- *Corresponding Author:
- M. S. Charde
Department of Pharmaceutical Sciences, Nagpur University Campus, Amrawati Road, Nagpur-440033, India
E-mail: manojudps@ rediffmail.com
| Date of Submission | 16 October 2004 |
| Date of Revision | 19 May 2005 |
| Date of Acceptance | 5 February 2006 |
| Indian J Pharm Sci, 2006, 68 (1): 26-31 |
Abstract
Madhu ghrita is a herbal formulation containing honey and ghee (clarified butterfat) as its constituents. The aim of present study is to verify the wound healing and antiinflammatory claims of Madhu ghrita. Incision and excision wound models were used for evaluation of wound healing activity followed by histopathological study in which healing markers like keratinization, epithelization, fibrosis, neovascularisation and collagenation were evaluated in male Wistar rats. The results of Madhu ghrita were compared with the results of untreated control group and results of framycetine sulphate cream, considered as a positive control. The formulation was also tested for antiinflammatory activity, using carrageenan-induced paw oedema in male Wistar rats. The test formulation Madhu ghrita enhanced the tensile strength, which significantly improved over untreated wounds. The tensile strength of untreated control wound was 281±5.82, while with the Madhu ghrita and framycetine sulphate cream 1% w/w, it was 328±8.9 and 398±6.32, respectively. Treatment with Madhu ghrita alone promoted wound contraction and reduced the wound closure time, so increase in tensile strength and wound contraction shows the wound healing potential of Madhu ghrita . Histopathological study shows that proliferation of epithelial tissue promotes angiogenesis, multiplication of fibrous connective tissue due to treatment with Madhu ghrita . The test formulation Madhu ghrita also shows significant antiinflammatory activity when the results are compared with the activity of ibuprofen gel as reference standard. The present study demonstrates the wound healing and antiinflammatory potential of Madhu ghrita .
The topical application of honey to wounds originated with ancient civilizations [1]. Its efficacy in wound healing remains largely anecdotal, with claims that it reduces inflammation, debrides necrotic tissue, reduces oedema and promotes angiogenesis, granulation and epithelization [2,3]. Antibacterial activity of honey has been established in vitro but the mechanism involved in wound healing is unexplained [4]. More recently, honey has become a topic of clinical and scientific research in wound care [5]. In Nigeria, used undiluted honey on a variety of wounds, including Fournier’s gangrene and burns. He found that burns reduce the number of positive swab culture, and he used it to debride slouchy and necrotic tissues [6]. Strong solution of honey or sugar and sugar paste inhibit microbial growth because of high osmolarity [7], but when used as dressing they become diluted to the point where its action ceases, especially in case of staphylococcus aureus [8,9]. Such wounds are rapidly rendered sterile by honey [10,13] because of its additional antimicrobial activity [14].
Wounds may be defined as loss or breaking of cellular and anatomic or functional continuity of living tissue [15]. Wound healing is a process that is fundamentally a connective tissue response. Initial stage of this process involves an acute inflammatory phase followed by synthesis of collagen and other extracellular macromolecules that are later remodeled to form scar [16,17]. Inflammation is often associated with pain and fever, which may be due to release of histamine, kinins, serotonin and prostaglandin [18]. The antiinflammatory agents are normally inhibiting the release of these inflammatory mediators to give relief from pain and inflammation.
The present study deals with the evaluation of wound healing and antiinflammatory potential of the test formulation. Madhu ghrita (MG) is a herbal formulation containing honey (50%) and clarified butterfat (50%) as its constituents. The wound healing study was evaluated by using incision and excision wound model followed by histopathological study. Antiinflammatory study was screened by carrageenan-induced rat paw oedema method [19]. The formulation MG belongs to panchagavya class of Ayurvedic texts. Panchagavya refers to the five important products of bovine origin, viz., milk, curd, ghee, urine and dung [20]. Ayurvedic texts describe several uses of panchagavya components. Ayurvedic medicines are largely based upon herbs, either single or in combination, having specific diagnostic and therapeutic principles [21]. Modern literature documents few pharmaceutical and clinical uses of panchagavya formulations [22,23].
