- *Corresponding Author:
- M. Jeyam
Biochematics Lab, Department of Bioinformatics, School of Life Sciences, Bharathiar University, Coimbatore-641 046, India
E-mail: jeyam@buc.edu.in
| Date of Submission | 10 February 2014 |
| Date of Revision | 04 February 2015 |
| Date of Acceptance | 20 September 2015 |
| Indian J Pharm Sci 2015;77(5):592-598 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The present study was designed to evaluate the antioxidant activity of 5 organic solvent extracts (petroleum ether, n-hexane, chloroform, ethyl acetate and methanol) of wheat grains, 3, 5 and 7 days old wheat seedlings. To determine the antioxidant activity of five extracts of four different samples, 1,1-diphenyl-2-picrylhydrazyl and 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging activity, total phenolic content and ferrous reducing power ability were carried out. 1,1-Diphenyl-2-picrylhydrazyl radical scavenging effect of chloroform and ethyl acetate extracts of 3 days old wheat seedlings was higher than wheat grains. Chloroform, ethyl acetate and methanol extracts of 3 days old wheat seedlings exhibited higher 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging effcet than extracts of other samples. The phenolic content was high in chloroform, ethyl acetate and methanol extract of 5 days old wheat seedlings. When compared with wheat grain, reducing power ability was high in chloroform, ethyl acetate and methanol extract of wheat seedlings, especially in 3 and 5 days old wheat seedlings. From the above results, it was concluded that chloroform, ethyl acetate and methanol extract of 3, 5 and 7 days old wheat seedlings showed better antioxidant activity than the wheat grain extracts. Hence, the results of the present study suggest the intake of wheat seedlings as a food supplement to combat the diseases caused by free radicals.
Keywords
Wheat grains, 1,1-diphenyl-2-picrylhydrazyl, 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid), phenolic content, reducing power ability
In the present era, people are affected by various diseases due to changes in culture and lifestyle. The changes in food habits and working pattern causes stress which leads to biochemical changes. Research findings relate oxidative stress to a number of diseases like cancer, diabetes, Alzheimer’s disease, coronary heart diseases, and aging [1,2]. Scientific reports emphasize the importance of dietary antioxidants as they may decrease the risk of these diseases and also improve general health [3]. Various parts of plants such as fruits, leaves, seeds, and oils contain many natural antioxidants like flavonoids, tannins, coumarins, curcuminoids, xanthones, phenols, and terpenoids [4]. Phenols which are good sources of natural antioxidants are present in cereals and legumes [5]. Slavin et al. [6] reported the whole grains to be an excellent source of carbohydrates, dietary fiber, protein, E and B-group vitamins, and minerals. Several research studies revealed that the whole grain diet reduces the body mass index. Moreover reduced risk of cardiovascular disease [7], colorectal cancer [8] and hypertension in women [9] is also related to the whole grain diet.
Wheat is one of the major cereals in India and constitutes the staple food in North India [10]. Wheat bran, germ, and endosperm are together known as wheat kernel. The antioxidants reported from wheat are carotenoids, tocopherols, flavonoids, and phenolic acids [11]. Ferulic acid is one of the phenolic compounds available in wheat which has strong antioxidant activity [12]. The reports of phytocompounds isolated from the wheat are only limited. Wheat germ extract contains 2,6-dimethoxy-1,4-benzoquinone (DMBQ) and 1,4-benzoquinone (BQ) [13] and the presence of hydroxybenzoic acids (p-hydroxybenzoic acid, vanillic acid and syringic acid), hydroxycinnamic acids (p-coumaric acid, ferulic acid and ferulic acid derivatives) and a flavonoid (apigenin) was reported by Hernández et al [14].
