- Corresponding Author:
- P. F. Builders
Department of Pharmaceutical Technology and Raw Material Development, National Institute for Pharmaceutical Research and Development, Abuja
E?mail: philsonsky@yahoo.com
| Date of Submission | 24 November 2011 |
| Date of Revision | 11 January 2013 |
| Date of Acceptance | 18 January 2013 |
| Indian J Pharm Sci 2013;75(1):45-52 |
Abstract
Wound healing agents support the natural healing process, reduce trauma and likelihood of secondary infections and hasten wound closure. The wound healing activities of water in oil cream of the methanol extract of Hibiscus sabdariffa L. (Malvaceae) was evaluated in rats with superficial skin excision wounds. Antibacterial activities against Pseudomonas aeroginosa, Staphylococcus aureus and Echerichia coli were determined. The total flavonoid content, antioxidant properties and thin layer chromatographic fingerprints of the extract were also evaluated. The extract demonstrated antioxidant properties with a total flavonoid content of 12.30±0.09 mg/g. Six reproducible spots were obtained using methanol:water (95:5) as the mobile phase. The extract showed no antimicrobial activity on the selected microorganisms, which are known to infect and retard wound healing. Creams containing H. sabdariffa extract showed significant (P<0.05) and concentration dependent wound healing activities. There was also evidence of synergism with creams containing a combination of gentamicin and H. sabdariffa extract. This study, thus, provides evidence of the wound healing potentials of the formulated extract of the calyces of H. sabdariffa and synergism when co-formulated with gentamicin.
Keywords
Antimicrobial activity, antioxidant activity, Hibiscus sabdariffa calyx, water in oil cream, wound healing
Wound healing consists of a complex, well?organised cascade of biochemical and cellular events that involves tissue repairs and regeneration[1,2]. It is fundamentally a connective tissue response and involves the activity of an intricate network of blood cells, cytokines and growth factors, which ultimately leads to the restoration of the injured skin or tissue to normal condition[3,4]. The aim of wound care, which must occur in a physiologic environment conducive to tissue repair and regeneration, is to promote healing in the shortest time possible, exclude secondary infections and minimize pain, discomfort and scarring[5]. The entire process of wound healing, which begins at the moment of injury and may continue for prolonged period, can be grouped into three distinct phases, namely: Inflammatory, proliferative and remodelling phase, each of these phases is characterised by a series of events[6]. These processes of healing are known to be influenced by several factors such as infections, nutrition, drugs and hormones, type and sites of wound, and certain disease conditions[7]. Agents with wound healing potential, which are obtained from natural and synthetic bioactive materials have the propensity for antioxidant, chelation and antimicrobial activities; and may act by one or more of these mechanisms[8]. A number medicinal plants such as Morinda citrifolia, Cassia alata, Jatropha curcas, Tridax procumbens, Wrightia tinctoria, Trigonella foenumgraceum among others have been identified and employed in folk medicine for wound care[9?16] and their efficacy is widely acclaimed[4,17]. Proper scientific evaluation of these herbal medicines is imperative in order to establish their efficacy and safety. H. sabdariffa is an annual dicotyledonous shrub, which grows to a height of about two meters with yellow or reddish flower and the leaves have three to five lobules. Although, native to India and Malaysia, H. sabdariffa is also widely available and must have being carried to Africa in early times[18]. Many parts of the plant are of value with the leaves, seeds and calyces widely used as either food or drug[19,20]. The water extract of the calyces of the red flowered specie of H. sabdariffa is widely used especially, as a beverage, which is taken hot or cold because of its unique and appealing characteristic colour and flavour, and its medicinal benefits[18]. Many phytochemical constituents and diverse biological activities have been attributed to this plant[21]. Phytochemical screening of the water and alcoholic extracts of the calyces showed the presence of such biochemicals as flavonoids, phenols, reducing sugars, combined reducing sugars among others[22]. It is also rich in ascorbic acid, riboflavin, niacin, copper, zinc, manganese and iron[23?26]. Both the water and alcoholic extracts of the calyces of H. sabdariffa has established antioxidant activities[27]. The effective free radical scavenging properties of the extracts is attributable to the presence of efficient free radical scavengers such as ascorbic acid, flavonoids and other phenolic compounds, and chelating metallic ions of copper, zinc, manganese and iron[27?29]. To our knowledge, H. sabdariffa is not directly indicated for wound healing in folkloric and orthodox applications. However, the presence of certain bioactive agents, such as phenolics, ascorbic acid, certain sugars and trace metallic elements with proven intrinsic wound healing activities make H. sabdariffa a potential candidate for wound healing[5]. Therefore, the aim of this study was to evaluate the wound healing potentials of the formulated methanol extract of the calyces of H. sabdariffa in rodents and the effect of co?formulating the extract with gentamicin.