Materials and Methods
Preparation of herbal formulation
MG was studied in present communication for its wound healing and antiinflammatory activity. The formulation MG was prepared by procedure mentioned in ancient Ayurvedic texts. This formulation contains two ingredients, viz., honey and clarified butterfat. The equal quantity of honey (50%) and clarified butterfat, previously liquefied (50%), in ratio of 1:1, were taken in mortar, stirred and triturated to get the homogeneous mixture [24]. This mixture, cooled and stored in a bottle, has been used for topical application to wounds. MG was obtained as a gift sample from Go-vigyan Anusandhan Kendra, Nagpur. All other chemicals and reagents used in the study were of AR/GR grade.
In the present experimental study the male Wistar rats (150-200 g) were used. They were housed and cared individually in clean polyethylene cages under standard environmental conditions of temperature 23±1°, 12 h light/ dark cycle and fed on normal pellet diet (Gold Mohur Brand, Lipton India Limited) and water ad libitum.
Wound healing activity
Animals were acclimatized under laboratory conditions before experiments were carried out. Animals were assigned into three groups containing six animals in each: Group 1, untreated control group; Group 2, application of MG formulation; and Group 3, application of framycetine sulphate cream (FSC) 1% w/w. The experimental protocols were approved by the Institute of Animal Ethics Committee. Except the drug under study, no topical or systematic therapy was given to animals. Animals showing infection/deterioration of wounds were excluded from the study and replaced with other animals. Animals received drugs daily from day ‘‘0’’ till complete wound healing or up to the 27th postoperative day, whichever was earlier.
Excision wounds
Animals were anaesthetized with ether and shaved on part to be exposed. A circulation piece (300 mm2 in area) was impressed on the dorsal thoracic region 5 cm away from the ears. Wound contraction was monitored by measuring wound area with 3-day gap till wounds were completely healed [25]. Wound contraction was studied by tracing the raw wound area on polythene paper every third day till wounds were completely healed. The animals were maintained individually in separate cages. Madhu ghrita, the test formulation, was applied on the wound once daily up to day 27, starting from first day of wounding.
Incision wounds
Animals were anaesthetized and paravertebral incision (2.5 cm) was made through the entire length of skin. After the incision was made, the part of skin was kept together and stitched with nylon thread, 0.5 cm apart, using a curved needle (Needle no. 11). The sutures were removed on the 8th day and complete healing (tensile) strength was measured on the 10th day of healing of incision wound [26]. The tensile strength was measured with the manually operated instrument. In this instrument, the strength developed in the 10-day healed incision wound was measured against the increasing weight to the other end.
Histopathological study
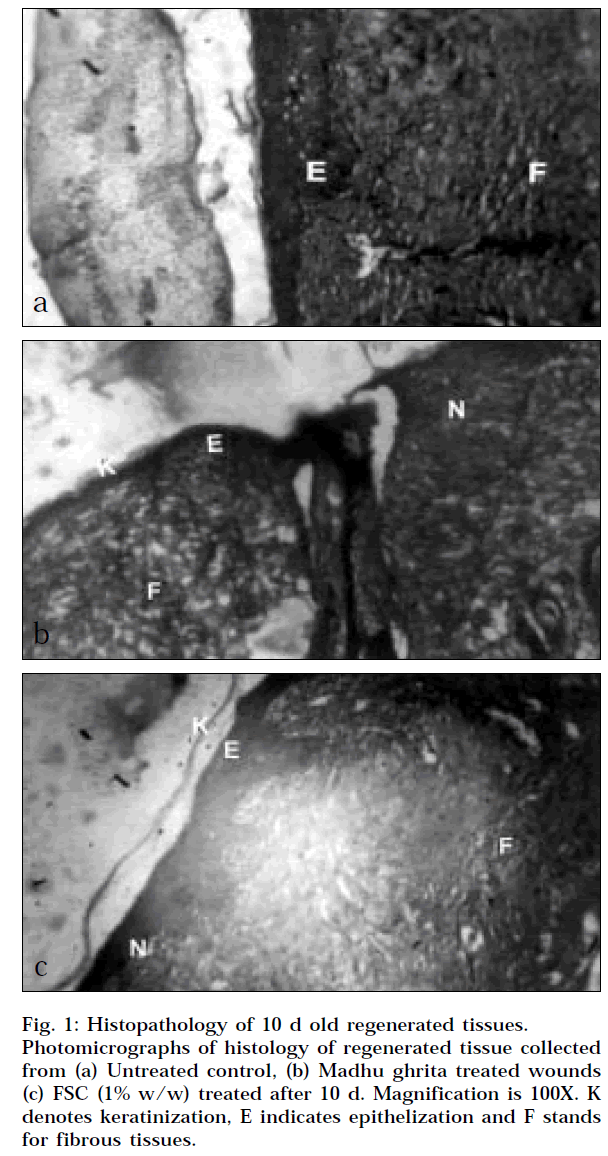
The present experimental study was supported with the histopathological study that involves the study of healing markers like keratinization, epithelization, collagenation, fibrosis and neovascularisation on the 10-day healed regenerated tissue. Immediately after taking the section, it was fixed with 10% formalin solution, and the tissues were dehydrated with 90% ethanol, embedded in paraffin, cut into thin sliced sections (7 mm thick), stained with haemotoxyline-eosin dye and observed under light microscope for healing markers like keratinization, epithelization, collagenation, fibrosis and neovascularisation. The results were studied and quantified by number from 1 to 5 where 5 stands for maximum similarity and 1 stands for least similarity from normal tissue round the wound area in group 1, 2 and 3.
