- *Corresponding Author:
- Preeta Kaur Chugh
Department of Pharmacology, Vardhaman Mahavir Medical College and Safdarjung Hospital, New Delhi-110 029, India
E-mail: docpreeta@yahoo.com
| Date of Submission | 15 December 2014 |
| Date of Revision | 22 November 2015 |
| Date of Acceptance | 07 February 2016 |
| Indian J Pharm Sci 2016;78(1):41-47 |
This is an open access article distributed under the terms of the Creative Commons Attribution?NonCommercial?ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non?commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
It is now known that vitamin D deficiency is a worldwide health problem. In our country, as food fortification is lacking, supplementation with pharmaceutical preparations is the only means of treatment of vitamin D deficiency. We aimed to study the composition and availability of various vitamin D preparations in the Indian market, data about which was collected from annual drug compendium. The preparations were assessed for total number, different formulations, constituents and amount of each constituent present in the formulation. Vitamin D3 is available in the form of cholecalciferol, alfacalcidiol and calcitriol as single ingredient products and in combination with calcium and other micronutrients. Most of the supplements contain calcitriol (46.5%) or alfacalcidiol (43%) as tablets (51.1%) and capsules (35.2%). Cholecalciferol, the preferred form for prophylaxis and treatment of vitamin D deficient states, constitutes only 10% of the available market preparations. High market sales of calcium supplements containing calcitriol indicate increasing intake of calcitriol rather than cholecalciferol; which could predispose to toxicity. There is a need for marketing and rational prescribing of the appropriate vitamin D supplement in ostensibly healthy Indian population. Implementation of population-based education and intervention programmes with enforcement of strict regulations could generate awareness and curb unsupervised intake of vitamin D containing dietary supplements. This health challenge mandates effective nutritional policies, fortification and supplementation programmes and partnership between government, healthcare and industry to safeguard the health of Indian population at large.
Keywords
Vitamin D, India, vitamin, supplement, nutrition, health
It has been widely accepted that vitamin D deficiency (VDD) is a global health problem that impacts not only musculoskeletal health but also varied acute and chronic diseases [1]. Low vitamin D has been associated with an increased risk of diabetes mellitus, cardiovascular disease, certain cancers, cognitive decline, autoimmune disorders and pregnancy complications [2]. It has been estimated that 20 to 80% of US, Canadian and European men and women are vitamin D deficient [1,3,4]. In Middle East and Asia, VDD is highly prevalent in both children and adults [1,2,5]. Even in India, numerous studies across various regions of the country indicate that approximately 70-90% of apparently healthy population is vitamin D deficient [6-9]. Low vitamin D status is prevalent irrespective of age, sex, profession, rural/urban settings or regional distribution [10,11] (Table 1).
| Study population | Number of participants | Age mean (SD) | State | Mean 25(OH) D serum levels (ng/ml) (SD) | References | |
|---|---|---|---|---|---|---|
| Young adults | ||||||
| Adults | 50 | 28.15 (4.9) years | Kashmir | 11.26 (9.65) | Zargaret al. [8] | |
| Adults | 90 | 22?23 years | Punjab and Haryana | 18.4 (4.3) | Tandonet al. [12] | |
| Urban adults | 31 | 25 (5) years | Delhi | 18.87 (4.69) | Goswamiet al. [13] | |
| Young adults | 186 | 18.6 (13) years | Delhi | 12.96 (9.84) | Marwahaet al. [14] | |
| Urban adults | 642 | 31.4 (13.4) years | Delhi | 7 (4.08) | Goswamiet al. [15] | |