Materials and Methods
The calyces of H. sabdariffa were collected from a red rosella farm in Jikwoyi village in Abuja Nigeria. The plant part was identified by the ethno botanist from Department of Medicinal Plant Research and Traditional Medicine, National Institute for Pharmaceutical Research and Development, (NIPRD), Abuja, Nigeria. A voucher specimen (NIPRD/H/6328) was deposited at the herbarium for reference. Sterile water was produced in the department of microbiology and biotechnology of NIPRD and used within 24 h of production. Methanol, gentamicin, glycerol, sorbitan monooleate (Span 80), Pseudomonas aeroginosa (ATCC 27853), Staphylococcus aureus (ATCC 28923) and Escherichia coli (ATCC 25922) were purchased from Sigma, Germany. Soft paraffin was from BDH, England. All solvents and chemicals used were of analytical grade.
Extraction of H. sabdariffa calyces
Six hundred grams of dry calyces of H. sabdariffa was rinsed with two 500 ml portions of distilled water to remove dust and adhering dirt. This was then transferred into 3 l of methanol maintained at 50° for 1 h. The extracted dye solution was obtained by filtering through a sieve of fine muslin cloth of 150 μm mesh size and the spent calyces rinsed with another 1 l of methanol. The extract was concentrated on a water bath set at 50°.
Total flavonoid content and fractionation fingerprinting by TLC
One milligram quantity of the extract was mixed with 1 ml distilled water and 75 µl of 5% sodium nitrite solution and allowed to stand for 5 min before 0.5 ml of 10% aluminium chloride solution was added, followed by 0.5 ml of 1M sodium hydroxide. The solution was mixed well and allowed to stand for 15 min. The relative absorbance was measured at 510 nm using UV/Vis spectrophotometer (Shimadzu 160A, Japan). The total flavonoids content was determined using standard quercetin calibration curve. The results were expressed as milligram (mg) of quercetin equivalents per gram (g)[30]. The thin layer chromatographic (TLC) analysis was conducted by testing combinations of polar and nonpolar liquid solvents suitable as mobile phases for TLC fingerprinting of the methanol extract of the calyces of H. sabdariffa using analytical TLC plates (silica gel G60 F254 TLC plates of E. Merck, layer thickness 0.2 mm) as the stationary phase. Of the solvent system tested and used as the mobile phase; the butanol:acetic:water (4:1:5) and methanol:water (95:5) solvent systems yielded the best result when methanol solution of the extract corresponding to 0.5 mg/ml were filled into a 10 µl capillary tube and spotted on the TLC plates. Spots were observed with the naked eye and under the UV light (Eagle Scientific; 365?254 nm, Britain) before spraying with ferric chloride for the methanol:water solvent system.
Antimicrobial assessment
Four hundred milligram quantity of the extract was dissolved in 10 ml of sterile water to produce the stock concentration of 40 mg/ml. The solution was stored in a refrigerator at 4° until required. The test organisms: P. aeruginosa, S. aureus and E. coli were subcultured from stock and grown for 24 h. The 24 h culture was diluted 1:200 to obtain a 105 cfu/ ml culture[31].
One millilitre quantity of the extract was added to 19 ml molten nutrient agar to give a final concentration of 2 mg/ml concentration. Molten agar containing 2 mg/ml gentamicin was also prepared to serve as positive control. The agar were respectively poured into sterile Petri dishes and allowed to gel and set. The dried agar plates were inoculated with the test organisms by surface technique. The plates were incubated at 37° for 24 h. The experiment was done in triplicate.
Formulation of creams
The topical creams are prepared according to the formula on Table 1. The soft paraffin was melted in a water bath at 70°. The surfactants sorbitan monolaurate and tween 80 were dispersed in the aqueous and oil phases respectively. Quantities of glycerol, methanol extract of the calyces of H. sabdariffa and/or gentamicin were accordingly mixed together to form the aqueous phase. The aqueous phase was slowly added to the oil phase with continuous stirring at 500 rpm with a Kenwood kitchen mixer (Kenwood, USA). On addition of all the aqueous phase the mixture was mixed for another 5 min before the cream was removed from the water bath and allowed to set.