Antiinflammatory activity
Antiinflammatory activity of MG formulation was studied by carrageenan-induced paw oedema method. The animals were divided into three groups containing six animals in each group. Group 1, (Control) Untreated group; Group 2, topical application with MG formulation; Group 3, topical application of ibuprofen gel. Group 2 and 3 received topical application of test formulation MG and ibuprofen gel, respectively, for comparison of antiinflammatory activity. One hour after the application of Madhu ghrita formulation, 0.1 ml of carrageenan (1%) was injected into sub-plantar region of hind paw of the rat. Measurements of paw volume (ml) were made by mercury displacing techniques using plethysmometer. Immediately before and 1, 2, 3, 4 h after carrageenan injection, percentage inhibition of inflammation after 1, 2, 3 and 4 h was calculated by Newbould method [27].
Statistical analysis
Data were analysed using one-way analysis variance (ANOVA) followed by Tukey-Kramer multiple comparison test (P<0.001), and this data were considered significant.
Results
In the present experimental study, the excision wound is considered as the model for evaluation of wound healing. In excision wound study, the wounds were treated with FSC 1% w/w cream and test formulation MG and the results show complete healing on day 24 and day 27 respectively. The results of wound healing were compared with untreated wounds (Group 1), which took more than 30 days for healing. The study was carried out till the fall of scar leaving no raw material behind. The results of excision wound studies are shown in Table 1.
| Post Wounding Days | Wounding Area (mm2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | F values | ||||||||
| 0 | 378.46 ± 6.08 | (0) | 399.08 ± 1.79 | (0) | 345.05 ± 5.45 | (0) | F(2,15)=26.698 | ||||
| 3 | 280.44 ± 7.72 | (25.94) | 328.28 ± 4.14 | (17.74) | 300.05 ± 3.06 | (13.04) | F(2,15)=20.669 | ||||
| 6 | 245.66 ± 5.98 | (35.08) | 271.35 ± 4.90* | (32.00) | 243.43 ± 8.16* | (29.45) | F(2,15)=6.133 | ||||
| 9 | 209.93 ± 5.78 | (44.53) | 224.45 ± 5.12* | (43.75) | 199.88 ± 7.31* | (45.07) | F(2,15)=4.258 | ||||
| 12 | 193.18 ± 2.63 | (48.95) | 179.78 ± 5.22* | (54.95) | 161.91 ± 4.98* | (53.07) | F(2,15)=12.475 | ||||
| 15 | 176.66 ± 1.79 | (53.40) | 130.61 ± 4.88 | (67.27) | 120.60 ± 4.78 | (65.04) | F(2,15)=52.449 | ||||
| 18 | 151.09 ± 3.07 | (60.07) | 90.23 ± 7.21 | (77.39) | 80.20 ± 2.86 | (76.75) | F(2,15)=62.487 | ||||
| 21 | 121.55 ± 2.77 | (67.88) | 31.73 ± 5.37 | (92.04) | 29.80 ± 2.53 | (91.36) | F(2,15)=190.14 | ||||
| 24 | 101.22 ± 2.62 | (73.25) | 20.07 ± 4.20 | (94.97) | 0.00 ± 0.00 | (100) | F(2,15)=346.33 | ||||
| 27 | 60.74 ± 2.86 | (83.96) | 0.00 ± 0.00 | (100) | 0.00 ± 0.00 | (100) | F(2,15)=448.53 | ||||
Table 1: Effect Of Topical Application Of Madhu Ghrita On Excision Wound
The incision wound study was also carried out to measure the tensile strength on day 10 of regenerated tissue. The incision wounds treated with MG formulation and FSC 1% w/w cream show tensile strength 328±8.90 g and 398±6.32 g, respectively, compared with mean ±SEM tensile strength of untreated group, i.e., 281±5.82 g. The above observation for tensile strength shows that the incision wounds treated with MG formulation show better tensile strength as compared to untreated group and the group treated with FSC 1% w/w cream. This shows wound healing potential of tested formulation. The results are mentioned in Table 2.