| Rural adults | 57 | 42–43 years | Delhi | 14.56 (9) | Goswamiet al. [7] | |
| Urban adults | 125 | 45.5 (0.95) years | Tirupathi | 13.52 (0.59) | Harinarayanet al. [16] | |
| Urban adults | 214 | 26–30 years | Mumbai | 12.80 (7.94) | Multaniet al. [17] | |
| Infants and children | ||||||
| Exclusively breastfed infants | 97 | 10 weeks | Delhi | 12.59 ( 8.37) | Agarwal et al. [18] | |
| Breastfed infants | 60 | 3.0 (0.14) months | Delhi | 9.03 (4.63) | Mehrotraet al. [19] | |
| Infants | 127 | 3 months | Delhi | 6.5 (4.0) | Agarwal et al. [20] | |
| Infants, urban area | 26 | 16±4.1 months | Delhi | 12.4 (7) | Agarwal et al. [11] | |
| Adolescent girls | 50 | 14.7 (0.10) years | Pune | 9.36 | Khadilkaret al. [21] | |
| Adolescents | 3089 | 10–18 years | Delhi | 10.4 (0.4) | Marwahaet al. [22] | |
| Pregnant and lactating women | ||||||
| Lactating mothers | 342 | 24.6 (2.8) years | Delhi | 7.84 (3.32) | Marwahaet al. [23] | |
| Lactating mothers | 180 | Delhi | 10.88 (5.8) | Seth et al. [24] | ||
| Pregnant women | 207 | 24.0 (4.1) years | Lucknow | 14.00 (9.3) | Sachanet al. [25] | |
| Pregnant women | 42 | 20–35 years | Mumbai | 22.99 (10.93) | Bhallaet al. [26] | |
| Pregnant women | 559 | 24 years | Mysore | 15.12 (9.6) | Farrantet al. [27] | |
| Pregnant women | 521 | 24.6 (2.8) years | Delhi | 9.28 (4.88) | Marwahaet al. [14] | |
Table 1: Vitamin D Serum Levels In Different Population Groups Across India
The major source of vitamin D is exposure to sunlight. It has been presumed that Indians are vitamin D sufficient due to adequate sunshine throughout the year [6]. However, reduced cutaneous synthesis of vitamin D could be attributed to limited UV exposure owing to increased skin pigmentation, topical application of sunscreen, certain socio cultural practices and urban life style [6]. Secondary sources include dietary intake of foods naturally rich in vitamin D such as salmon, cod liver oil, sun dried mushrooms or vitamin D fortified foods [2,28]. In our country, availability, acceptability and cost of these dietary products limits their widespread use by the general population. This complex interplay between lack of adequate sun exposure, deficient intake and effective food fortification strategies makes Asian Indian population particularly susceptible to vitamin D insufficiency/deficiency. Thus, vitamin D supplementation in the form of pharmaceutical preparations is one of the most effective ways to prevent and treat VDD in high-risk groups [29]. We, therefore undertook a study to ascertain that availability and composition of various pharmaceutical preparations of vitamin D in the Indian market.
Materials and Methods
This study was conducted to determine the number and composition of the various vitamin D pharmaceutical preparations. Data for the study was collected from an annual Drug Compendium entitled The Drug Today 2013 (October–December 2013 issue) and product labels. The preparations were assessed for total number, different formulations, constituents and amount of each constituent present in the formulation.
Results
Analysis of various vitamin D preparations
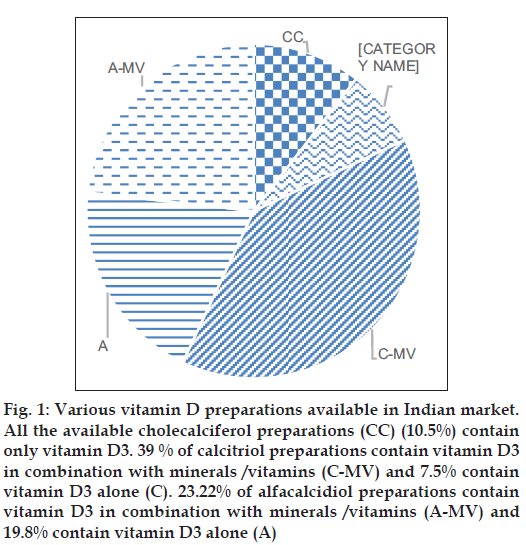
A total of 258 vitamin D formulations are available in the Indian market. Vitamin D is commonly available in two forms: Vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Only two preparations contain vitamin D2 (ergocalciferol). More than 99.9% of the preparations contain vitamin D3 in the form of alfacalcidiol (25 hydroxycholecalciferol), calcitriol (1,25 dihydroxycholecalciferol) or cholecalciferol (inactive vitamin D). Most of the preparations contain calcitriol (n=120, 46.5%) or alfacacidiol (n=111; 43.02%). Approximately 10% of preparations contain cholecalciferol (n=27, 10.5%) (fig. 1, Tables 2 and 3).