Stability assessment of creams
The freshly prepared cooled cream samples were assessed for the appearance and colour by visual assessment with the unaided eyes, the cream consistency was also assessed by rubbing between the fore and first fingers and the homogeneity of the creams assessed in terms of creaming and phase separation. Quantities of each cream sample placed in a wide mouthed plastic container and stored at –5°, 27° (room temperature) and 40°. Colour/appearance, consistency and homogeneity of these samples were observed daily for 14 days for any changes. The pHs of the freshly prepared creams were determined using the pH meter (Accumet Research AR10, Singapore). The creams were stored at 27° (room temperature) and their pH monitored daily for 14 days[32]: Five gram quantities of each cream sample were transferred into a 10 ml capacity beaker. The pH of the creams was determined by immersing the probe of the pH meter into the creams.
| Components | A (g) | B (g) | C (g) | D (g) | E (g) | F (g) |
|---|---|---|---|---|---|---|
| H. sabdariffaextract | 0.5 | 2.5 | 5 | 0.5 | - | - |
| Gentamicin | - | - | - | 0.25 | 0.5 | - |
| Petrolatum | 21.16 | 21.16 | 21.16 | 21.16 | 21.16 | 21.16 |
| Glycerol | 4.67 | 4.67 | 4.67 | 4.67 | 4.67 | 4.67 |
| Sorbitanmonolaurate | 5 | 5 | 5 | 5 | 5 | 5 |
| Tween 80 | 2 | 2 | 2 | 2 | 2 | 2 |
| Water | 16.67 | 14.67 | 12.17 | 16.42 | 16.67 | 17.17 |
TABLE 1: Formula For Hibiscus Sabdariffa And Gentamicin Creams.
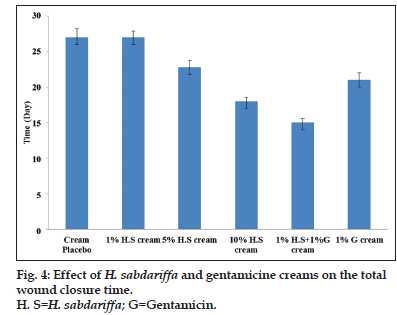
The cream sample formulations are A-1%w/w H. sabdariffa, B-5%w/w, H. sabdariffa, C-10%w/w H. sabdariffa, D-1%w/w H. sabdariffa-1%w/w Gentamicin, E-Gentamicin, F-Placebo cream, H. sabdariffa=Hibiscus sabdariffa
Wound healing studies
Wistar albino rats (180?200 g) of either sex maintained at the Animal Facility Centre of NIPRD, Abuja, were used. They were housed under standard conditions of temperature, (25±2°) and light, (approximately12/12 h light?dark cycle), fed on standard diet and given water ad libitum. The animal study was carried out with prior approval of the animal ethical committee of NIPRD Abuja Nigeria, the principles of good laboratory practice and animal handling (National Institutes of Health guide for the care and use of laboratory animals; Publication No. 85?23, revised 1985) was followed.
The wound healing studies was carried out by randomly dividing the rats into six groups of five per group. Of the 30 rats used twelve were female; two females were included in each group. A round seal of 4 cm diameter was impressed on the sides of the central trunk of each rat, depilated using a mini laser shaver (IPL450, Germany) and sterilised with ethanol. Excision wound was inflicted immediately on the rats under light chloroform anaesthesia[4,33,34]. Full skin thickness was excised with surgical blade from the back of the central trunk marked area to get a wound measuring about 4 cm2. After achieving complete haemostasis by blotting the wound with cotton swab soaked in warm saline, the animals were placed singly in individual cages. Animals were treated once daily for a 21 days period by applying the creams liberally on the wounds as follows: Group A rats served as the negative control and were treated with the blank ointment formulation, while groups B, C and D rats were treated with the cream containing 1.0, 5.0 and 10.0% w/w of H. sabdariffa extract. Groups E and F were treated with cream containing 1% w/w gentamicin and 1% w/w H. sabdariffa+1% w/w gentamicin, respectively. The wound area was measured with a divider and thereafter estimated on a transparent meter rule every 3 days. The time taken for epithelialization and complete wound closure to be achieved was determined. Epithelisation corresponds to the accelerated regeneration of epidermal structures from the undamaged epithelial cells at the wound margin by the process of epithelial mitosis and complete wound closure is the time taken for the healing to complete. Wound closure was calculated as a percentage of the original wound size using the equation as % Wound closure=(IDW-DW)IDW×100, where, IDW (Day 0) is initial diameter of wound and DW (Day X) is diameter of wound after day X.