The antioxidant activities of whole wheat and milling fractions have been widely elucidated [15] against oxidation of DNA, proteins, and membrane lipids which are biologically important molecules [16]. The different parts of wheat are also tested for their antioxidant activity. Wheat bran extracts are proved to scavenge free radicals of 1,1-diphenyl- 2-picrylhydrazyl (DPPH), 2,2’-azinobis-(3- ethylbenzothiazoline-6-sulfonic acid) (ABTS+), and peroxide anion (O2 -) [11]. They also have been reported to inhibit the peroxidation of human LDL cholesterol [16], phospholipid liposomes, and hydrogen peroxide [17]. The roasted defatted wheat germ [5,18], the oil from wheat germ [19] and wheat germ protein hydrolysates [20] have also been reported to exhibit antioxidant activity and the antioxidant activity of the coloured wheat seeds may be attributed to the natural pigments of wheat seeds [21-27]. Onyenecho and Hettiarachchy [28] demonstrated that oxidation of bulk oils was inhibited by phenols from ethanol extracts of wheat bran. Species, genotypes, and growing locations are also having pronounced effect on the difference in the antioxidant properties of wheat [29-31].
Increase in antioxidant content of 3 to 5 days wheat sprouts was due to germination [32] and the content of fatty acids like palmitic acid, linoleic acid, and oleic acid were also found to be high in wheat sprouts [33]. Previous findings reported that aqueous and ethanol extracts of wheat grass have potent antioxidant activity and inhibit proliferation of leukemia cells [34] and the antioxidant properties were changed by lighting effects [35].
The objective of the present study was to evaluate the antioxidant activities of five different extracts (petroleum ether, n-hexane, chloroform, ethyl acetate, and methanol) of wheat grains (WG), 3 days, 5 days and 7 days old wheat seedlings (WS) using DPPH, ABTS, Phenolic content and Ferrous reducing power ability.
Materials and Methods
1,1-Diphenyl-2-picrylhydrazyl (DPPH), gallic acid, sodium carbonate, ascorbic acid, potassium dihydrogen phosphate, potassium hydrogen phosphate, potassium ferricyanide, trichloro acetic acid, ferric chloride, 2,2’-azinobis-(3-ethylbenzothiazoline- 6-sulfonic acid) (ABTS), potassium persulfate, mercuric chloride, potassium iodide, sulfuric acid, hydrochloric acid, a-naphthol, magnesium ribbons, acetic anhydride, sodium hydroxide, and ninhydrin were purchased from HiMedia Laboratories Pvt. Ltd., India. Folin-Ciocalteu’s reagent was obtained from S d fine-Chem. Ltd., India. All the solvents were of analytical grade and purchased from Sisco Research Laboratories Pvt. Ltd., India.
Collection and preparation of plant material
Wheat (Triticum aestivum) seeds (Variety: HD2833- Pusa Tripti) were obtained from the Indian Agricultural Research Institute (IARI), Wellington, The Nilgiris, India. One thousand five hundred grams of wheat seeds were taken, washed and soaked in distilled water for 12 h and kept for germination up to 3, 5 and 7 days as each 500 g and WG (without germination, 500 g) were taken after thoroughly washed with distilled water. The WG and germinated WS were shade dried and homogenized to fine powder which was used for further processing.
Preparation of extracts
Wheat grains and different days (3, 5 and 7 days) of wheat seedlings powder were taken and extracted separately with solvents of increasing polarity (petroleum ether, n-hexane, chloroform, ethyl acetate, and methanol) using a Soxhlet apparatus for 48 h, filtered and the extracts were concentrated to 2% of the original volume using Rotavapor, (Buchi-R210, Switzerland). The final yields of the extracts were used for studies.
DPPH radical scavenging activity
The antioxidant activities were determined using DPPH as a free radical [36]. Sixty microliters each of all the five extracts of WG, 3, 5 and 7 d old wheat seedlings (30 mg/ml concentration) were made up to 0.1 ml with methanol to which 3.9 ml (0.025 g/l) of the DPPH solution was added. A control solution was prepared without the addition of the extract and methanol was used as blank. The absorbance was measured at 515 nm. The analysis was performed in triplicate and the scavenging activities of the extracts were calculated using the following equation: DPPH radical scavenging activity (%)= [(Abscontrol-Abssample)/Abscontrol]×100. Where Abscontrol=absorbance of DPPH+methanol; Abssample=absorbance of DPPH+WG/WS extracts.