Fig. 1: Various vitamin D preparations available in Indian market. All the available cholecalciferol preparations (CC) (10.5%) contain only vitamin D3. 39 % of calcitriol preparations contain vitamin D3 in combination with minerals /vitamins (C-MV) and 7.5% contain vitamin D3 alone (C). 23.22% of alfacalcidiol preparations contain vitamin D3 in combination with minerals /vitamins (A-MV) and 19.8% contain vitamin D3 alone (A)
| Alfacalcidiol (25 hydroxy cholecalciferol) | Calcitriol (1,25 dihydroxy cholecalciferol) | Cholecalciferol (inactive Vitamin D) | Total | |
|---|---|---|---|---|
| Total number of preparations (%)* | 111 (43.02) | 120 (46.5) | 27 (10.5) | 258 (100)* |
| Number of preparations that contain only | 22 (19.8) | 9 (7.5) | 27 (100) | 58 (22.4)* |
| Vitamin D3 (%)** | ||||
| Number of preparation that contain Vitamin D3 in | 85 (76.5) | 26 (21.6) | 0 | 111 (43)* |
| combination with calcium (%)** | ||||
| Number of preparations that contain Vitamin D3 | 10 (9.0) | 87 (72.5) | 0 | 97 (37.6)* |
| in combination with zinc (%)** | ||||
| Number of constituents (range) | 1–14 | 1–12 | 1 | 1–14 |
| Number of preparations available as tablets (%)** | 68 (61.2) | 60 (50) | 4 (14.8) | 132 (51.1)* |
| Number of preparations available as capsules (%)** | 35 (31.5) | 53 (44.1) | 3 (11.1) | 91 (35.2)* |
| Number of preparations available as injectable (%) | 0 | 2 (1.6) | 4 (14.8) | 6 (2.3)* |
| Number of preparations available as sachets (%) | 0 | 0 | 17 (62.9) | 17 (6.5)* |
Table 2: Vitamin D3 Preparations Available In The Market
| Brand name | Company | Vitamin D biochemical form | Formulation | Amount of Vitamin D | Available as | Cost (INR) |
|---|---|---|---|---|---|---|
| Rocaitrol | Abott Healthcare Pvt. Ltd. | Cholecalciferol | Capsules | 0.25 µg | 10 capsules | 175.4/17.5* |
| Calosto | Abott Healthcare Pvt. Ltd. | Calcitriol | Capsules | 0.25 µg | 15 capsules | 198.36/13.22* |
| Alpha calcirol | Cadila Pharmaceutical Ltd. | Alfacalcidiol | Capsules | 0.25 µg | 10 capsules | 48.87/4.8* |
| Alfarich | Cipla Ltd. | Alfacalcidiol | Capsules | 0.25 µg | 10 capsules | 54.30/5.4* |
| Alfado | GlaxoSmithKline Pharmaceuticals Ltd. | Alfacalcidiol | Capsules | 0.25 µg | 10 capsules | 54.30/5.4* |
| Alfa D3 | GlaxoSmithKline Pharmaceuticals Ltd. | Alfacalcidiol | Capsules | 0.5 µg | 10 capsules | 268/26.8* |
| Rocaltrol | Sun Pharmaceutical Industries Ltd. | Calcitriol | Capsules | 0.25 µg | 10 capsules | 123/12.3* |
| Alphadol | Panacea Biotec Ltd. | Alfacalcidiol | Capsules | 0.25 µg | 10 capsules | 169/16.9* |
| Vitanova SG | Zuventus Healthcare Ltd. | Cholecalciferol | Capsules | 60,000 IU | 10 capsules | 250/25* |
| Quente D3 | Stanley Healthcare | Cholecalciferol | Capsules | 60,000 IU | 4 capsules | 86.25/21.5* |
| Minroset | Win Medicare Ltd. | Alfacalcidiol | Capsules | 0.25 µg | 10 capsules | 56.8/5.6* |
| Calcit SG | ZudusCadila Healthcare Ltd. | Alfacalcidiol | Tablets | 0.25 µg | 10 tablets | 94.40/9.4# |
| Vitalcal | Genesis Biotec Inc. | Cholecalciferol | Tablets | 60,000 IU | 4 tablets | 96.00/24# |
| Hira D3 | Hiral Lab Ltd. | Cholecalciferol | Tablets | 60,000 IU | 4 tablets | 80/20# |
| Calcit SG | ZudusCadila | Alfacalcidiol | Tablets | 0.25 µg | 10 tablets | 94.40/9.4# |
| Arachitol | Solvay Pharma India Pvt. Ltd. | Cholecalciferol | Injection | 600,000 IU | 1 injection (2 ml) | 34 |
| Fovit D3 | Forgo Pharmaceuticals (P) Ltd. | Cholecalciferol | Injection | 600,000 IU | 1 injection (1 ml) | 21.90 |
| D3 UP | Abott Healthcare Pvt Ltd. | Cholecalciferol | Granules | 60,000 IU | 1 sachet | 21 |
| Caldikind | Mankind Pharmaceutical Pvt. Ltd. | Cholecalciferol | Granules | 60,000 IU | 1 sachet | 21 |
| Calotec D3 | Lupin Laboratories Ltd. | Cholecalciferol | Powder | 60,000 IU | 1 g | 29 |
| Sorvate | Glenmark | Calcitriol | Ointment | 0.0003% | 20 g | 175 |
| Rosical | Sun Pharma | Calcitriol | Ointment | 3 µg | 15 g | 213 |
Table 3: Commonly Available Vitamin D Branded Preparations In The Market
