT extract were identified by LC-MS/MS. According to the molecular weights of precursor ion and their corresponded fragment ions in MS/MS spectra referring to published literature. The base peak chromatogram (BPC) of positive and negative ions of the YCTMT extract by LC-MS/MS analysis was shown in Figure 2. Albiflorin, paeoniflorin, palmatine, berberine, daidzein, formononetin, gallic acid, caffeic acid, puerarin, ferulic acid, and glycyrrhizin were identified in YCTMT extract by LC-MS/MS analysis according to MS and MS/MS ions (Table 1).
| Peak no. | Selected ion | MS (ESI) | MS/MS (ESI) | Molecular formula | Proposed compound |
|---|---|---|---|---|---|
| 1 | [M+H]+ | 481.2 | 319.2, 196.9 | C23H28O11 | Albiflorin |
| 2 | [M+NH4]+ | 498.3 | 301.1, 178.9 | C23H28O11 | Paeoniflorin |
| 3 | [M]+ | 352.5 | 337.1, 308.1 | C21H22NO4 | Palmatine |
| 4 | [M]+ | 336.1 | 321.1, 292.1 | C20H18NO4 | Berberine |
| 5 | [M]+ | 254.1 | 198.9, 137.0 | C15H10O4 | Daidzein |
| 6 | [M+H]+ | 823.5 | 647.5, 453.4 | C42H62O16 | Glycyrrhizin |
| 7 | [M]+ | 268.3 | 254.0, 237.0, 213.0 | C16H12O4 | Formononetin |
| 8 | [M-H]- | 169 | 125.0 | C7H6O5 | Gallic acid |
| 9 | [M-H]- | 415.2 | 295.1, 267.0 | C21H20O9 | Puerarin |
| 10 | [M-H]- | 179.1 | 135.0 | C9H8O4 | Caffeic acid |
| 11 | [M-H]- | 193.0 | 177.9, 149.0, 134.0 | C10H10O4 | Ferulic acid |
| 12 | [M-H]- | 821.5 | 627.1, 361.1 | C42H62O16 | Glycyrrhizin |
Table 1: Hplc-Ms-Ms Identification of the Constituents in Yctmt Extract
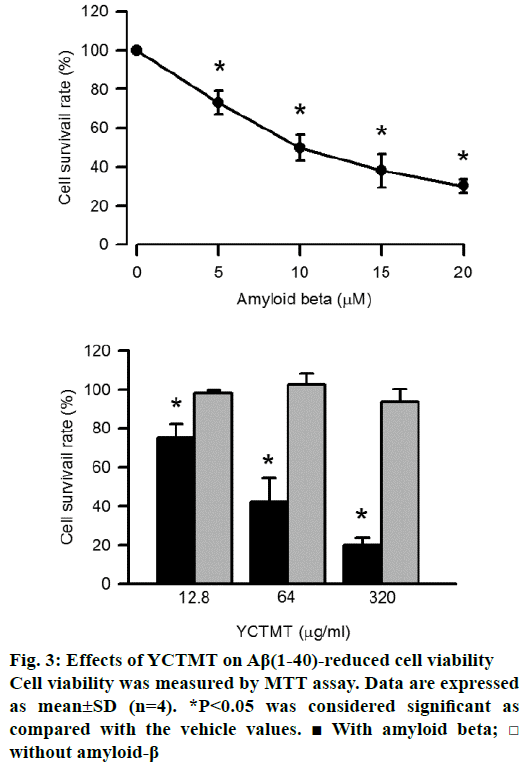
The viability of primary cortical neurons was measured by MTT assay. As shown in Figure 3A, Aβ(1-40) reduced cell viability in a concentration-dependent manner, significant effect started at 5 μM. Meanwhile, neurons incubated with various concentrations of YCTMT alone showed no significant effect on viability, even when the concentration of YCTMT was up to 320 μg/ml. But neurons pretreated with YCTMT attenuated Aβ(1-40)-impaired cell viability in a concentrationdependent manner starting from 12.8 μg/ml and up (P<0.05) (Figure 3B).