ABTS radical scavenging assay
The total antioxidant activity of all the five extracts was measured by ABTS radical cation decolorization assay according to the method of Re et al. [37] described by Siddhuraju and Manian [38]. ABTS radical was produced by reacting 7 mM ABTS aqueous solution with 2.4 mM potassium persulfate in the dark for 12- 16 h at room temperature. Prior to assay, this solution was diluted in methanol and equilibrated at 30o to give an absorbance at 734 nm of 0.700±0.02 and in the present study, the stock solution was obtained in the ratio of 1:53 v/v. One ml of the ABTS stock solution was added to 4 µl of test sample (10 mg/ml concentration). The control was prepared without test sample solution. After the initial mixing, all the tubes were kept at 30º exactly for 30 min and absorbance was measured at 734 nm and here, methanol was used as blank. The analysis was done in triplicate and the percentage scavenging activities of the extracts were calculated using the following equation: ABTS radical scavenging activity (%)= [(Abscontrol-Abssample)/ Abscontrol]×100. Where Abscontrol=absorbance of ABTS solution+methanol; Abssample=absorbance of ABTS solution+WG/WS extracts.
Total phenolic content
The total phenolic content was determined according to the method described by Siddhuraju and Becker [39]. In the test tubes, 40 µl each of all the five extracts of WG, 3, 5 and 7 d old wheat seedlings (30 mg/ml concentration) were taken and made up to volume of 1 ml with distilled water. Then 0.5 ml of Folin-Ciocalteu phenol reagent (1:1 with water) and 2.5 ml of sodium carbonate solution (20%) were added sequentially in each tube. Soon after vortexing the reaction mixture, the test tubes were placed in dark for 40 min and the absorbance was recorded at 725 nm. The analysis was performed in triplicate and the results were expressed as gallic acid equivalents (GAE) using the following linear equation based on the calibration curve: y=mx+c (y=absorbance, x=phenolic content, and c=intercept). The values of the equation was found to be Y=0.045x-0.033 with R2=0.997.
Reducing power ability
The reducing power of the extracts was determined according to the method described by Oyaizu [40]. In the five test tubes, 40 µl each of all the five extracts of WG, 3, 5 and 7 days old wheat seedlings (30 mg/ml concentrations) were taken and made up to 1 ml with methanol, further mixed with phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 ml, 1%). After incubating the mixture at 50° for 20 min, 2.5 ml of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 3000 rpm for 10 min. The upper layer of the solution (2.5 ml) was mixed with distilled water (2.5 ml) and FeCl3 (0.5 ml, 0.1%). The absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power. Ascorbic acid was used as standard. A control solution was prepared without the addition of the extract. The analysis was performed in triplicate and the results were expressed as mean±standard deviation.
Statistical analysis
All the values were presented as mean±standard deviation and the statistical analyses were done using the SPSS (version20) package for windows. Using this software, one way ANOVA followed by Tukey’s method was performed to find the significant differences among the samples.
Results
DPPH radical scavenging activities
Lower the absorbance of the reaction mixture, higher the free radical scavenging activity and is expressed in percentage scavenging. When the DPPH free radical scavenging activities of petroleum ether and hexane extracts of wheat grains (WG) and 3, 5 and 7 days old wheat seedlings (WS) were analysed, no significant difference was observed (fig. 1). The chloroform (65.60, 55.83 and 45.28%) extract of 3, 5 and 7 days WS and methanol extract (72.76, 48.49 and 47.8%) of 5, 3 and 7 days WS, respectively, exhibited better activity than WG (38.96 and 33.69%) whereas ethyl acetate extract of 3 days WS (64.83%) only had higher activity than WG (50.53%). Of the seedlings, chloroform, ethyl acetate of 3 day WS and methanol extract of 5 day WS had higher DPPH radical scavenging activity and there was a decreased activity in 7 day WS. The observed results showed significant (P<0.05) differences at 5% level.