| Groups | Treatment | Tensile Strength (g) |
|---|---|---|
| 1 | Untreated | 281 ± 2.87 |
| 2 | MG formulation | 328 ± 3.63* |
| 3 | FSC 1% w/w cream | 398 ± 3.92* |
| F Values | F (2,15) = 282.20 |
Table 2: Effect Of Topical Application Of Madhu Ghrita On Incision Wound
The last parameter studied for wound healing activity was histopathological study. In this study, some healing markers were selected and evaluated like epithelization, keratinization, fibrosis, collagenation and neovascularisation. After interpretation of histopathological slide, it was observed that the tested formulation MG promotes keratinization, fibrosis, collagenation and neovascularisation. The histopathological results of test formulation compared with the results of untreated group and results of FSC 1% w/w cream, which is considered as positive control. The results are shown in Table 3 with photomicrograph in Fig. 1a, 1b and 1c.
| Parameters | Group 1 | Group 2 | Group 3 | F Values |
|---|---|---|---|---|
| Keratinization | 0.3 ± 0.02 | 4.1 ± 0.05 | 4.0 ± 0.13 | F(2,15) =603.00 |
| Epithelization | 1.7 ± 0.13 | 3.3 ± 0.08 | 4.2 ± 0.12 | F(2,15) =116.37 |
| Fibrosis | 2.3 ± 0.15 | 4.2 ± 0.05 | 4.1 ± 0.16 | F(2,15) =57.918 |
| Collagen | 2.8 ± 0.15 | 4.4 ± 0.08 | 4.4 ± 0.14 | F(2,15) =49.870 |
| Neovascularisation | 0.5 ± 0.06 | 3.7 ± 0.05 | 4.4 ± 0.12 | F(2,15) =540.42 |
Table 3: Histopathological Examinations Of Wound Treated With Madhu Ghrita At End Of 10 Days
The topical antiinflammatory activity of the MG formulation was also carried out. The percentage protection (inhibition) of oedema for MG formulation and ibuprofen gel was found to be 29.97 (1 h), 60.94 (2 h), 55.00 (3 h), 57.17 (4 h); and 36.18 (1 h), 67.17 (2 h), 63.93 (3 h), 67.06 (4 h) respectively. The tested formulation MG showed significant antiinflammatory activity when the results were compared with untreated group and decrease in inflammation by ibuprofen gel was taken as reference standard. The results are significant (P<0.001) and are shown in Tables 4, 5.
| Groups | Average weight of Animals (g) | Dose (mg/kg) | Mean value ± SEM of Odema Volume at different intervals | ||||
|---|---|---|---|---|---|---|---|
| 1h | 2h | 3h | 4h | ||||
| Group 1 (Untreated) | 160 | - | 0.3156 ± 3.03 | 0.7576 ± 7.74 | 0.8835 ± 7.53 | 0.8602 ± 7.22 | |
| Group 2 (MG formulation) | 178 | 100 | 0.2210 ± 22.10 | 0.2959* ± 2.39 | 0.3975* ± 2.46 | 0.3684* ± 1.22 | |
| Group 3 (Standard) | 197 | 100 | 0.2014 ± 1.77 | 0.2487 ± 1.78 | 0.3186 ± 2.26 | 0.2833 ± 2.02 | |
| F values | (2,15)= 5.201 | (2,15)=34.401 | (2,15)=41.297 | (2,15)=50.337 | |||
Mean value of odema volume at different intervals
Table 4: Data Showing The Anti inflammatory Activity Of Formulation.
| Groups | Dose (mg/kg) | Percent inhibition of paw volume at different time intervals | |||
|---|---|---|---|---|---|
| 1h | 2h | 3h | 4h | ||
| Standard (Ibuprofen) Test | 100 | 36.18 | 67.17 | 63.93 | 67.06 |
| (MG Formulation) | 100 | 29.97 | 60.94 | 55.00 | 57.17 |
Percent inhibition of paw volume at different intervals
Table 5: Topical Anti inflammatory Activity Of Mg Formulation.