Statistics
All experiments were performed in replicates (at least n=3) for validity of statistical analysis. Results were expressed as mean±SEM. ANOVA and Student t?tests were performed on data set. Differences were considered significant for P values <0.05.
Results and Discussion
The solvents used for the extraction of active biochemical from plant materials are important to the amount and type of phytochemicals contained in the plant material and is related to the deferential and preferential solubility of the phytochemicals contained in the plant materials[35]. Many of the health benefits of H. sabdariffa have been linked to its flavonoids, phenolic compounds and trace metallic elements. Methanol has been used to achieve a high recovery of flavonoids and phenolic compounds from plant materials[36,37]. Apart from the high throughput of extract yield, other attributes of methanol extraction includes low toxicity, ease of evaporation, promotion of rapid physiologic absorption of the extract, preservative action and prevention of the extract to complex or dissociate[38,39]. A solid mass of 11.6±0.5% w/w of H. sabdariffa extract was obtained by the methanol extraction and used for the formulation of the creams.
The total flavonoids content of the methanol extract was 12.30±0.09 mg/g. Although, the calyces of H. sabdariffa contain a variety of bioactive constituents, the flavonoid content has been tracked because of its implication in wound healing activities[8]. The assay of individual flavonoid is complex and expensive, thus, the measurement of the total flavonoid becomes an attractive alternative. In this regard flavonoid can be used as a chemical marker for assessing the bioactive content and quality control the creams. TLC is a simple but reliable technique for detection of phytochemical constituents in plant extracts and has been used to establish the fingerprints of the methanol extract of H. sabdariffa. Butanol:acetic:water (4:1:5) and methanol:water (95:5) were selected as the mobile phase because they produced the best result in terms of the number of spots, resolution and reproducibility. The butanol:acetic:water and methanol:water solvent systems yielded five and six spots, respectively. When the TLC plates were viewed under the UV lamp at 265 nm spots with Rfs 0.34 and 0.57 were detected for plates developed in butanol:acetic:water and methanol:water mobile phases, respectively. When the plates developed in methanol:water were derivatised with ferric chloride, two spots was imparted blue and one brown which is characteristic of flavonoids and phenols, respectively[40]. Thus, fingerprint profile was established with five and six spots for the respective solvent systems. The characteristics of the TLC separations are presented in Table 2.
Antimicrobial activity is one of the mechanisms by which some bioactive substances effect wound healing hence the investigation of the antibacterial potential of the extract in relation to gentamicin. Most infections on wounds are typically caused by common body bacteria flora[41]. Pseudomonas aeroginosa and Echerichia coli, and Staphylococcus aureus were selected as they comprise the bacteria that commonly colonize open wounds to cause poor healing (Table 3). The benefits of antimicrobials in wound management have been established in numerous studies suggesting accelerated healing with either systemic or topical application of antibiotics[42], thus, endorse antimicrobial activity as a mechanism of wound healing[41,43?45]. The extract does not have antibacterial effect against the selected organisms in this study suggesting that the pro?wound healing activity of H. sabdariffa extract may be by a mechanism other than antibacterial activity.
| Solvents | Spots | Colour | Retention Rf |
|---|---|---|---|
| Butanol:aceticacids:water (5:1:2) | 1 | Brown | 0.2 |
| 2 | Purple | 0.3 | |
| 3 | Blue | 0.34 (florescence, 265 nm) | |
| 4 | Pink | 0.4 | |
| 5 | Yellow | 0.8 | |
| Methanol:water (95:5) | 1 | Brown | 0.20 (brown) |
| 2 | Purple | 0.45 | |
| 3 | Blue | 0.57 (florescence, 265 nm blue/green:Ferric chloride) | |
| 4 | Pink | 0.69 (blue/green:Ferric chloride) | |
| 5 | Yellow | 0.77 | |
| 6 | Yellow | 0.81 |
Table 2: Characteristics Of Finger print Of Methanol Extract Of The Calyces Of Hibiscussabdariffa
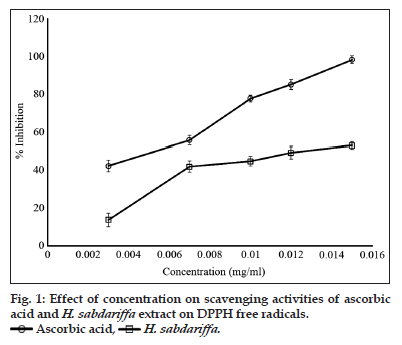
Due to its numerous applications, H. sabdariffa is a plant of interest in several cultures of the world[19,45,46]. The decoctions and infusions of the calyces of this plant are commonly used as a food drink and as medicine[22,47]. The presence of sugars, flavonoids, phenolic compounds and ascorbic acid which are well known for their antioxidant properties has been established in extracts of H. sabdariffa[22]. Furthermore, established are the presence ionised trace metallic elements such as zinc, manganese, iron, and copper. The antioxidant potential of the methanol extract, determined by the inhibition of the free radical scavenging activities of DPPH is presented in fig. 1. The free radical scavenging potency of the extract has been attributed to the presence of flavonoids, phenolic compounds, ascorbic and the trace metallic elements[22]. The relative ratios of the DPPH’s free radical mopping efficiency of ascorbic acid and the extract of H. sabdariffa are 1.6 and 1, respectively.