Different formulations of vitamin D
The most common formulation for oral administration is in the form of tablets (n=132, 51.1%) and capsules (n=91, 35.2%). Though alfacalcidiol and calcitriol are commonly available as tablets and capsules; cholecalciferol is in the form of granules in sachets (n=17, 62.9%). Other dosage forms include syrups and softgel capsules. Though oral administration in the form of drops is commonly recommended for infants and children, adolescents and adults are usually prescribed tablets, capsules or granules for supplementation. While all cholecalciferol preparations are available as a single constituent; more than 75% of alfacalcidiol preparations also contain calcium (n=85, 76.5%). Majority of calcitriol preparations are combined with zinc or zinc sulphate (n=87, 72.5%). Vitamin D preparations also contain various other minerals/vitamins such as magnesium, cupric, boron, methylcobalamin, vitamin E, vitamin K, vitamin C, pyridoxine, folic acid, beta-carotene, glutamic acid, manganese, omega 3 and docosapentaenoic acid. Tablets and capsules contain 10 IU (0.25 μg) -10000 IU (25 mg) of vitamin D and are usually administered on a daily basis. Sachets of cholecalciferol containing granules of vitamin D amount to 60,000 IU of vitamin D are administered weekly. Vitamin D2 (doxercalciferol) preparation is available in tablet form (two formulations) containing 20 IU (0.5 μg) and 100 IU (2.5 μg) respectively (Table 3).
Cost analysis
Cost analysis reveals that tablets/capsules of alfacalcidiol (equivalent to 10 IU of vitamin D) and calcitriol (0.25 μg) commonly costs Indian National Rupees (INR) 5.5 and INR 7, respectively (Table 4). Whereas, cholecalciferol granules (equivalent to 60,000 IU) costs INR 20. The common treatment regime for VDD is 60000 IU weekly for eight weeks, which costs approximately INR 160. Furthermore, vitamin D given monthly as maintenance therapy for a year would cost INR 240.
| Alfacalcidiol | Calcitriol | Cholecalciferol (60,000 IU) | |
|---|---|---|---|
| (0.25 µg) 10 IU | (0.25 µg) 10 IU | ||
| Average cost | 4.3 | 5.15 | 10.10 |
| Modal | 5.5 | 7 | 20 |
| Cost range of | 0.75–7.5 (10 IU) | 3–13.5 (10 IU) | 4.9 (5000 IU) |
| tablets (strength) | |||
| Cost range of | 3–22.6 (10 IU) | 4–12.9 (10 IU) | 5.9 (1000 IU) |
| capsules (strength) | |||
| Cost of granules | 16 (60,000 IU) | NA | 0.73–23.23 |
| (strength) | (60,000 IU) |
Table 4: The cost (in Indian national rupees) Of various dosage forms of vitamin D3 Preparations
Discussion
Vitamin D deficient state has become one of the most prevalent and underdiagnosed medical conditions in the world [1,30]. Recent evidence suggests that lack of adequate sun exposure is the most important factor for this global pandemic as very few foods naturally contain vitamin D (wild caught salmon and UV exposed mushrooms) [2]. Analysis in children and adults indicate that dietary sources are grossly inadequate in providing the Recommended Dietary Allowances (RDA) for vitamin D. In our population, cutaneous production of vitamin D is further limited by increased melanin content of skin or sun avoidance by use of sunscreens, extensive clothing cover due to sociocultural practices or staying indoors for most of the day [6]. Deficient vitamin D status can be corrected either by vitamin D fortification (addition of micronutrients to processed foods) or supplementation (the provision of relatively large doses of micronutrients, usually in form of pills, capsules or syrup). Vitamin D fortification is an effective and passive way to increase vitamin D intake in both general population and vulnerable groups [31-33]. However, it mandates political commitment and involvement of various ministries (health, agriculture and social welfare) to develop nationwide strategies for a better vitamin D status in the population. In our country, where vitamin D fortification initiative is lacking, supplementation is the only alternative [34].