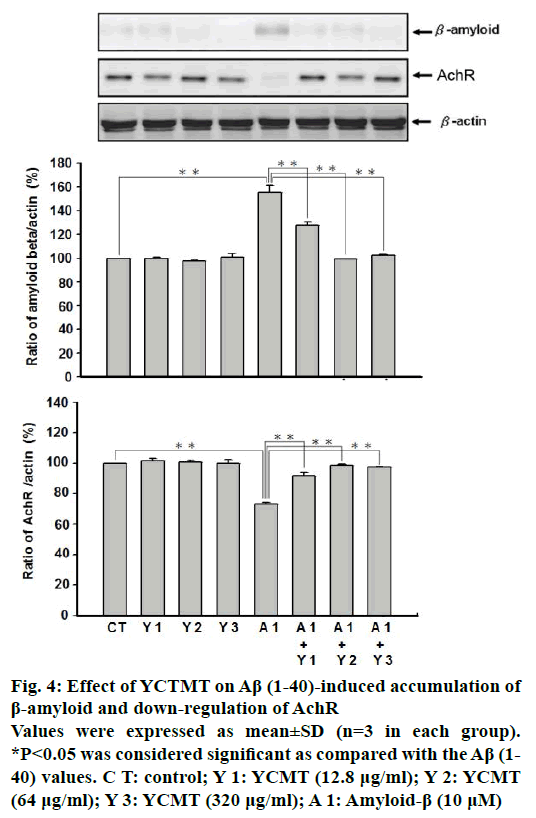
To explore the protective effect of YCTMT on cortical neurons, the expression of β-amyloid and AchR was measured using western blot. As shown in Figure 4, administration of Aβ(1-40) markedly increased the expression of β-amyloid as compared with the control. However, pre-incubation with YCTMT for 1 h inhibited the expression of β-amyloid in Aβ(1-40)-stimulated cortical neurons in a concentration-dependent manner starting at 12.8 μg/ml (P<0.05). Administration of Aβ(1-40) for 4 h markedly reduced the expression of AchR as compared with the control. β-actin plays as a stable and constitutive internal control and has the same expression. With 1 h pre-incubation of YCTMT, the reduced expression of AchR in Aβ(1-40)-stimulated cortical neurons was reversed in a concentrationdependent manner also starting at 12.8 μg/ml (P<0.05).
Figure 4: Effect of YCTMT on Aβ (1-40)-induced accumulation of β-amyloid and down-regulation of AchR
Values were expressed as mean±SD (n=3 in each group).
*P<0.05 was considered significant as compared with the Aβ (1-40) values. C T: control; Y 1: YCMT (12.8 μg/ml); Y 2: YCMT (64 μg/ml); Y 3: YCMT (320 μg/ml); A 1: Amyloid-β (10 μM)
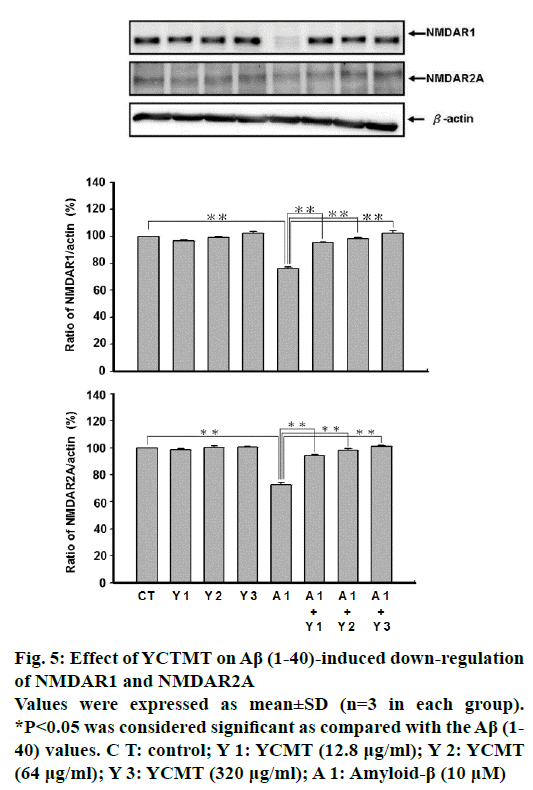
Administration of Aβ(1-40) markedly reduced the expression of NMDAR1 and NMDAR2A as compared with the control. However, pre-incubated with YCTMT for 1 h reversed the reduced expression of NMDAR1 and NMDAR2A in Aβ(1-40)-stimulated cortical neurons in a concentration-dependent manner starting from 12.8 μg/ml and up (P<0.05, Figure 5).
