- *Corresponding Author:
- Xianyan Zhang
Department of Gastroenterology, Health Management Center, West China Fourth Hospital, Sichuan University, Chengdu, Sichuan 610017, China
E-mail: xinghe2734946@163
| This article was originally published in a special issue, “Exploring the Role of Biomedicine in Pharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(1) Spl Issue “294-299” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To conduct a study on the value of serum gastrin-17 plus pepsinogen I, II and Helicobacter pylori C14 breath test in the early screening of gastric carcinoma. The clinical data of 96 patients with epigastric distress requiring gastroscopic pathological examination from January 2019 to June 2020 were analyzed retrospectively. 49 patients diagnosed with chronic superficial gastritis and chronic atrophic gastritis were included in the gastritis group, and 47 patients diagnosed with early gastric carcinoma were included in the gastric carcinoma group; the serum levels of gastrin-17, pepsinogen I, pepsinogen II, and pepsinogen ratio, as well as Helicobacter pylori positive rate, were compared between the two groups. The diagnostic value of various indicators and their combination was analyzed by the receiver operating characteristic curve. The patients in the gastric carcinoma group had a higher level of gastrin-17 than the gastritis group (p<0.05), whereas the patients in the gastric carcinoma group had a lower level of pepsinogen ratio than the gastritis group (p<0.05); for the early stage of gastric carcinoma, the diagnostic results of Helicobacter pylori positive, pepsinogen I, and pepsinogen II showed moderate consistency with the gold standard (p<0.05), the diagnostic results of serum gastrin-17 and pepsinogen ratio for the early stage of gastric carcinoma showed good consistency with the gold standard (p<0.05), and the diagnostic results of the combination of various indicators showed superior consistency with the gold standard (p<0.05); the positive rate of Helicobacter pylori detected by serum gastrin-17, pepsinogen I and pepsinogen II levels, pepsinogen ratio, and C14 breath test showed a moderate screening value for the early stage of gastric carcinoma (p<0.05), and the combination of various indicators embraced high screening value (p<0.05). Serum gastrin-17 combined with pepsinogen I, II, and Helicobacter pylori C14 shows a high application value for early screening of gastric carcinoma. Its advantage lies in convenient operation, good repeatability, and wide application. Moreover, it can provide a necessary reference for the screening and early diagnosis of gastric carcinoma.
Keywords
Gastric carcinoma, serum gastrin-17, pepsinogen I, pepsinogen II, Helicobacter pylori
Gastric Cancer (GC) is one of the most often diagnosed cancers around the world. In China, GC comes in second in terms of mortality behind other malignancies, and its incidence comes in second when compared to other types of malignant tumors[1-3]. Early GC mostly lacks specific manifestations and tends to be confused with chronic superficial gastritis, leaving its clinical diagnosis difficult. Most of them have progressed to the intermediate and advanced stages of GC when patients present with significant manifestations followed by gastroscopy, thus losing the timing of surgical treatment[4-6]. The combined detection of serum Gastrin-17 (G-17), Pepsinogen (PG) I, and PGII has been applied in gastric precancerous lesions and GC screening in Japan[7,8]. Serological screening for GC diagnosis in China is still in its infancy. GC is one of the most often diagnosed cancers around the world. Helicobacter pylori (H. pylori) infection is the first type of carcinogenic factor recognized by the World Health Organization as an independent risk factor for the development and progression of GC. Our hospital intended to improve the accuracy of early diagnosis of GC by combined detection of serological detection and H. pylori infection. The diagnostic value was analyzed in this study. Specific studies are described below.
Materials and Methods
General data:
From January 2019 to June 2020, the clinical data of 96 patients with epigastric distress who needed a gastroscopic pathological examination were reviewed retrospectively.
Inclusion criteria: Patients at our hospital were diagnosed with early GC, chronic superficial gastritis, or chronic atrophic gastritis after a gastroscopic pathological examination; adult patients aged ≥18 y and patients with complete relevant examinations and clinical data in our hospital.
Exclusion criteria: Patients complicated with other malignant tumors; patients who failed to tolerate gastroscopy; patients with a history of anti-H. pylori therapy and gastric acid secretion inhibitors, gastric mucosal protective agents, anticoagulants, antibiotics, and other drugs 2 w before admission; patients with clotting disorder or hemorrhagic disorder and hemorrhagic diathesis and patients who were free of being fully diagnosed by gastroscopic pathological examination.