In our analysis, we found that multiple supplements of vitamin D are available. The two common forms are vitamin D3 (alfacalcidiol, cholecalciferol and calcitriol) and vitamin D2 (ergocalciferol). Evidence suggests that cholecalciferol is superior to ergocalciferol in terms of potency, elevating and sustaining 25 (OH) D concentrations and maintaining the storage form of vitamin D [35,36]. In our analysis, we were not able to ascertain the cause of lack of availability of vitamin D2 preparations in the Indian market. Majority of preparations available in the market contain vitamin D3 (99.9%). About half of the preparations (46.5%) contain calcitriol in the form of tablets or capsules of 0.25 mcg. Calcitriol has a rapid onset of action with short half-life of 6 h. It is most useful in chronic kidney disease and type I and type II vitamin D deficient rickets (VDDR) with decreased synthesis of calcitriol. Though calcitriol is the most commonly available form, it is not the preferred agent for treatment of nutritional deficiency or stoss therapy (single large oral/ intramuscular therapy). It is associated with a high incidence of hypercalcemia and requires serum calcium monitoring. Furthermore, when calcitriol is used as a supplement, 25(OH) D levels do not indicate clinical vitamin D status. Calcitriol does not build up stores and is an expensive preparation [37]. Around 43% of the preparations contained alfacalcidiol (25 dihydroxy cholecalciferol). It is most commonly available as tablets or capsules of 10 IU, usually in combination with calcium (76.5% of alfacalcidiol preparations). Alfacalcidiol has a rapid onset of action with a half life of 2-3 weeks. It does not require hepatic 25-hydroxylation, and is therefore most useful in patients with liver disease. Approximately 10% of vitamin D3 preparations are available as cholecalciferol. It is the inactive, unhydroxylated form of vitamin D3, synthesized in skin from 7 dehydrocholesterol. It has a slow onset of action with a half-life of 12-30 days. Thus, it is the preferred form for prophylaxis or treatment of vitamin D deficient states [37].
Vitamin D can be administered daily, weekly, monthly, or every 4 months to sustain an adequate serum 25 (OH) D concentrations [38-40]. A high bolus dose of vitamin D (up to 300,000 IU) can be used initially in persons with extreme VDD. Repeated boluses of high-dose vitamin D at 6- to 12-month intervals have been used in a nursing home setting, but a steady-state serum 25(OH) D concentration is likely to be maintained by more frequent, lower doses of vitamin D. An effective strategy to treat vitamin D deficient state in children and adults is to administer 50,000 IU of vitamin D3 once a week for 6-8 weeks respectively [41,42]. To prevent recurrence, administration of 600 to 1000 IU/day is effective [43]. In our country, most market preparations contain 60, 000 IU, and are usually recommended for 6 to 8 weeks for obtaining adequate serum 25 (OH) D concentrations. A monthly maintenance therapy is usually required and should be continued for over one year [44]. Another study suggested that a single high dose of 120,000-180,000 IU of oral cholecalciferol was adequate to elevate 25(OH) D out of the deficiency range. However, maintenance dose is required for sustaining the desired concentration of 25(OH) D [44,45]. Our analysis suggests that this treatment regime would initially cost approx. INR 160 and a maintenance therapy for a year would cost INR 240. However, the treatment costs are usually higher as calcium supplements are coadministered with vitamin D therapy. Vitamin D supplementation
Vitamin D supplementation varies with the RDA, tolerable upper levels in different age groups and in certain circumstances [46]. The Institute of Medicine (IOM), USA guidelines suggest a vitamin D sufficient level of 20 ng/ml to optimize bone health [47]. In contrast, US Endocrine Society recommends that serum 25 (OH) D levels of 30 ng/ml (vitamin D sufficiency) should be attained for children and adults to optimize the probability of good health and avoid other risk associated with vitamin D deficient status (Table 5). Furthermore, it is now acknowledged that previously recommended vitamin D intake of 200 IU/day in the American recommended intakes or 400 IU/day in the WHO report are grossly inadequate [46]. Thus, RDA of 600-800 IU is recommended to maintain adequate levels of vitamin D [46]. In our country, Indian Council of Medical Research (ICMR) recommends a daily supplement of 400 IU/day of vitamin D for Indians under situations of minimal exposure to sunlight [34]. However, in light of recent evidence, there is a need to update these guidelines regarding vitamin D intake and supplementation in adults, vulnerable population and susceptible groups [48-50]. It is now recognized that vitamin D is not as toxic as once thought to be. IOM recommends that up to 4,000 IUs of vitamin D/day is safe for most children and adults. Studies in various populations have shown that adults can tolerate 10,000 IU of vitamin D/day for at least five months without altering their serum calcium or urinary calcium output [50]. However, in rare cases, vitamin D toxicity can cause hypercalcemia, hyperphosphatemia, nephrocalcinosis, and soft tissue calcification, thus contributing to high risk of mortality [51].
