- *Corresponding Author:
- B. K. Manjunatha
Department of Botany and Biotechnology, S. R. N. M. N. College of Applied Sciences, Shimoga - 577 201, India
E-mail: doctor_bkm@Yahoo.com
| Date of Submission | 24 November 2005 |
| Date of Decision | 10 July 2006 |
| Date of Acceptance | 7 April 2007 |
| Indian J Pharm Sci, 2007, 69 (2): 283-287 |
Abstract
Wound healing activity of aqueous and ethanol leaf extracts of L. serratum was studied by excision, incision and dead space wound models on rats. As compared to aqueous and control group of animals, ethanol extract showed significant wound healing activity which was evidenced by significant decrease in the period of epithelialisation (17.84±0.06) and increase in wound contraction rate, skin breaking strength (581.45±4.98), granulation tissue breaking strength (512.80±5.08), dry weight of granulation tissue (47.23±0.10) and elevated concentration of hydroxyproline (2322.83±8.49). Histopathalogy of the granulation tissue of the ethanol extract treated animals showed few macrophages with increase in collagenation indicating the potency of the ethanol extract in promoting the process of wound healing. The present finding provides a scientific base to the ethno medicinal use of L. serratum.
Lycopodium serratum is a pteridophyte (Lycopodiaceae) commonly known as Club moss. The plant is found distributed all along the moist deciduous forests of the Western Ghats. The plant is used in the homeopathic preparations to increase efficiency of learning and memory, in treating Alzheimer’s disease [1], cancer [2] and oitis media in children [3]. The plant is reported to contain alkaloids like serratezomines A-C [4], lycoposerramine-A [5], lycoposerramine F-O [6], quinolizine or pyridine and alpha- pyridone type alkaloids which are the potent inhibitors of acetylcholineesterase [7] and triterpenoids [8]. The tribal groups of western Ghats of Chikamagalur region use this plant for treating wounds. The whole plant is ground in hot water and the thick paste thus obtained is applied externally to sores, cuts, wounds and burns (Personal communication). This paper reports the wound healing efficacy of the pteridophytic plant Lycopodium serratum.
Plants were collected from the Western Ghat range of Chikmagalur district of Karnataka State, during September to October 2003 and voucher specimen (BKM-456) was authenticated by referring the specimen to Sri Krishna Devaraya University herbaria and Kuvempu University herbaria and a voucher specimen was deposited in the departmental herbaria, SRNMN College, Shimoga for future reference.
The plants were shade dried and powdered mechanically (sieve size 10/45). About 250 g of powdered material was exhaustively extracted with 70% ethanol for about 48 h in a Soxhlet extractor. The extract was filtered and concentrated in vacuum under reduced pressure using rotary flash evaporator (Buchi, Flawil, Switzerland) (yield 20.8% w/w). For aqueous extract, 250 g of powdered material was macerated with 1000 ml of distilled water for three days with intermittent stirring, filtered and concentrated (yield 15% w/w). Both the extracts were subjected to preliminary phytochemical tests [9].
Two types of drug formulations were prepared from each of the extracts. For topical administration, 5% w/w ointment was prepared in 2% sodium alginate. For oral administration, suspensions of 30 mg/ml and 20 mg/ml of aqueous and ethanol extracts were prepared in 1% gum tragacanth.
Wistar rats of either sex weighing 150-200 g were procured from the National College of Pharmacy, Shimoga, Karnataka and were maintained at standard housing conditions. The animals were fed with commercial diet (Hindustan Lever Ltd., Bangalore) and water ad libitum during the experiment. The study was approved by the Institutional Animal Ethical Committee, National College of Pharmacy, Shimoga. Acute toxicity study was conducted for both ethanol and aqueous extracts by stair case method [10] following OECD 2002 norms. The LD50 of aqueous and ethanol extracts were found to be 300 and 200 mg/kg. One tenth of the dose was selected for the evaluation of wound healing activity i.e., 30 and 20 mg/kg, respectively [11].
The rats were inflicted with excision wounds as described by Morton and Malone [12], under light ether anesthesia. A circular wound of about 500 mm2 was made on depilated ethanol sterilized dorsal thoracic region of rats. The animals were divided into 4 groups of 6 each. The animals of group I were left untreated and considered as the control, the group II served as reference standard and treated with 1% w/w framycetin sulphate cream (FSC), the group III and IV animals were treated with 50 mg of ointment prepared from aqueous and ethanol extracts of L. serratum. The ointment was topically applied once a day till epithelialisation was complete, starting from the day of the operation. The wounds were traced on graph paper on 4th, 8th, 12th and 16th post wound days and thereafter daily until healing was complete. The parameters studied were percentage of wound closure and period of epithelialisation.