ABTS radical scavenging activities
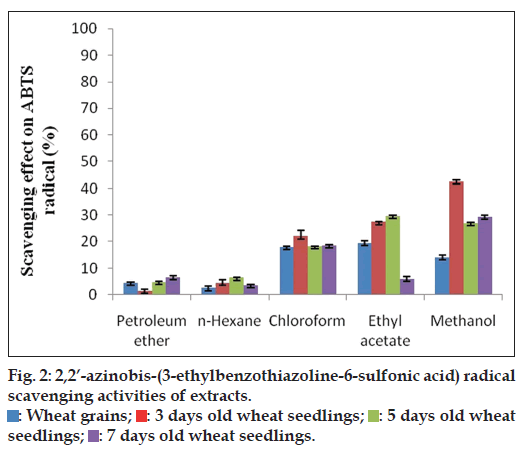
The decolourization of ABTS radical cation assay is used to determine the antioxidant activity. ABTS with potassium persulfate in water at overnight results in the generation of ABTS radical cations [37]. Reduction in color is directly proportional to the reduction of ABTS radical [41]. In this study, ABTS radical scavenging activities of five extracts of four (WG, 3, 5 and 7 day WS) samples were evaluated to assess the antioxidant activity. From the results (fig. 2), it was inferred that chloroform (22%), ethyl acetate (27.4%) and methanol extracts (42.47%) of 3 day old wheat seedlings exhibited higher ABTS radical scavenging activity than the WG (18.08, 19.49 and 14.06%), 5 day (18.31, 29.6 and 27.08%) and 7 day (18.59, 6.07 and 29.34%) old wheat seedlings, respectively and petroleum ether (4.58, 1.54, 4.91 and 6.71%) and n-hexane extracts (2.57, 4.61, 6.46 and 3.66%) of WG, 3, 5 and 7 day old wheat seedlings exhibited trivial ABTS radical scavenging activity. The observed results showed significant (P<0.05) differences at 5% level.
Total phenolic content
Total phenolic content of all the five extracts of WG, 3, 5 and 7 day old WS were evaluated and the results were tabulated (Table 1). Phenolic content was high in chloroform, ethyl acetate and methanol extract of 5 d WS. When compared with WG, the total phenolic content started to increase in 3 and 5 d WS then decresed in 7 d WS, whereas petroleum ether and n-hexane extracts of WS had very low level of phenols. While comparing all the samples based on the polarity, significantly the chloroform extract had high phenolic content and it decreased when go for methanol extract. Further, the significant differences were not observed between chloroform and ethyl acetate extract of all the four samples. The observed results showed significant (P<0.05) differences at 5% level.
| Samples | Petroleum ether | Hexane | Chloroform | Ethyl acetate | Methanol |
|---|---|---|---|---|---|
| Wheat grains | 3.82±0.28 | 5.55±0.08 | 8.41±0.06 | 7.4±0.09 | 3.54±0.05 |
| 3 days old wheat seedlings | 3.48±0.04 | 2.16±0.08 | 10.29±0.06 | 10±0.09 | 8.46±0.07 |
| 5 days old wheat seedlings | 1.86±0.07 | 2.12±0.03 | 12.05±0.06 | 12.19±0.08 | 10.62±0.09 |
| 7 days old wheat seedlings | 2.32±0.04 | 1.91±0.05 | 10.77±0.04 | 10.44±0.05 | 6.19±0.05 |
Results are expressed as mean±SD in mg GAE/g of extract (n=4, P<0.05), significant differences at 5% level. GAE: Gallic acid equivalent, SD: standard deviation
Table 1: Total Phenolic Contents Of Extracts
Reducing power ability
The reducing power of all five extracts of WG and different days of wheat seedlings were investigated and results were given in Table 2. From the observed results, among the 4 samples, reducing power ability was higher in chloroform, ethyl acetate and methanol extracts of WS than in WG. There was a gradual increase in the reducing power ability of WS from 3 to 5 day and thereafter decline in 7 day WS. Further, the higher reducing power ability was observed in hexane and chloroform extracts of 3 d WS than 5 and 7 day WS. The petroleum ether and n-hexane extracts of WG and 7 day WS did not show significant activity but petroleum ether extract of 5 day WS and n-hexane extract of 3 day WS showed reducing power ability. There is no significant (P>0.05) difference between the samples at 5% level.