| US IOM classification* [30] | US Endocrine Society classification [3] |
|---|---|
| Severe deficiency: <5 | Deficiency: <20 |
| Deficiency: <15 | Insufficiency: 21–29 |
| Sufficiency: >20 | Sufficiency: >30 |
| Risk of toxicity: >50 | Toxicity: >150 |
Table 5: Vitamin D status in relation to 25?Hydroxy vitamin D levels
Studies indicate general and widespread use of dietary supplements across populations [52,53]. Though these supplements often are used with the intention of attaining health benefits by preventing chronic diseases, cumulative effects of widespread supplement use, together with food fortification, have raised concern regarding exceeding upper recommended levels and, thus, long-term safety [53]. In case of vitamin D, unsupervised intake of alfacalcidiol and calcitriol, which are not recommended for vitamin D deficient states, could result in adverse effects/toxicity [54]. Thus, it is recommended that supplements should only be used with strong medically based cause, such as symptomatic nutrient deficiency disease [53]. ‘Blanket prescription’ of these supplements in all patients for prolonged use should be stopped. Physicians need to evaluate the patient’s dietary nutrient consumption, intake of any other multivitamin supplement, potential interactions and prescribe them only on an individual basis.
Widespread VDD in the Indian population is a cause for grave concern. Adequate measures are imperative to prevent VDD at the outset. There is a need for educating the masses about sensible sun exposure for vitamin D synthesis and dietary intake of vitamin D rich foods. Fortification of commonly available foods could prevent vitamin D insufficiency in our large population. There is an urgent need to prioritize development of national level programs to provide regulated vitamin D fortified foods at affordable prices for Indian population at large. As far as vitamin D supplements are concerned, their easy availability and chronic self-administration mandates awareness on the part of the consumer, pharmacist, chemists, physicians and regulatory bodies to prevent misuse and serious harm. As evident by high market sales, high intake of calcitriol in the form of dietary calcium supplement in the general population could result in serious hypercalcemia, calcium stones and metastatic calcification. The prescribing physicians need to be aware of this potential risk and should prescribe supplements only on an individual basis for requisite duration. These supplements should be adequately labeled indicating the amount of each constituent with special instructions/precautions/warnings, if any. Stringent regulations are required to keep a check on their marketing and availability. Adequate legal actions are required for pharmaceutical companies/ drug firms not adhering to regulatory provisions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Hossein-nezhad A, Holick MF. Vitamin D for health: A global perspective. Mayo ClinProc 2013;88:720-55.
- Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266-8.
- Ganji V, Zhang X, Tangpricha V. Serum 25-hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay-adjusted data. J Nutr 2012;142:498-507.
- Greene-Finestone LS, Berger C, de Groh M, Hanley DA, Hidiroglou N, Sarafin K, et al. 25-Hydroxyvitamin D in Canadian adults: Biological, environmental, and behavioral correlates. OsteoporosInt 2011;22:1389-99.
- Fields J, Trivedi NJ, Horton E, Mechanick JI. Vitamin D in the Persian Gulf: Integrative physiology and socioeconomic factors. CurrOsteoporos Rep 2011;9:243-50.
- Gupta A, Gupta R. Vitamin D deficiency in India: Prevalence, causalities and interventions. Nutrients 2014;6:729-75.
- Goswami R, Kochupillai N, Gupta N, Goswami D, Singh N, Dudha A. Presence of 25(OH) D deficiency in a rural North Indian village despite abundant sunshine. J Assoc Physicians India 2008;56:755-7.
- Zargar AH, Ahmad S, Masoodi SR, Wani AI, Bashir MI, Laway BA, et al. Vitamin D status in apparently healthy adults in Kashmir Valleyof Indian subcontinent. Postgrad Med J 2007;83:713-6.
- Harinarayan CV, Ramalakshmi T, Prasad UV, Sudhakar D, Srinivasarao PV, Sarma KV, et al. High prevalence of low dietary calcium, high phytate consumption, and Vitamin D deficiency in healthy South Indians. Am J ClinNutr 2007;85:1062-7.
- Garg MK, Marwaha RK, Khadgawat R, Ramot R, Obroi AK,Mehan N, et al. Efficacy of Vitamin D loading doses on serum 25-hydroxy Vitamin D levels in school going adolescents: An open label non-randomized prospective trial. J PediatrEndocrinolMetab 2013;26:515-23.