In the incision wound model, 6 cm long paravertebral incisions were made through full thickness of the skin on either side of the vertebral column of the rat as described by Ehrlich and Hunt [13]. The wounds were closed with interrupted sutures of 1 cm apart. The grouping of the animals was similar to excision wound model. The ointment was topically applied once in a day. The sutures were removed on 8th post wound day. The skin breaking strength of the wounds were measured on 10th day as described by the method of Lee [14].
Under light ether anesthesia, dead space wounds were created by subcutaneous implantation of sterilized cylindrical grass piths (2.5 cm × 0.3 cm), one on either side of the dorsal paravertebral surface of rat [15]. The animals were divided into 3 groups of 6 rats in each group. The group I served as control, which received 1 ml of 1% gum tragacanth/kg, p.o. The animals of group II and III received oral suspensions of aqueous and ethanol extracts, respectively (30, and 20 mg/kg p.o., respectively). The granulation tissues formed on the grass piths were excised on 10th post wound day and the tissue breaking strength was measured. Simultaneously, granulation tissue so harvested was subjected to hydroxyproline estimation following the method of Woessner [16] and histopathological study was carried out to evaluate the effect of the extracts on collagen formation. The data were subjected to ANOVA followed by Turkey’s multiple comparison test and the values of P ≤0.01 were considered statistically significant.
Preliminary phytochemical analysis of aqueous extract revealed the presence of flavonoids, saponins, tannins, and glycosides where as ethanol extract showed positive test to alkaloids, flavonoids, saponins, tannins, glycosides and triterpenoids.
In excision wound model, the ethanol extract treated animals showed faster epithelialisation of wound (17.84±0.06) which is more or less similar to the values of standard drug treated group (17.37±0.17). While the period of epithelialisation was 22.61±0.15 in case of control and 19.28±0.01 in case of the animals treated with aqueous extract (Table 1). In incision wound model, the mean skin breaking strength was significant in animals treated with ethanol and aqueous extracts 581.45±4.98 and 488.62±6.33, respectively when compared to control (408.98±7.39) (Table 2).
| Groups (N) | 4th day | 8th day | 12th day | 16th day | Mean time of epithelialisation in days |
|---|---|---|---|---|---|
| Control | 13.91±0.31 | 26.35±0.19 | 58.58±0.18 | 76.52±0.19 | 22.61±0.15 |
| Framycetinsulphate cream | 38.58±0.15* | 75.29±0.26* | 83.58±0.16* | 95.15±0.27* | 17.37±0.17* |
| Aqueous extract | 32.27±0.23* | 62.01±0.22* | 75.22±0.15* | 86.30±0.12* | 19.28±0.01* |
| Ethanol extract | 35.55±0.09* | 69.50±0.14* | 80.23±0.07* | 92.47±0.14* | 17.84±0.06* |
| ANOVA | 2708.0 | 1.137 | 5791.0 | 1941.0 | 386.8 |
| F, df | 3,20 | 3,20 | 3,20 | 3,20 | 3,20 |
N = 6 animals in each group. *P≤0.01 indicates significant when compared to control. Values are expressed as mean±SE.
Table 1: Effect of Topical Application of Aqueous and Ethanol Extracts of Lycopodium Serratum on Excision Wound Models
| Group (N) | Tissue breaking strength (g) |
|---|---|
| Control | 408.98±7.39 |
| Framycetinsulphate cream | 655.70±13.03* |
| Aqueous extract | 488.62±6.33* |
| Ethanol extract | 581.45±4.98* |
| ANOVA | |
| F | 160.2 |
| df | 3,20 |
N = 6 animals in each group.*P≤ 0.01 indicates significant when compared to control. Values are expressed as mean±SE.
Table 2: Effect of topical application of aqueous and ethanol extracts of Lycopodium Serratum on incision wound models.