Discussion
Wound healing involves a highly dynamic integrated series of cellular physiological and biochemical processes that occur in living organisms. Repair through regeneration is very common in unicellular and the lower metazoan animal group, while it is highly restricted in the higher animals [28]. Proper and timely wound healing is a vexing problem faced by all clinicians. In majority of patients, normal healing establishes tissues integrity quickly and effectively. However at times, this healing is delayed and the ability to accelerate the wound healing becomes highly desirable [29].
In excision wound study, from above observations, it was concluded that the test formulation MG showed better and faster healing as compared to the untreated group. During the initiation of study from day ‘‘0’’, there was not much difference in the healing of wounds in all the three groups, i.e., Group 1, Group 2 and Group 3 (days 0-9). But after day 9, the healing process was faster with Group 2, i.e., wounds treated with MG formulation. When the results are compared with Group 1, Group 2 shows faster wound closure and wound contraction. When the results were interpreted in higher phase (days 12-21), the healing potential of MG was clearly seen from the results in comparison with the untreated group. The healing potential of Group 3, i.e., wounds treated with FSC 1% w/w cream, was taken as positive control and we observed that the results shown by Group 2 and Group 3 are comparatively similar indicating similar potential of MG formulation over FSC 1% w/w cream for wound healing activity. The wounds treated with MG formulation (Group 2) shows complete healing on day 27, and day 24 for wounds treated with FSC 1% w/w cream (Group 3). The untreated group took more than 30 days for complete healing of wounds. So from the results, it can be concluded that MG takes less time for the complete healing of wound as compared to untreated group, hence showing better and faster wound healing activity.
In the incision wound study, after treatment with MG, on 10th day, regenerated tissue shows increase in tensile strength as compared to untreated group. Both FSC 1% w/w cream and MG treatment increase tensile strength, ultimately, showing enhancement of wound healing activity in male Wistar rat. The increase in tensile strength may be due to promotion of collages formation which significantly contributes to better and effective healing.
Wound healing involves different phases such as contraction, epithelization, granulation and collagenation [30]. In the present study, various markers were studied for evaluation histopathological study like keratinization, epithelization, fibrosis, collagenation and neovascularisation. The results that were observed and interpreted evidenced that the wounds treated with MG and FSC 1% w/w cream were promoting the healing marker and showing better wound healing activity compared to wound healing activity of untreated control group. MG promotes keratinization, fibrosis, collagen formation and neovascularisation to a greater extent. The wounds treated with MG formulation and FSC 1% w/w cream promoted keratinization to a greater extent as compared to untreated control group. In case of epithelization as healing marker, MG formulation showed a quite lesser enhancement of epithelization as compared the positive control, but better than that of untreated control group. When other healing markers like fibrosis, collagenation and neovascularisation were taken into consideration, MG showed improved results over the results of untreated group, and the results shown by MG group was comparable with the results of FSC 1% w/w cream treated group. This evidenced the promotion of all healing markers, i.e., wound healing activity shows healing potential of tested formulation.
The observed results for MG treated group (Group 2) shows the promotion of wound healing in all the three models when results are compared with the untreated control group (Group 1). This verifies the stated claim of MG formulation for the promotion of wound healing activity.
Determination of antiinflammatory activity is based on plethysmographic measurement of oedema produced by sub planar injection of carrageenan in the hind paw of rat. The increase in oedema in animals treated with standard drug (ibuprofen) and MG formulation were compared with increase in oedema of untreated control animals at constant intervals of 1, 2, 3 and 4 h. Thus percentage inhibition of oedema at known intervals in treated animals was used for the purpose of calculating percent inhibition of oedema of control. The present study revealed that the MG formulation showed better antiinflammatory activity. The maximum activity was observed during 3rd and 4th h, and the results are significant (P<0.001) and are comparable to standard Ibuprofen. The antiinflammatory activity may be due to the inhibition of release of histamine, serotonin and kinins in first hour after the injection of carrageenan, and this also retarded the release of prostaglandin-like substance in 2-4 h [31], showing antiinflammatory potential of MG formulation. The above obtained evidences for the wound healing and antiinflammatory study verify the objective of the present study.