- Agarwal KS, Mughal MZ, Upadhyay P, Berry JL, Mawer EB,
- Tandon N, Marwaha RK, Kalra S, Gupta N, Dudha A, Kochupillai N. Bone mineral parameters in healthy young Indian adults with optimal Vitamin D availability. Natl Med J India 2003;16:298-302.
- Goswami R, Gupta N, Goswami D, Marwaha RK, Tandon N,
- Marwaha RK, Tandon N, Garg MK, Kanwar R, Narang A, Sastry A, et al. Vitamin D status in healthy Indians aged 50 years and above. J Assoc Physicians India 2011;59:706-9.
- Goswami R, Marwaha RK, Gupta N, Tandon N, Sreenivas V, Tomar N, et al. Prevalence of Vitamin D deficiency and its relationship withthyroid autoimmunity in Asian Indians: A community-based survey. Br J Nutr 2009;102:382-6.
- Harinarayan CV, Ramalakshmi T, Prasad UV, Sudhakar D. Vitamin D status in Andhra Pradesh: A population based study. Indian J Med Res 2008;127:211-8.
- Multani SK, Sarathi V, Shivane V, Bandgar TR, Menon PS, Shah NS. Study of bone mineral density in resident doctors working at a teaching hospital. J Postgrad Med 2010;56:65-70.
- Agarwal N, Faridi MM, Aggarwal A, Singh O. Vitamin D Status of term exclusively breastfed infants and their mothers from India. ActaPaediatr 2010;99:1671-4.
- Mehrotra P, Marwaha RK, Aneja S, Seth A, Singla BM, Ashraf G, et al. Hypovitaminosis d and hypocalcemic seizures in infancy. IndianPediatr 2010;47:581-6.
- Agarwal R, Virmani D, Jaipal ML, Gupta S, Gupta N, Sankar MJ, et al. Vitamin D status of low birth weight infants in Delhi: Acomparative study. J Trop Pediatr 2012;58:446-50.
- Khadilkar A, Crabtree NJ, Ward KA, Khadilkar V, Shaw NJ,
- Marwaha RK, Tandon N, Reddy DR, Aggarwal R, Singh R, Sawhney RC, et al. Vitamin D and bone mineral density status of healthy schoolchildren in northern India. Am J ClinNutr 2005;82:477-82.
- Marwaha RK, Tandon N, Chopra S, Agarwal N, Garg MK, Sharma B, et al. Vitamin D status in pregnant Indian women across trimestersand different seasons and its correlation with neonatal serum 25-hydroxyvitamin D levels. Br J Nutr 2011;106:1383-9.
- Seth A, Marwaha RK, Singla B, Aneja S, Mehrotra P, Sastry A, et al. Vitamin D nutritional status of exclusively breast fed infants and their mothers. J PediatrEndocrinolMetab 2009;22:241-6.
- Sachan A, Gupta R, Das V, Agarwal A, Awasthi PK, Bhatia V. High prevalence of Vitamin D deficiency among pregnant women and their newborns in northern India. Am J ClinNutr 2005;81:1060-4.
- Bhalala U, Desai M, Parekh P, Mokal R, Chheda B. Subclinical hypovitaminosis D among exclusively breastfed young infants. Indian Pediatr 2007;44:897-901.
- Farrant HJ, Krishnaveni GV, Hill JC, Boucher BJ, Fisher DJ, Noonan K, et al.Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. Eur J ClinNutr 2009;63:646-52.
- Biancuzzo RM, Young A, Bibuld D, Cai MH, Winter MR, Klein EK, et al. Fortification of orange juice with Vitamin D2 or Vitamin D3 isas effective as an oral supplement in maintaining Vitamin D status in adults. Am J ClinNutr 2010;91:1621-6.
- Vieth R, Carter G. Difficulties with Vitamin D nutrition research: Objective targets of adequacy, and assays for 25-hydroxyvitamin D. Eur J ClinNutr 2001;55:221-2.
- Ben-Shoshan M. Vitamin D deficiency/insufficiency and challenges in developing global Vitamin D fortification and supplementation policy in adults. Int J VitamNutr Res 2012;82:237-59.
- Babu US, Calvo MS. Modern India and the Vitamin D dilemma: Evidence for the need of a national food fortification program.Mol Nutr Food Res 2010;54:1134-47.