Figure 5: Effect of YCTMT on Aβ (1-40)-induced down-regulation of NMDAR1 and NMDAR2A
Values were expressed as mean±SD (n=3 in each group).
*P<0.05 was considered significant as compared with the Aβ (1-40) values. C T: control; Y 1: YCMT (12.8 μg/ml); Y 2: YCMT (64 μg/ml); Y 3: YCMT (320 μg/ml); A 1: Amyloid-β (10 μM)
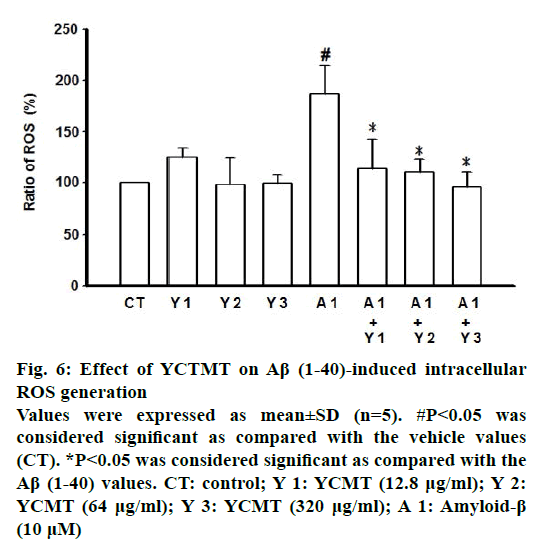
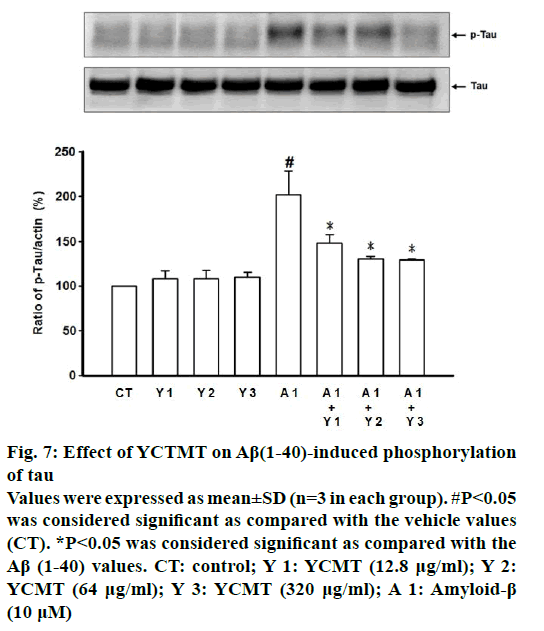
Administration of Aβ(1-40) markedly induced intracellular ROS generation as compared with the control. β-actin plays as a stable and constitutive internal control and has the same expression, as shown in Figure 5. However, pre-incubated with YCTMT for 1 h inhibited intracellular ROS generation in Aβ(1-40)- stimulated cortical neurons in a concentrationdependent manner, significant effect starting at 12.8 μg/ml (P<0.05) (Figure 6). Administration of Aβ(1-40) markedly induced phosphorylation of tau as compared with the control. However, pre-incubated with YCTMT for 1 h inhibited the phosphorylation of tau in Aβ(1-40)-stimulated cortical neurons in a concentration-dependent manner, significant effect starting at 12.8 μg/ml (P<0.05, Figure 7).