Acknowledgements
The authors are thankful to Dr. S. G. Wadodkar, Department Pharmaceutical Sciences; and Nagpur University, Nagpur for providing working facilities and to Dr. N. V. Kurkure for his constant help in proceeding with this work.
References
- Forrest, R.D., J. Roy. Soc. Med., 1982, 75, 198.
- Malon, P.C., Primary Intention, 1998, 6, 148.
- Malon, P.C., J. Wound Care, 1999, 8, 415.
- Tonks, A., Cooper, R.A., Price, A.J., Malon, P.C. and Jones, K.P., Cytokines, 2001, 14, 240.
- Subrahmanyam, M.A., Burns, 1998, 24, 157.
- Dunford, C., Nursing Standard, 2000, 15, 63.
- Chirife, J., Scarmato, G. and Herszage, L., Lancet, 1982, 560.
- Chirife, J., Herszage, L., Joseph, A. and Kohn, E. S., AntimicrobAg. Chemother.,1983, 23, 766.
- Herszage, L., Montenegro, J.R. and Joseph, A.C., Bol. Tra. Soc.Argent Ci.,1980, 41, 315.
- Efem, S.E., Brit. J. Surg., 1988, 75, 679.
- Cavanagh, D., Beazley, J. and Osta, P., J. obstet. Gynaecol. Br.Comm. 1970, 77, 1037.
- Armon, P.J., Trop. Doct., 1980, 10, 91.
- Braniki, F.J., Ann. R. Coll. Surg. Engl.,1981, 63, 348.
- Cooper, R.A. and Molan. P.C., J. R. Soc. Med., 1999, 92, 283.
- Patil, M.B., Jalalpure, S.S. and Ali, A., Indian drugs, 2001, 38, 288
- Chithra, P., Sajithal, G.B. and Chandra K.G., Indian. J. Exp. Bio.,1998, 36, 896.
- Jaswanth, A., Akilandewari, L.V., Manimaran, S. and Ruckmani.,Indian J. Pharm. Sci., 2001, 63, 41.
- Rang, H.P., In; Textbook of pharmacology, International StudentEdn., Churchill Livingstone, 1995, 246.
- Thirupathy, K.P., Ananda, V.A., Kumar, P.R. and Rajasekaran, A., Indian Drugs, 2001, 38, 426.
- Charde, M.S., Fulzele, S.V., Satturwar, P.M. and Dorle A.K., Indiandrugs,2003, 40, 115.
- Sai, P.K., Reddy, N.P. and Babu, M.,Indian. J. Exp. Bio., 1995,3, 673.
- Oyebola, D.D. and Ariwodola, J.O., Afr. J. Med. Sci., 1985, 14, 9.
- Adekile, A.D., Odebio, O. and Ojewole, A.O., J. Trop. Pediatr.,1983, 29, 299.
- Preparation of Madhughrita, Ancient Ayurvedic Text, 1, 130.
- Shobha, N.S. and Rao, G.S., Indian Drugs, 2000, 37, 412.
- Charde, M.S., Fulzele, S.V., Satturwar, P.M. and Dorle, A.K., IndianJ. Pharm. Sci.,2003, 65, 482.
- Thomas, C.A. and Rama sharma, G.V.S., Indian Drugs, 1999, 6,203.
- Purna, K.S., Neelakanta, P.R. and Batu, M., Indian. J. Exp. Bio.,1995, 33, 673.
- Bisht, D., Mehrotra, R., Singh, P.A., and Ashok, K., Indian. J. Exp.Bio.,1996, 37, 187.
- Hemalatha, S., Sobramanian, N., Ravichandran, V. and ChinnaswamyK., Indian. J. Pharm. Sci., 2001, 331.
- Thirupathy, K.P., AnandaVijaya Kumar, P.R. and Rajasekaran, A.,Indian Drugs, 2001, 38, 426