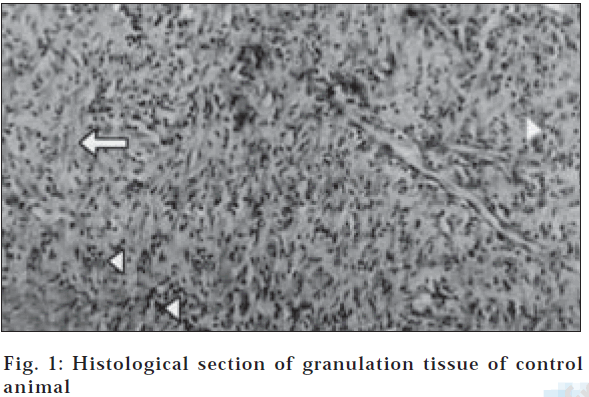
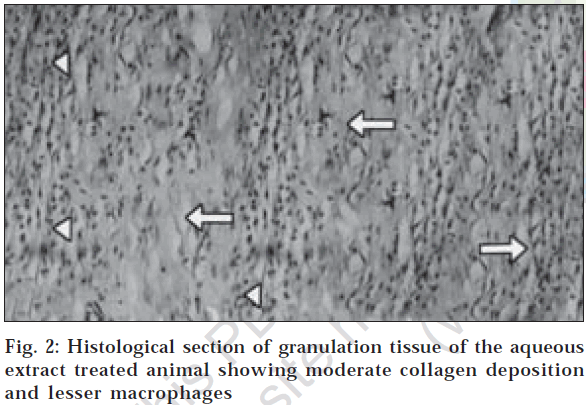
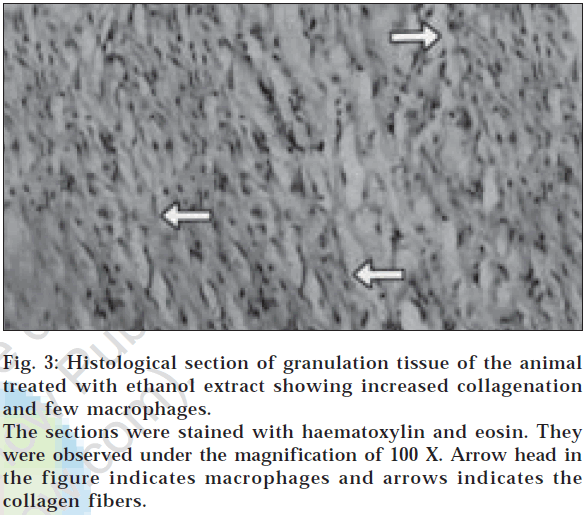
In dead space wound model also, ethanol extract treated animals showed significant increase in dry weight of granulation tissue (47.23±0.10) and tissue breaking strength (512.80±5.08) followed by aqueous extract treated group of animals (Table 3). Estimation of hydroxyproline content in the granulation tissue revealed that the higher concentration of hydroxyproline was noticed in ethanol and aqueous extract treated groups (2322.83±8.49 and 1871.00±17.23, respectively). The histological profile of granulation tissue in control group of animals revealed clumping of macrophages with poor collagenation is observed (fig. 1), while in aqueous extract treated animals moderate collagen deposition with scattered macrophages have been noticed (fig. 2). However in the animals treated with ethanol extract revealed increased collagen fibers with few macrophages (fig. 3) indicating the effect of ethanol on collagen maturation.
| Groups (N) | Granulation tissue dry weight (mg/100g) | Breaking strength (g) | Hydroxyproline (µg/100mg) |
|---|---|---|---|
| Control (1 ml of 1% gum tragacanth / kg b.w.) | 33.45±0.15 | 399.20±8.39 | 1285.00±21.98 |
| Aqueous extract | 39.36±0.13* | 478.18±6.04* | 1871.00±17.23* |
| Ethanol extract | 47.23±0.10* | 512.80±5.08* | 2322.83±8.49* |
| ANOVA | |||
| F | 2960.0 | 76.6 | 137.9 |
| Df | 2,15 | 2,15 | 2,15 |
N = 6 animals in each group. *P≤0.01 indicates significant when compared to control. Values are expressed as mean±SE.
Table 3: Effect of topical application of aqueous and ethanol extracts of Lycopodium Serratum on dead space wound models.
Fig. 3: Histological section of granulation tissue of the animal treated with ethanol extract showing increased collagenation and few macrophages.
The sections were stained with haematoxylin and eosin. They were observed under the magnification of 100 X. Arrow head in the figure indicates macrophages and arrows indicates the collagen fibers.
Collagen is a major protein of the extracellular matrix and is the component that ultimately contributes to wound strength. Increase in breaking strength of granulation tissue indicates the enhanced collagen maturation by increased crosslinking. In addition, increase in dry granulation tissue weight indicates the presence of higher protein content [17]. Breakdown of collagen liberates free hydroxyproline and measurement of the hydroxyproline could be used as an index for collagen turnover [18]. The results revealed that, animals treated with aqueous and ethanol extracts showed faster rate of epithelialization in excision wound model which may be attributed to the phytoconstituents like tannins [19], flavonoids [20] and triterpenoids [21] which are known to promote the wound healing process mainly due to their antimicrobial property. Increase in skin breaking strength and tissue breaking strength in incision and dead space wound model respectively indicated enhanced collagen maturation. Increase in the granulation tissue dry weight and hydroxyproline content indicated the high collagen turnover which may be due to the activity of some phytoconstituents like flavonoids which are known to reduce lipid peroxidation not only by preventing or slowing the onset of cell necrosis but also by improving vascularity.