| Samples | Petroleum ether | Hexane | Chloroform | Ethyl acetate | Methanol |
|---|---|---|---|---|---|
| Wheat grains | 1.6±1.26 | 6.6±0.67 | 11.81±0.43 | 7.22±1.05 | 0.9±0.64 |
| 3 days old wheat seedlings | 5.27±0.43 | 33.19±1.05 | 35.97±0.64 | 13.4±0.84 | 19.31±0.43 |
| 5 days old wheat seedlings | 12.01±0.84 | 9.24±0.64 | 20.49±1.05 | 14.24±0.84 | 26.6±1.26 |
| 7 days old wheat seedlings | 2.32±0.04 | 1.91±0.05 | 10.77±0.04 | 10.44±0.05 | 6.19±0.05 |
Results are expressed as mean±SD in mg AAE/g of extract (n=4, P<0.05), significant differences at 5% level. AAE: Ascorbic acid equivalent, SD: standard deviation
Table 2: Reducing Power Ability Of Extracts
Discussion
Natural antioxidants are present in many plants which play a major role in the reduction of free radicals and lead to the prevention of mutagenesis, carcinogenesis and aging [42]. Further, they reduce the risk of many diseases including cancer, diabetes, Alzheimer’s disease, coronary heart diseases [1-3]. In the present study, radical scavenging activity of wheat grain, 3, 5 and 7 day old wheat seedlings were evaluated using DPPH and ABTS radical scavening assay, the results of which showed that the chloroform, ethyl acetate and methanol extracts of WG, 3, 5 and 7 day old wheat seedlings had significant free radical scavenging activity. Further, petroleum ether and hexane extract of four samples showed very low free radical scavenging activity. From the results, chloroform, ethyl acetate and methanol extract of wheat seedlings showed better activity than the WG and of all the four samples, mostly 3 day old wheat seedlings showed higher radical scavenging activity.
In the present study, compared to WG, 3, 5 and 7 day WS had higher radical scavenging activity. It is interesting to note an earlier report which stated that thermal processing of 2 day wheat sprouts and 10 day seedlings increased the DDPH radical scavenging activity [43]. Total phenolic content is directly proportional to the antioxidant activity because phenolics are the main antioxidant components present in the plant extracts [44,45]. Previous report states that the single group of plant phenolics and the flavonoids were used in many applications like antibiotics, antiulcer, and antiinflammatory agents [46]. The present investigation demonstrated that chloroform extracts of all the four samples have more phenolics. Phenolic content of 2 day wheat sprouts (9%) and 10 day wheat seedlings (8%) were increased by thermal processing [43]. In the current study, when comparing with WG, phenolics are high in WS. Further, the results of this study showed that phenolics are high in 3 and 5 day seedlings and it started to decrease in 7 day wheat seedlings. From the earlier reports it was observed that 10 day old wheat seedlings have the lower content of phenolics than 2 day wheat sprouts which is in consistent with the results of present study.
Qingming et al. [47] reported that reducing power of the extracts significantly play a major role in exhibiting the antioxidant activity and it leads to the reduction of Fe3+/Ferricyanide complex to Fe2+ form. The present study about reducing power ability of all the five extracts of 4 samples revealed that chloroform extracts of all the 4 samples have higher reducing power ability than the other extracts. Among these 4 samples, chloroform extract of 3 day old wheat seedlings had high reducing power ability. Liyana-Pathirana and Shahidi [15] stated that wheat grain has better reducing power capacity than wheat flours but in the present experiment, WG have lower reducing power ability than the wheat seedlings.