Figure 6: Effect of YCTMT on Aβ (1-40)-induced intracellular ROS generation
Values were expressed as mean±SD (n=5). #P<0.05 was considered significant as compared with the vehicle values (CT). *P<0.05 was considered significant as compared with the Aβ (1-40) values. CT: control; Y 1: YCMT (12.8 μg/ml); Y 2: YCMT (64 μg/ml); Y 3: YCMT (320 μg/ml); A 1: Amyloid-β (10 μM)
Figure 7: Effect of YCTMT on Aβ(1-40)-induced phosphorylation of tau
Values were expressed as mean±SD (n=3 in each group). #P<0.05 was considered significant as compared with the vehicle values (CT). *P<0.05 was considered significant as compared with the Aβ (1-40) values. CT: control; Y 1: YCMT (12.8 μg/ml); Y 2: YCMT (64 μg/ml); Y 3: YCMT (320 μg/ml); A 1: Amyloid-β (10 μM)
YCTMT is one of the most important traditional Chinese medicines and widely used in Asian countries such as China, Japan, and Korea. However, there are several pieces of evidence, which demonstrate that some of the YCTMT composites, such as A. membranaceus (Fisch.) Bunge, G. quinquefolium (L.) Alph. Wood, P. lobata (Willd.) Ohwi, and G. uralensis, have the ability to protect against AD [17-20]. Moreover, YCTMT improves Aβ(1-40)-induced AD-like phenotype, including learning and memory loss [12]. The death of neuron in cerebral cortex is an important feature of AD [3]. In the present study, we also found Aβ(1-40), the most abundant of Aβ isoform-induced cytotoxicity in primary cortical neurons. Though YCTMT alone has no effect on cell viability of cortical neurons, Aβ(1- 40)-induced neuron death is inhibited by pretreatment of YCTMT in a concentration-dependent manner. These results suggested that AD could be improved by YCTMT via reduced death of cortical neuron.
The imbalance between clearance and production of Aβ causes its intracellular accumulation, which is the initiating factor of AD. These accumulated intracellular Aβ will further deposit and assemble into fibrils, which arrange themselves into parallel β-pleated sheets to form insoluble plaques [8]. These Aβ plaques are the conspicuous pathological feature in brains of AD patients. In addition, there are evidences proposed that among the Aβ isoforms, Aβ(1-40) and Aβ(1-42) are the most toxic species. In AD caused by APP gene duplication, the intracellular Aβ(1-40) has shown prominent existence in patients’ neurons [21,22]. Murine model of AD showed that intracellular Aβ accumulation results in neuron death [23]. At present, we had demonstrated the increased expression of Aβ in Aβ(1-40)-treated primary cortical neurons, and which was attenuated by YCTMT in a dose-dependent manner. These results were in accordance with pervious study, which demonstrated that Aβ plague accumulation was reduced by YCTMT in brain of Aβ(1-40)-induced AD rats [12]. In addition, the Aβ-induced toxic effect appeared more rapidly on cholinergic axon terminals than on cell bodies [24]. The activation of choline acetyltransferase and acetylcholinesterase are significantly reduced in AD patients [25]. In Aβ(1-40)-induced AD rats, the expression of acetylcholine in brain was recovered by YCTMT [12]. The results of present study indicated that the Aβ(1-40)-induced AchR expression was inhibited by YCTMT in primary cortical neurons. All these data gathered had made it clear that YCTMT could improve Aβ-induced neuronal death via reduction of Aβ accumulation and increased AchR expression.
NMDARs are the glutamate-gated cation channels with high calcium permeability and widely expressed in the central nervous system and play an important role in both excitatory synaptic transmission under physiological conditions and excitotoxic neuronal death under pathological conditions [26]. NMDARs are tetrameric complexes form by several heteromeric complexes from three NMDAR classes and their various subunits, which are NMDAR1, NMDAR2 with subunits A, B, C, and D, and NMDAR3 with subunits A and B. NMDAR1/2A, the di-heteromeric component of NMDARs, is widely and abundantly expressed in CNS in the mammalian [27]. It has been demonstrated that during the progression process of AD, mRNA and DNA expression of NMDAR1 subunit was down regulated in human [28]. In AD, NMDAR1 containing an N-terminal splice cassette appears to be reduced significantly, which may result in susceptible cells accession [29]. In the hippocampus and cortex of post-mortem br ain from AD patients, levels of NMDAR2A mRNA and protein similar to NMDAR1 are also decreased [30]. It was found that while the expression of NMDAR1/2A was reduced by Aβ(1-40) in primary cortical neurons, pretreatment with YCTMT reversed the effect in a dose-dependent manner. These results indicated that YCTMT could improve Aβ- induced neuronal death via destructing the diminishing expression of NMDAR1/2A.