Hence, any drug that inhibits lipid peroxidation is believed to increase the viability of collagen fibrils by increasing the strength of collagen fibers, by increasing the circulation, by preventing the cell damage and by promoting the DNA synthesis [22]. Hence, the wound healing promoting activity of L. serratum may also be attributed to the antioxidant and antibacterial potency of the active constituents present in it. The present study revealed that ethanol extract possesses significant wound healing promoting activity compared to aqueous extract. The present finding provides scientific evidence to the ethnomedicinal property of Lycopodium serratum in healing the wounds.
Acknowledgements
The authors are grateful to Sri. Girimaji N. Rajgopal, Sri. S. V. Thimmaiah, Prof. Darmananda Rao (National Education Society) and Prof. T. S. Ramkumar (Principal, S. R. N. M. N. College of Applied Sciences, Shimoga) for financial support and to Prof. B. Abdul Rahiman (Department of Biotechnology, Kuvempu University) Dr. Y. N. Manohara (National College of Pharmacy, Shimoga) for their support and encouragement.
References
- Xiao, X.Q., Zhang, H.Y. and Tang, X.C., J. Neurosci. Res., 2002, 67, 30.
- Rajendran, E.S., Homeopathy, 2004, 93, 99.
- Friese, K.H., Kruse, S., Ludtke, R. and Moeller, H., Int. J. Clin.Pharmacol. Ther.,1997, 35, 296.
- Morita, H., Hirasawa, Y. and Kobayashi, J., J. Org. Chem., 2003, 68, 4563.
- Takayama, H., Katakawa, K., Kitajima, M., Yamaguchi, K. and Aimi,N., Org. Lett., 2002, 4, 1243.
- Takayama, H., Katakawa, K., Kitajima, M., Yamaguchi, K. and Aimi,N., Chem. Pharm. Bull. (Tokyo), 2003, 51, 1163.
- Tang, X.T., Tan, C.H., Ma, X.Q., Wang, B.D., Jiang, S.H. and Zhu, D.Y., Planta Med., 2003, 69, 576.
- Tsuda, Y., Fujimoto, T., Isobe, K., Sano, T. and Kobayashi, M.,YakugakuZasshi, 1974, 94, 970.
- Kokate, C.K., Purohith, A.P. and Gokhale, S.B., In; Pharmacognosy,NiraliPrakashan, Pune, 1990, 120.
- Jalalpure, S.S., Patil, M.B., Prakash, N.S., Hemalatha, K. and Manvi,F.V., Indian J. Pharm. Sci., 2003, 65, 363.
- Ghosh, M.N., In; Fundamentals of experimental pharmacology,Scientific Book Agency, Calcutta, 1984, 153.
- Morton, J.P. and Malone, M.H., Arch. Int. Pharmacodyn., 1972, 196, 117.
- Ehrlich, H.P. and Hunt, T.K., Ann. Surg., 1968, 167, 324.
- Lee, K.H., J. Pharm. Sci., 1968, 57, 1042.
- Patil, M.B., Jalalpure, S.S. and Nagoor, V.S., Indian Drugs, 2004, 41, 40.
- Woessner, J.F., Archiv. Biochem.,1963, 93, 440.
- Azad, S., In; Essentials of surgery, Paras Medical Publishers,
- Madhura, M.R. and Sushma, A.M., Fitoterapia, 2003, 74, 553.Hyderabad, 2002, 1.
- Ya, C., Gaffney, S.H., Lilley, T.H., Haslam, E., In; Hemingway, R.W.and Karchesy, J., Eds., Chemistry and significance of condensedtannins, Plenum Press, New York, 1988, 553.
- Tsuchiya, H., Sato, M., Miyazaki, T., Fujiwara, S., Tanigaki, S.,Ohyama, M., Tanaka, T. and Iinuma M., J. Ethnopharmacol.,1996, 50, 27.
- Scortichini, M. and Pia Rossi, M., J. Appl. Bacteriol., 1991, 71, 109.
- Getie, M., Gebre Mariam, T., Reitz, R. and Neubert, R.H.,Pharmazie, 2002, 57, 320.