| Agent | Test micro-organisms | ||
|---|---|---|---|
| Staphylococcus aureus (ATCC 28923) | Pseudomonas aeroginosa (ATCC 27853) | Escherichia coli (ATCC 25922) | |
| H. sabdariffa | - | - | - |
| Gentamicin | + | + | + |
Table 3: Antibacterial activities of the methanol extract of hibiscus sabdariffa. -=none inhibition of growth of microorganism,+=inhibition of growth of microorganism, h. Sabdariffa=hibiscus sabdariffa.
The majority of topical pharmaceutical products comprise of semisolid formulations which include ointments, creams, lotions and gels. The type of formulation is important to the efficacy of the active component and anatomy of body parts hence the need to choose the appropriate formulation that will give optimum drug delivery. An appropriate formulation for treating open wounds should be nonirritant and also have excellent spreading and emollient properties. These attributes influence the choice of formulations for different kinds of wounds. The methanol extract of H. sabdariffa calyces was formulated as a cream using the principles of water in oil emulsions[48]. The formula and the components used in the formulation of the cream are presented in Table 1. The type of base used in formulating a topical dermatologic product greatly influences its effectiveness. Water and glycerol which forms the hydrophilic component of the formulation were serve as moisturizer and vehicle to solubilize and disperse the extract in the nonaqueous phase of the emulsion. The soft paraffin in the formula was added to impart emollient effect on the cream and ameliorate dryness and irritation of the injured skin by forming occlusive barrier on the skin to prevent the escape of moisture from the skin into the environment thereby causing moisture to accumulate between the skin and the cream layer to prevent dehydration. Hydration of the stratum corneum also allows the opening up of intra and intercellular channels and pathways for easier passage of drug molecules into cells and damaged tissues[49]. Other attributes of the water in oil based cream includes easy washability and high skin pores occlusion efficiency. Generally, occlusion of wounds has been identified to significantly reduce inflammation which corresponds to reduction in pains and scaring[50]. Reduction of pain and inflammation as well as acceleration of wound healing has been enhanced with moist healing environment and such exhilarating pro?wound healing environment has been achieved using water in oil creams which corresponds to an occlusive formulation[51,52]. Apart from the intrinsic potency of the extract in relation to its bioactive components, the effectiveness of its formulation is also critical as this corresponds to the functional effectiveness of the extract. The stability of the cream is critical to its efficacy and safety. The appearance and consistency of the formulations was used to evaluate the stability of the creams as obvious instability may appear as change in colour and/or consistency. With this regards there were no signs of coalescence, change in colour or consistency in the creams under the different stress conditions. Spoilage or instability in certain creams has also occurred as changes in pH which has resulted in such adverse reactions as skin irritation[53]. The effect of storage time on the pH of the creams is presented in fig. 2. The pH of the creams showed differences in pH that can be related to either to the presence or concentration of H. sabdariffa extract in the cream. The creams containing the extract showed lower pH, the pH of the creams increased with decrease in the concentration of the extract (fig. 2). A pH of 2.1±0.6 has been reported for the aqueous extract which corroborates the low pH of the creams. On storage the creams containing the extract did not show significant changes in pH within 14 days. However, creams containing gentamicin only, combination of gentamicin and H. sabdariffa and the placebo cream showed variable increases in pH. The maintenance of a stable pH by creams containing the different concentration of extract is an evidence of the stability of the cream.