Excessive activation of NMDARs is stimulated by Aβ, which in turn leads to calcium influx. The abnormally increased concentration of calcium in cytosol and mitochondria leads to mitochondria disruption. In addition, intracellular Aβ causes damage to cytochrome c oxidase, α-ketoglutarate and pyruvate dehydrogenase, and DNA in mitochondria. Therefore, Aβ directly or indirectly harms the mitochondrial functions and results in overproduction of ROS [8,31]. Oxidative stress generated from these ROS plays an important role in Aβ-induced excitotoxicity [32]. Aβ- induced generation of oxidative stress is inhibited by NMDAR inhibitors [33]. It was found that the generation of ROS was stimulated by Aβ(1-40) in primary cortical neurons, while pretreatment with YCTMT reversed the effect in a dose-dependent manner. These results indicated that YCTMT could improve Aβ-induced excitotoxicity via reducing the generation of oxidative stress.
Tau, the highly soluble microtubule-associated protein, promotes tubulin assembly into microtubules and stabilizes formed microtubules [34]. There are several critical phosphorylation sites on serine, threonine, and tyrosine residues for regulating the affinity of microtubules [34,35]. Normally, tau stabilizes microtubule structure, but such stabilization is converted into disruption when hyper phosphorylation of tau is induced by several kinases, including glycogen synthase kinase-3β, cyclin-dependent kinase-5, mitogen-activated protein kinase, Akt, Fyn, protein kinase A, calcium-calmodulin protein kinase 2, and microtubule affinity-regulating kinase [36]. The sites of hyperphosphorylation on tau form paired helical filaments, which result in neurofibrillary lesions in brains of AD patients [8]. It has been proposed that phosphorylation of tau is induced by Aβ in cortical neurons [37]. It was demonstrated that Aβ(1-40)-induced tau phosphorylation in primary cortical neurons, of which neurons treated with YCTMT alone has no effect. On the other hand, pretreatment with YCTMT reversed the Aβ(1-40)-induced tau phosphorylation in a dose-dependent manner. The evidence suggested that Aβ-induced hyper phosphorylation of tau via NMDAR activation through glycogen synthase kinase-3β, and the positive feedback from hyper phosphorylation of tau activated the NMDAR via tyrosine kinase, Fyn [38]. All these results indicated that YCTMT could improve Aβ-induced excitotoxicity via the reduction of tau phosphorylation.
In present study, we have demonstrated that YCTMT effectively attenuates Aβ-induced neuronal death. The mechanisms underlying this protective effect include improvement of β-amyloid accumulation and AchR reduction; reversal of NMDAR1 and NMDAR2A expression; reduction of intracellular ROS generation; and inhibition of tau phosphorylation. Experimental findings support the potential use of YCTMT as a therapeutic agent for AD prevention. However, Aβ(1-42) is the other toxic species in AD. The further study about Aβ(1–42)-induced neurocytotoxicity reduced by YCTMT will be more confirm the hypothesis.
Acknowledgments
Authors express their gratitude to Prof. Hsieh Ming- Tsuen of the Department of Chinese Pharmaceutical Sciences and Chinese Medicine Resources, China Medical University, Taichung, Taiwan for identifying and certifying the plant material used. This study was supported by the Show Chwan Memorial Hospital, Changhua, Taiwan (Grant: RD99008, RD98017, RD103013) and Ministry of Science and Technology of Taiwan (MOST 103-2314-B-212-001-).