- Allen L, de Benoist B, Dary O, Hurrel R, editors. Guidelines on food fortification with micronutrients. In: Food and Agricultural Organization of the United Nations. Geneva: World Health Organization; 2009. Available from: http://www.who.int/nutrition/publications/ micronutrients/9241594012/en/. [Last accessed on 2014 Jul 14].
- Calvo MS, Whiting SJ. Public health strategies to overcome barriers to optimal Vitamin D status in populations with special needs. J Nutr 2006;136:1135-9.
- A Report of the Expert Group of the Indian Council of Medical Research. Jamai-Osmania PO, Hyderabad: National Institute of Nutrition, Indian Council of Medical Research, Nutrient Requirements and Recommended Dietary Allowances for Indians; 2009. Available from: http://www.pfndai.com/Draft_RDA-2010.pdf. [Last accessed on 2014 Jul 12].
- Biancuzzo RM, Clarke N, Reitz RE, Travison TG, Holick MF. Serum concentrations of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 in response to Vitamin D2 and Vitamin D3 supplementation. J ClinEndocrinolMetab 2013;98:973-9.
- Park SY, Murphy SP, Wilkens LR, Yamamoto JF, Kolonel LN. Allowing for variations in multivitamin supplement composition improves nutrient intake estimates for epidemiologic studies. J Nutr 2006;136:1359-64.
- Malluche HH, Monier-Faugere MC, Koszewski NJ. Use and indication of Vitamin D and Vitamin D analogues in patients with renal bone disease. Nephrol Dial Transplant 2002;17Suppl 10:6-9.
- Demetriou ET, Travison TG, Holick MF. Treatment with 50,000 IU Vitamin D2 every other week and effect on serum 25-hydroxyvitamin D2, 25-hydroxyvitamin D3, and total 25-hydroxyvitamin D in a clinical setting.EndocrPract 2012;18:399-402.
- Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high-dose oral Vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA 2010;303:1815-22.
- Heikinheimo RJ, Inkovaara JA, Harju EJ, Haavisto MV, Kaarela RH, Kataja JM, et al. Annual injection of Vitamin D and fractures of aged bones. Calcif Tissue Int 1992;51:105-10.
- Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral Vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: Randomised double blind controlled trial. BMJ 2003;326:469.
- Gordon CM, Williams AL, Feldman HA, May J, Sinclair L, Vasquez A, et al. Treatment of hypovitaminosis D in infants and toddlers. J ClinEndocrinolMetab 2008;93:2716-21.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Guidelines for preventing and treating Vitamin D deficiency and insufficiency revisited. J ClinEndocrinolMetab 2012;97:1153-8.
- Goswami R, Gupta N, Ray D, Singh N, Tomar N. Pattern of 25-hydroxy vitamin D response at short (2 month) and long (1 year) interval after 8 weeks of oral supplementation with cholecalciferol in Asian Indians with chronic hypovitaminosis D. Br J Nutr 2008;100:526-9.
- Pietras SM, Obayan BK, Cai MH, Holick MF. Vitamin D2 treatment for Vitamin D deficiency and insufficiency for up to 6 years. Arch Intern Med 2009;169:1806-8.
- Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and Vitamin D from the Institute of Medicine: What clinicians need to know. J ClinEndocrinolMetab 2011;96:53-8.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of Vitamin D deficiency: An Endocrine Society clinical practice guideline. J ClinEndocrinolMetab 2011;96:1911-30.
- Marwaha RK, Tandon N, Agarwal N, Puri S, Agarwal R, Singh S, et al. Impact of two regimens of Vitamin D supplementation oncalcium – Vitamin D-PTH axis of schoolgirls of Delhi. Indian Pediatr 2010;47:761-9.
- Haga HJ, Schmedes A, Naderi Y, Moreno AM, Peen E. Severe deficiency of 25-hydroxyvitamin D3 (25-OH-D3) is associated with high disease activity of rheumatoid arthritis. ClinRheumatol 2013;32:629-33.
- Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J ClinNutr 2003;77:204-10.
- Tripathi KD. Essentials of Medical Pharmacology. 7th ed. New Delhi: Jaypee Brothers Medical Publisher; 2014. p. 335-46.
- Mileva-Peceva R, Zafirova-Ivanovska B, Milev M, Bogdanovska A,
- Mursu J, Robien K, Harnack LJ, Park K, Jacobs DR Jr. Dietary supplements and mortality rate in older women: The Iowa Women’s Health Study. Arch Intern Med 2011;171:1625-33.
- Chugh PK, Lhamo Y. An assessment of vitamin supplements in the Indian market. Indian J Pharm Sci 2012;74:469-73.