Wounds may occur as a result of mechanical abrasions, surgical procedures, cuts, burns, infectious diseases and other pathological conditions[2]. All wounds follow roughly the same healing process, which consists of an orderly progression of events that re?establish the integrity of the damaged tissue. Though, many wounds can heal naturally, there is however the need to accelerate the healing process as several complications may arise when wound healing is delayed[41]. Prolongation of wound healing often results in prolonged trauma and secondary infections[43]. Further complication may occur when wound are infected with tetanus, or colonising bacteria escape from the primary location of the wound and enter the blood leading to septicemia. Severity of the wounds and/or poor state of health of the individual are among the factors that prolong wound healing[9]. Different pro?wound healing techniques has been proposed and used for managing cutaneous wounds. The plant kingdom remains a major source of biochemicals with potent wound healing abilities. A large number of plants with antimicrobial activity have shown effective wound healing activities others without no antimicrobial potential has also modulated wound repairs[9,43,54]. The wound healing characteristics of the creams are presented in figs. 3 and 4. Though, the methanol extract did not show any antimicrobial activity, the cream however showed significant (P<0.05) wound healing activity. The creams containing 1 and 5% w/w of the extract as well as the placebo cream all showed an initial increase in the wound diameter before a subsequent progressive wound closure. Noticeable healing started after day 2, of applications for the gentamicin cream and creams containing 1 and 5% w/w of the extract. However, for the placebo cream wound closure commenced after 15 days of treatment (fig. 3). The creams containing 10% w/w of the extract and 10% w/w of the extract/gentamicin combination showed an enhanced progressive wound healing. Wound healing started with the first cream application. The progression of wound closure with the gentamicin cream and the cream containing the 10% w/w extract were comparable. The effect of the creams containing the extract on the total wound closure was typically concentration dependent (fig. 4). The creams containing the combination of the extract and gentamicin showed an obvious synergism in wound healing progression and the time required for achieving complete wound closure.
References
- Esimone CO, Ekong US, Nworu CS, Okereke BC. Evaluation of the antiseptic properties of Cassia alata-based herbal soap. Int J Alternat Med 2008;6:117-24.
- Diegelmann RF, Evans MC. Wound healing: An overview of acute, fibrotic and delayed healing. Front Biosci 2004;9:283-9.
- Clark RA. Cutaneous wound repairs. In: Goldsmith LA, editor. Physiology, Biochemistry and Molecular Biology of Skin. New York: Oxford University Press; 1991.
- Shetty S, Udupa S, Udupa L. Evaluation of antioxidant and wound healing effects of alcoholic and aqueous extract of Ocimum sanctum Linn in Rats. Evid Based Complement Alternat Med 2008;5:95-101.
- Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. ClinMicrobiol Rev 2001;14:244-69.
- Thomas JC. Veterinary Pathology. 6th ed. Maryland, USA: William’s and Wilkins; 1997.
- Karl M, Lacrix JV, Peterson HH. Canine Surgery. 4th ed. California: American Veterinary Publications; 1995. p. 42-5.
- Mallefet P, Dweck CA. Mechanisms involved in wound healing. Biomed Sci 2008;7:609-15.
- Palu A, Su C, Zhou BN, West B, Jensen J. Wound healing effects of noni (Morindacitrifolia L.) leaves: A mechanism involving its PDGF/A2A receptor ligand binding and promotion of wound closure. Phytother Res 2010;24:1437-41.
- Udupa AI, Kulkumi DR, Udupa SI. Effect of Tridaxprocumbens extracts on wound healing. Int J Pharmacogn 1995;33:37-40.
- Taranalli AD, Kuppast IJ. Study of wound healing activity of seeds of Trigonellafoenumgraceum in rats. Indian J Pharm Sci 1996;58:117-9.
- Saha K, Mukherjee PK, Das J, Pal M, Saha BP. Wound healing activity of Leucaslavandulaefolia Rees. J Ethnopharmacol 1997;56:139-44.
- Carson CF, Riley TV, Cookson BD. Efficacy and safety of tea tree oil as a topical antimicrobial agent. J Hosp Infect 1998;40:175-8.
- Chithra P, Sajithlal GB, Chandrakasan G. Influence of Aloe vera on the glycosaminoglycans in the matrix of healing dermal wounds in rats.J Ethnopharmacol 1998;59:179-86.
- Veerapur VP, Palkar MB, Srinivasa H, Kumar MS, Patra S, Rao PG, et al. Effect of ethanol extract of Wrightiatinctoria bark on wound healing in rats. J Nat Remed 2004;4:155-9.
- Rathi B, Pathi PA, Baheti AM. Evaluation of aqueous extract of pulp and seeds of Moringaoleifera for wound healing in albino rats. J Nat Remed 2004;4:145-9.
- Thaker AM, Anjaria JV. Antimicrobial and infected wound healing response of some traditional drugs. Indian J Pharmacol 1986;18:171-4.