Conflict of interest
All authors declare no conflict of interests.
Financial support
Nil.
References
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med 2012;367:795-804.
- Rocca WA, Petersen RC, Knopman DS, Hebert LE, Evans DA, Hall KS, et al. Trends in the incidence and prevalence of Alzheimer's disease, dementia, and cognitive impairment in the United States. Alzheimers Dement 2011;7:80-93.
- Franco R, Cedazo-Minguez A. Successful therapies for Alzheimer's disease: why so many in animal models and none in humans? Front Pharmacol 2014;5:146.
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring HarbPerspect Med 2011;1:a006189.
- Hsieh MT, Peng WH, Wu CR, Ng KY, Cheng CL, Xu HX. Review on experimental research of herbal medicines with anti-amnesic activity. Planta Med 2010;76:203-17.
- Yankner BA. Mechanisms of neuronal degeneration in Alzheimer's disease. Neuron 1996;16:921-32.
- Benilova I, Karran E, De Strooper B. The toxic Aβ oligomer and Alzheimer's disease: an emperor in need of clothes. Nat Neurosci 2012;15:349-57.
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med 2010;362:329-44.
- Li Y. Effect of Yi-Qi-Tsung-Ming-Tangand its derived formula on tinnitus and deafness on 68 cases. Yunnan J Tradit Chin Med Mater Med 2013;4:87.
- Gao XG, Liu J. Effect of Yi-Chi-Tsung-Ming-Tangand its derived formula on symptoms of conscious aging diseases. J PractTradit Chin Int Med 2007;4:60.
- Gui MJ, Bao YC, Zhang B. Effect of Yi-Chi-Tsung-Ming-Tang and its derived formula on neurology diseases. Clin J Tradit Chin Med 2012;3:260-2.
- Yeh CH, Hsieh MT, Hsueh CM, Wu CR, Huang YC, Liao JW, et al. Therapeutic effect of Yi-Chi-Tsung-Ming-Tangon amyloid beta-induced Alzheimer's disease-like phenotype via an increase of acetylcholine and decrease of amyloid beta. Evid Based Complement Alternat Med 2012;2012:414536.
- Chen CJ, Chen JH, Chen SY, Liao SL, Raung SL. Upregulation of RANTES gene expression in neuroglia by Japanese encephalitis virus infection. J Virol 2004;78:12107-19.
- Huang FM, Kuan YH, Lee SS, Chang YC. Cytotoxicity and genotoxicity of triethyleneglycol-dimethacrylate in macrophages involved in DNA damage and caspases activation. Environ Toxicol 2015;30:581-8.
- Huang FM, Chang YC, Lee SS, Yeh CH, Lee KG, Huang YC, et al. BisGMA-induced cytotoxicity and genotoxicity in macrophages are attenuated by wogonin via reduction of intrinsic caspase pathway activation. Environ Toxicol 2016;31:176-84.
- Lin RH, Yang ML, Li YC, Chang HM, Kuan YH. Indium chloride-induced micronuclei via reactive oxygen species in Chinese hamster lung fibroblast V79 cells. Environ Toxicol 2013;28:595-600.
- Sun M, Zhou T, Zhou L, Chen Q, Yu Y, Yang H, et al. Formononetin protects neurons against hypoxia-induced cytotoxicity through upregulation of ADAM10 and sAβPPα. J Alzheimers Dis 2012;28:795-808.
- Choi RC, Zhu JT, Leung KW, Chu GK, Xie HQ, Chen VP, et al. A flavonol glycoside, isolated from roots of Panaxnotoginseng, reduces amyloid-beta-induced neurotoxicity in cultured neurons: signaling transduction and drug development for Alzheimer's disease. J Alzheimers Dis 2010;19:795-811.
- Wang JZ, Xia YY, Grundke-Iqbal I, Iqbal K. Abnormal hyperphosphorylation of tau: sites, regulation, and molecular mechanism of neurofibrillary degeneration. J Alzheimers Dis 2013;33:S123-39.