ABTS radical scavenging was not observed in the flour samples of 3 hard winter wheat varieties grown at five different locations of Eastern Colorado [31] but Lv et al. [48] reported that wheat flour of ten wheat varieties grown at maryland showed good ABTS radical scavenging activity. In the present study, WG and WS grown in India have potential ABTS radical scavenging capacity. The results of the present study is in confirmation with the report of Lv et al. [48] but contradict the report of Yu et al. [31]. Hence, it was concluded that the growing locations and varieties of wheat may have an effect on the radical scavenging activity.
From the results of all the assays, chloroform extract of all the four samples showed better antioxidant activity than the other extracts of 4 samples. When compared with WG, 3 and 5 day old wheat seedlings showed better antioxidant activity. The results also revealed that the antioxidant activity increased gradually when the wheat grains germinate up to 5 day and started to decrease in 7 day. Hence, it was concluded that 3 day old wheat seedlings may help to prevent/combat diseases caused by oxidative stress.
The results of the present study recommend the 3 day old wheat seedlings as food supplement in the treatment of diseases caused by the free radicals. As wheat is already a staple diet, 3 day WS can be advised to the patients and can be tested for synergism with regular medications in combating free radicals.
Acknowledgements
The authors are very grateful to the University Grants Commission (UGC), New Delhi, India for providing the financial support of this work under the scheme of UGC Major Research Project (F. No.36-51/2008 (SR)).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Ames BN. Identifying environmental chemicals causing mutations and cancer. Science 1979;204:587-93.
- Baublis AJ, Clydesdale EM, Decker EA. Antioxidant in wheat-based breakfast cereals. Cereal Food World 2000;45:71-4.
- Yu L, Scanlin L, Wilson J, Schmidt G. Rosemary extracts as inhibitors of lipid oxidation and color change in cooked turkey products during refrigerated storage. J Food Sci 2002;67:582-5.
- Duthie G, Crozier A. Plant-derived phenolic antioxidants. CurrOpinLipidol 2000;11:43-7.
- Krings U, EI-saharty YS, EI-Zeany BA, Pabel B, Berger RG. Antioxidant activity of extracts from roasted wheat germ. Food Chem 2000;71:91-5.
- Slavin JL, Jacobs D, Marquart L, Wiemer K. The role of whole grains in disease prevention. J Am Diet Assoc 2001;101:780-5.
- Katcher HI, Legro RS, Kunselman AR, Gillies PJ, Demers LM, Bagshaw DM, et al. The effects of a whole grain-enriched hypocaloricdiet on cardiovascular disease risk factors in men and women with metabolic syndrome. Am J ClinNutr 2008;87:79-90.
- Schatzkin A, Mouw T, Park Y, Subar AF, Kipnis V, Hollenbeck A, et al. Dietary fiber and whole-grain consumption in relation tocolorectal cancer in the NIH-AARP Diet and Health Study. Am J ClinNutr 2007;85:1353-60.
- Wang L, Gaziano JM, Liu S, Manson JE, Buring JE, Sesso HD. Whole- and refined-grain intakes and the risk of hypertension in women. Am J ClinNutr 2007;86:472-9.
- Khetarpaul N, Goyal R. Effect of composite flour fortification to wheat flour on the quality characteristics of unleavened bread. Br Food J 2009;111:554-64.
- Zhou K, Su L, Yu LL. Phytochemicals and antioxidant properties in wheat bran. J Agric Food Chem 2004;52:6108-14.
- Graf E. Antioxidant potential of ferulic acid. Free RadicBiol Med 1992;13:435-48.
- Kim MH, Jo SH, Ha KS, Song JH, Jang HD, Kwon YI. Antimicrobial activities of 1,4-benzoquinones and wheat germ extract. J MicrobiolBiotechnol 2010;20:1204-9.