- Fasoyiro SB, Ashaye OA, Adeola A, Samuel FO. Chemical and storability of fruit-flavoured (Hibiscus sabdariffa) drinks. World J AgricSci 2005;1:165-8.
- Aliyu L. Roselle. (Hibiscus sabdariffa L.). J ApplAgricTechnol 2000;6:16-20.
- Parkouda C, Diawara IB, Ouoba IL. Technology and physicochemical characteristics of Bikalga, alkaline fermented seeds of Hibiscus sabdariffa. Afr J Biotechnol 2008;7:916-22.
- Chifundera K, Balagizi K, Kizungu B. Les empoisonnementsetleurs antidotes en médecinetraditionnelle au Bushi, Zaire. Fitoterapia 1994;65:307-13.
- Builders PF, Ezeobi CR, Tarfa FD, Builders MI. Assessment of the intrinsic and stability properties of the freeze-dried and formulated extract of Hibiscus sabdariffa (Linn). Afr J Pharm Pharmacol2010;4:304-13.
- Qi Y, Chin K, Malekian LF, Berhame M, Gager J. Biological characteristics, nutritional and medicinal value of Roselle Hibiscus sabdariffa. Circular-urban forestry. Nat Resour Environ 2005;604:1-2.
- Babalola SO, Babalola AO, Aworh OC. Compositional attributes of the calyces of roselle (Hibiscus sabdariffa.L). J Food TechnolAfr 2000;6:133-4.
- Wong P, Salmah YH, Cheman, YB. Physico-chemical characteristics of roselle (Hibiscus sabdariffa l.). Nutr Food Sci 2002;32:68-73.
- Sena LP, Vanderjagt DJ, Rivera C, Tsin AT, Muhamadu I, Mahamadou O, et al. Analysis of nutritional components of eight famine foods of the Republic of Niger. Plant Foods Hum Nutr1998;52:17-30.
- Poustka F, Irani NG, Feller A, Lu Y, Pourcel L, Frame K, et al. A trafficking pathway for anthocyanins overlaps with the endoplasmic reticulum-to-vacuole protein-sorting route in Arabidopsis and contributes to the formation of vacuolar inclusions. Plant Physiol 2007;145:1323-35.
- Atta S, Diallo AB, Bakasso Y, Sarr B, Saadou M, Glew RH. Micro-element contents in roselle (Hibiscus sabdariffa L.)at different growth stages. Afr J Food AgricNutr Dev 2010;10:2615-28.
- Wang CJ, Wang JM, Lin WL, Chu CY, Chou FP, Tseng TH. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food ChemToxicol 2000;38:411-6.
- Siddique NA, Mujeeb M, Najmi AK, Akram M. Evaluation of antioxidant activity, quantitative estimation of phenols and flavonoids in different parts of Aeglemarmelos. Afr J Plant Sci 2010;4:1-5.
- National Institute for Pharmaceutical Research and Development (NIPRD). Quality Manual 2012 – Part II – Microbiology Quality/11A/SOPs.
- Akhtar N, Shahiq-uz-zaman, Khan BA, Haji M, Khan S, Ahmad M, et al. Evaluation of various functional skin parameters using a topical cream of Calendula officinalis extract. Afr J Pharm Pharmacol 2011;5:199-206.
- Morton JJ, Malone MH. Evaluation of vulneray activity by an open wound procedure in rats. Arch IntPharmacodynTher 1972;196:117-26.
- Esimone CO, Nworu CS, Jackson CL. Cutaneous wound healing activity of a herbal ointment containing the leaf extract of Jatrophacurcas L. (Euphorbiaceae). Int J Appl Res Nat Prod 2008;1:1-4.
- Green RJ. Antioxidant activity of peanut plant tissues. Master Thesis. USA: North Carolina State University; 2004.
- Chatha SA, Anwar F, Manzoor M, Bajwa JR. Evaluation of the antioxidant activity of rice bran extracts using different antioxidant assays. GrasasAceitesSevilla 2006;57:328-35.
- Anwar F, Jamil A, Iqbal S, Sheikh MA. Antioxidant activity of various plant extracts under ambient and accelerated storage of sunflower oil.GrasasAceitesSevilla 2006;57:189-97.
- Lourens AC, Reddy D, Baser KH, Viljoen AM, Van Vuuren SF. In vitro biological activity and essential oil composition of four indigenous South African Helichrysum species. J Ethnopharmacol 2004;95:253-8.