- Ahn J, Um M, Choi W, Kim S, Ha T. Protective effects of GlycyrrhizauralensisFisch. on the cognitive deficits caused by beta-amyloid peptide 25-35 in young mice. Biogerontology 2006;7:239-47.
- Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerriere A, Vital A, et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet 2006;38:24-6.
- Cabrejo L, Guyant-Maréchal L, Laquerrière A, Vercelletto M, De la Fournière F, Thomas-Antérion C, et al. Phenotype associated with APP duplication in five families. Brain 2006;129:2966-76.
- Magrane J, Rosen KM, Smith RC, Walsh K, Gouras GK, Querfurth HW. Intraneuronal beta-amyloid expression downregulates the Akt survival pathway and blunts the stress response. J Neurosci 2005;25:10960-9.
- Kasa P Sr., Papp H, Kasa P Jr., Pakaski M, Balaspiri L. Effects of amyloid-beta on cholinergic and acetylcholinesterase-positive cells in cultured basal forebrain neurons of embryonic rat brain. Brain Res 2004;998:73-82.
- Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, Perry RH. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br Med J 1978;2:1457-9.
- Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. CurrOpinPharmacol 2007;7:39-47.
- Tackenberg C, Grinschgl S, Trutzel A, Santuccione AC, Frey MC, Konietzko U, et al. NMDA receptor subunit composition determines beta-amyloid-induced neurodegeneration and synaptic loss. Cell Death Dis 2013;4:e608.
- Jacob CP, Koutsilieri E, Bartl J, Neuen-Jacob E, Arzberger T, Zander N, et al. Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer's disease. J Alzheimers Dis 2007;11:97-116.
- Hynd MR, Scott HL, Dodd PR. Selective loss of NMDA receptor NR1 subunit isoforms in Alzheimer's disease. J Neurochem 2004;89:240-7.
- Mishizen-Eberz AJ, Rissman RA, Carter TL, Ikonomovic MD, Wolfe BB, Armstrong DM. Biochemical and molecular studies of NMDA receptor subunits NR1/2A/2B in hippocampal subregions throughout progression of Alzheimer's disease pathology. Neurobiol Dis 2004;15:80-92.
- Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks. Nat Neurosci 2010;13:812-8.
- Sultana R, Butterfield DA. Role of oxidative stress in the progression of Alzheimer's disease. J Alzheimers Dis 2010;19:341-53.
- Parks JK, Smith TS, Trimmer PA, Bennett JP Jr, Parker WD Jr. Neurotoxic Aβ peptides increase oxidative stress in vivo through NMDA-receptor and nitric-oxide-synthase mechanisms, and inhibit complex IV activity and induce a mitochondrial permeability transition in vitro. J Neurochem 2001;76:1050-6.
- Avila J, Lucas JJ, Perez M, Hernandez F. Role of tau protein in both physiological and pathological conditions. Physiol Rev 2004;84:361-384.
- Williamson R, Scales T, Clark BR, Gibb G, Reynolds CH, Kellie S. Rapid tyrosine phosphorylation of neuronal proteins including tau and focal adhesion kinase in response to amyloid-beta peptide exposure: involvement of Src family protein kinases. J Neurosci 2002;22:10-20.
- Wang K, Zhu L, Zhu X, Zhang K, Huang B, Zhang J Protective effect of paeoniflorin on Abeta25-35-induced SH-SY5Y cell injury by preventing mitochondrial dysfunction. Cell MolNeurobiol 2014;34:227-34.
- Kim EA, Cho CH, Hahn HG, Choi SY, Cho SW. 2-cyclopropylimino-3-methyl- 1,3-thiazoline hydrochloride protects against beta-amyloid-induced activation of the apoptotic cascade in cultured cortical neurons. Cell MolNeurobiol 2014;34:963-72.
- Mondragón-Rodríguez S, Trillaud-Doppia E, Dudilot A, Bourgeois C, Lauzon M, Leclerc N, et al. Interaction of endogenous tau protein with synaptic proteins is regulated by N-methyl-D-aspartate receptor-dependent tau phosphorylation. J BiolChem 2012;287:32040-53.