- Hernández L, Afonso D, Rodríguez EM, Díaz C. Phenolic compounds in wheat grain cultivars. Plant Foods Hum Nutr 2011;66:408-15.
- Liyana-Pathirana CM, Shahidi F. Antioxidant and free radical scavenging activities of whole wheat and milling fraction. Food Chem 2007;101:1151-7.
- Yu L, Zhou K, Parry JW. Inhibitory effects of wheat bran extracts on human LDL oxidation and free radical. LWT Food SciTechnol2005;38:463-70.
- Martínez-Tomé M, Murcia MA, Frega N, Ruggieri S, Jiménez AM, Roses F, et al. Evaluation of antioxidant capacity of cereal brans. J Agric Food Chem 2004;52:4690-9.
- Gelmez N, Kincal NS, Yener ME. Optimization of supercritical carbon dioxide extraction of antioxidants from roasted wheat germ based on yield, total phenolic and tocopherol content, and antioxidant activities of the extracts. J Supercrit Fluid 2009;48:217-24.
- Malecka M. Antioxidant properties of the unsaponification matter isolated from tomato seeds, oat grains and wheat germ oil. Food Chem 2002;79:327-30.
- Zhu KX, Zhou HM, Qian HF. Antioxidant and free radical-scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase. Process Biochem 2006;41:1296-302.
- Sun Q, Sun BQ. Dynamic of seed pigment content of black kernel wheat at different seed development stages. SciAgric Sin 2001;34:461-4.
- Li XP, Lan SQ, Liu YP. Studies on pigment and its related physio-biochemical properties of blue or purple grain wheat. ActaAgron Sin 2003;29:157-8.
- Zong XF, Zhang JK, Li BX, Yu GD, Shi YM, Wang SG. Relationship between antioxidant and grain colors of wheat (TriticumaestivumL.).ActaAgron Sin 2006;2:237-42.
- Li W, Pickard MD, Beta T. Effect of thermal processing on antioxidant properties of purple wheat bran. Food Chem 2007;104:1080-6.
- Tang XZ, Li QH, Ma D, Jiang Y, Sun LZ, Yin YP. Technological conditions for extraction of the pigments from green-wheat-bran by acidified alcohol. Food Ferment Ind 2008;9:190-4.
- Hosseinian FS, Li W, Beta T. Measurement of anthocyanins and other phytochemicals in purple wheat. Food Chem 2008;109:916-24.
- Knievel DC, Abdel-Aal ES, Rabalski I, Nakamura T, Hucl P. Grain color development and the inheritance of high anthocyanin blue aleurone and purple pericarp in spring wheat (TriticumaestivumL.). J Cereal Sci 2009;50:113-20.
- Onyenecho SN, Hettiarachchy NS. Antioxidant activity of durum wheat bran. J Agric Food Chem 1992;40:1496-500.
- Saleem A, Ahotupa M, Pihlaja K. Total phenolics concentration and antioxidant potential of extracts of medicinal plants of Pakistan. Z Naturforsch C 2001;56:973-8.
- Kaur C, Kapoor HC. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int J Food SciTechnol 2002;37:153-61.
- Yu L, Haley S, Perret J, Harris M. Comparison of wheat flour grown at different locations for their antioxidant properties. Food Chem 2004;86:11-6.
- Falcioni G, Fedeli D, Tiano L, Calzuola I, Mancinelli L, Marsili V, et al. Antioxidant activity of wheat sprouts extract in vitro: Inhibitionof DNA oxidative damage. J Food Sci 2002;67:2918-22.
- Marton M, Mandoki Z, Csapo J. Evaluation of biological value of sprouts I. Fat content, fatty acid composition. ActaUnivSapientiae Aliment 2010;3:53-65.
- Aydos OS, Avci A, Ozkan T, Karadag A, Gurleyik E, Altinok B, et al.Antiproliferative, apoptotic and antioxidant activities of wheatgrass (TriticumaestivumL.) extract on CML (K562) cell line. Turk J MedSci 2011;41:657-63.