- Parekh J, Karathia N, Chanda S. Screening of some traditionally used medicinal plants for potential antibacterial activity. Indian J Pharm Sci2006;68:832-4.
- Vairavasundaram RP, Senthil K. Effect of sample preparation and TLC methods on the quantitation of quercetin content in asthma weeds. IntJ Drug Dev Res 2010;2:15-9.
- Gbedema SY, Emelia K, Francis A, Kofi A, Eric W. Wound healing properties and kill kinetics of Clerodendronsplendens G. Don, a Ghanaian wound healing plant. Pharmacogn Res 2010;2:63-8.
- Langford JH, Artemi P, Benrimoj SI. Topical antimicrobial prophylaxis in minor wounds. Ann Pharmacother 1997;31:559-63.
- Ofori-Kwakye K, Kwapong AA, Adu F. Antimicrobial activity of extracts and topical products of the stem bark of Spathodeacampanulata for wound healing. AfrJ Tradit Complement Altern Med 2009;6:168-74.
- Mbosso EJ, Ngouela S, Nguedia JC, Penlap V, Rohmer M, Tsamo E. Spathoside, a cerebroside and other antibacterial constituents of the stem bark of Spathodeacampanulata. Nat Prod Res 2008;22:296-304.
- Mensah AY, Houghton PJ, Dickson RA, Fleischer TC, Heinrich M, Bremner P. In vitro evaluation of effects of two Ghanaian plants relevant to wound healing. Phytother Res 2006;20:941-4.
- Du CT, Francis FJ. Anthocyanin of rossele Hibiscus sabdaraffa L. J Food Sci 1973;38:310-2.
- Abu-Tarboush HM, Ahmed AA, Kahtani HA. Some nutritional andfunctional properties of Karkade (Hibiscus sabdariffa) seed products. Cereal Chem 1997;74:352-5.
- De Vringer T, de Ronde HA. Preparation and structure of a water-in-oil cream containing lipid nanoparticles. J Pharm Sci 1995;84:466-72.
- Roberts MS, Pugh WJ, Hadgraft J. Epidermal permeability: Penetrant structure relationships. 2. The effect of h-bonding groups in penetrants on their diffusion through the stratum corneum. Int J Pharm 1996;132:23-32. Moist healing and wound care including burns. Burnsugery.org, 2000. Available from: http://www.burnsurgery.org/Betaweb/Modules/moisthealing/part_2bc.htm [Last accessed on 11 January 2013].
- Hultén L. Dressings for surgical wounds. Am J Surg 1994;167:42S-4.
- Metzger S. Clinical and financial advantages of moist wound management. Home Healthc Nurse 2004;22:586-90.
- Atiyeh BS, Amm CA, El Musa KA. Improved scar quality following primary and secondary healing of cutaneous wounds. Aesthetic PlastSurg 2003;27:411-7.
- Kikwai L, Babu RJ, Prado R, Kolot A, Armstrong CA, Ansel JC, et al. In vitro and in vivo evaluation of topical formulations of spantide II. AAPS PharmSciTech 2005;6:E565-72.
- Woods RK, Dellinger EP. Current guidelines for antibiotic prophylaxis of surgical wounds. Am Fam Physician 1998;57:2731-40.
 Ascorbic acid,
Ascorbic acid, H. sabdariffa.
H. sabdariffa.
 F,
F, E,
E, 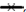 , D
, D  , C
, C B,
B, A. A=1%w/w H. sabdariffa; B=5%w/w H. sabdariffa; C=10%w/w H. sabdariffa; D=1%w/w H. sabdariffa+1%w/w Gentamicin; E=Gentamicin; F=Placebo cream.
A. A=1%w/w H. sabdariffa; B=5%w/w H. sabdariffa; C=10%w/w H. sabdariffa; D=1%w/w H. sabdariffa+1%w/w Gentamicin; E=Gentamicin; F=Placebo cream.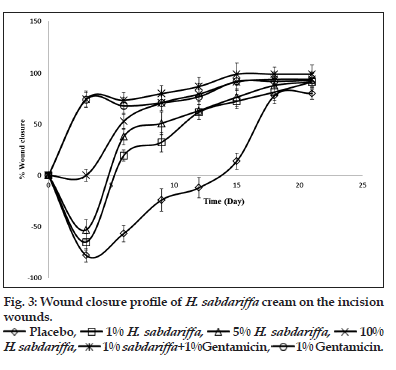
 1% sabdariffa+1%Gentamicin,
1% sabdariffa+1%Gentamicin, 1% Gentamicin.
1% Gentamicin.