- Urbonavi A, Samuolien G, Brazaityt A, Duchovskis P, Ruzgas V, Zukauskas A. The effect of variety and lighting quality on wheat grass antioxidant properties. Zemdirbyste-Agriculture 2009;96:119-28.
- Brand-williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food SciTechnol1995;28:25-30.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cationdecolorization assay. Free RadicBiol Med 1999;26:1231-7.
- Siddhuraju R, Manian S. The antioxidant activity and free radical scavenging capacity of dietary phenolic extracts from horse gram (Macrotylomauniflorum(Lam. Verdc.) seeds. Food Chem2007;105:950-8.
- Siddhuraju R, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimaticorgins of Drumstick tree (Moringaolifera Lam.) leaves. J Agric Food Chem 2003;51:2144-55.
- Oyaizu M. Studies on products of browning reaction: Antioxidative activity of products browning reaction prepared from glucosamine. Jpn J Nutr 1986;44:307-15.
- Adedapo AA, Jimoh FO, Koduru S, Masika PJ, Afolayan AJ. Evaluation of the medicinal potentials of the methanol extracts of the leaves and stems of Hallerialucida. BioresourTechnol 2008;99:4158-63.
- Liu XL, Zhao MM, Wang JS, Yang B, Jiang YM. Antioxidant activity of methanolic extract of emblica fruit (PhyllanthusemblicaL.) from sixregions in China. J Food Compost Anal 2008;21:295-7.
- Randhir R, Kwon YI, Shetty K. Effect of thermal processing on phenolics, antioxidant activity and health-relevant functionality of select grain sprouts and seedlings. Innov Food SciEmerg 2008;9:355-64.
- Chew YL, Goh JK, Lim YY. Assessment of in vitroantioxidantcapacity and polyphenolic composition of selected medicinal herbs from Leguminosaefamily in Peninsular Malaysia. Food Chem 2009;116:13-8.
- Liu SC, Lin JT, Wang CK, Chen HY, Yang DJ. Antioxidant properties of various solvent extracts from lychee (Litchi chinenesisSonn.) flowers. Food Chem 2009;114:577-81.
- Subba Rao MV, Muralikrishna G. Evaluation of the antioxidant properties of free and bound phenolic acids from native and malted finger millet (ragi, EleusinecoracanaIndaf-15). J Agric Food Chem2002;50:889-92.
- Qingming Y, Xianhui P, Weibao K, Hong Y, Yidan S, Li Z, et al. Antioxidant activities of malt extract from barley (HordeumvulgareL.) toward various oxidative stress in vitro and in vivo. Food Chem 2010;118:84-9.
- Lv J, Yu L, Lu Y, Niu Y, Liu L, Costa J, et al.Phytochemicalcompositions, and antioxidant properties, and antiproliferative activities of wheat flour. Food Chem 2012;135:325-31.
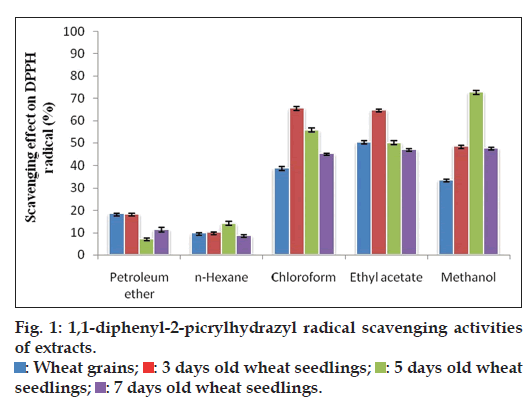
 : Wheat grains;
: Wheat grains;  : 3 days old wheat seedlings;
: 3 days old wheat seedlings;  : 5 days old wheat seedlings;
: 5 days old wheat seedlings;  : 7 days old wheat seedlings.
: 7 days old wheat seedlings.