According to the results of the gastroscopic pathological examination, 49 patients diagnosed with chronic superficial gastritis and chronic atrophic gastritis among the 96 patients were included in the gastritis group, and 47 patients diagnosed with early GC were included in the GC group. Patients in the gastritis group were 33 y-71 y of age, with an average of (49.65±8.96) y; the maximum diameter of lesions was 1.1-2.13 cm, with an average of (1.61±0.26) cm; there were 16 patients with chronic superficial gastritis and 33 patients with chronic atrophic gastritis. Patients in the GC group were 36 y-70 y of age, with an average of (50.14±8.71) y; the maximum diameter of lesions was 1.0-2.16 cm, with an average of (1.64±0.31) cm.
Methods:
Serological tests: All patients had 3 ml of blood drawn from the cubital vein under fasting conditions, which were then allowed to stand for 20 min before being placed in a centrifuge and centrifuged at 3000 r/min for 10 min to obtain serum for testing. Serum samples were measured for G-17, PGI, and PGII levels by immune enzymelinked immunosorbent assay, and the PGI-to-PGII Ratio (PGR) was calculated.
C14 breath test: 2 h after fasting or feeding, all patients were given one urea 14C capsule with a cup of water orally. The insufflation nozzle and accumulator card were opened after 15 min of sitting quietly, and the insufflation nozzle was loaded with the accumulator card in the direction indicated by the arrow. Later, the patient began to insufflate smoothly with an insufflation nozzle and blow as long as possible, and the air could be exchanged in the middle but the suck-back was prohibited. The insufflation was maintained for about 1-3 min until the indicator changed from orange-red to yellow. The accumulator card was inserted into the instrument card slot to read the automatic measurement result on the instrument.
Diagnostic criteria: In our hospital, all patients had a gastroscopic pathological examination, and the laboratory results were accepted as the gold standard. The serological positive criterion was described as; PGI <70 μg/l, PGII >15 μg/l, PGR <7 and G-17 >7 pmol/l [9,10].
Methodology:
The serum levels of G-17, PGI, and PGII as well as the positive rate of H. pylori were compared between the two groups. The consistency between the diagnostic results of various indicators and their combination for early GC and the gold standard was analyzed. The diagnostic efficacy indicators (sensitivity, specificity and accuracy) were calculated. The Receiver Operating characteristic (ROC) curve parameter and Area under the Curve (AUC) were utilized to analyze the diagnostic value of various indicators and their combination.
Statistical analysis:
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) 21.0 software. Enumeration data were compared using Chi-Square (χ2) test and expressed as n (%); measurement data were compared using t-test and expressed as (x̄ ±s); AUC grading criteria was displayed below; AUC >0.9 signified a high diagnostic value, 0.7<AUC≤0.9 indicated a moderate diagnostic value, 0.5≤AUC≤0.7 suggested a low diagnostic value, and AUC <0.5 was deemed as the absence of diagnostic value basically; the consistency was analyzed by kappa, with grading criteria; excellent for 0.8<kappa≤1.0, good for 0.6<kappa≤0.8, moderate for 0.4≤kappa≤0.6, and poor for kappa<0.4; p<0.05 indicated statistically significant differences. p<0.05 suggested a statistically significant difference.
Results and Discussion
In this section, we will discuss the comparison of serological indicators, positive rate of H. pylori, consistency and diagnostic efficacy indicators, and analysis of diagnostic value in detail.
As shown in Table 1, the patients in the GC group had higher levels of G-17 and PGI than the gastritis group (p<0.05), whereas the patients in the GC group had lower levels of PGII and PGR than the gastritis group (p<0.05).
| Group | n | G-17 (pmol/l) | PGI (μg/l) | PGII (μg/l) | PGR |
|---|---|---|---|---|---|
| Gastritis | 49 | 9.13±2.97 | 169.65±21.26 | 16.03±6.78 | 11.01±3.42 |
| GC | 47 | 18.74±4.85 | 73.21±14.13 | 19.27±7.69 | 3.76±1.01 |
| t | 11.762 | 26.061 | 2.192 | 14.530 | |
| p | ≤0.001 | ≤0.001 | 0.031 | ≤0.001 |
Table 1: Comparison of Serological Indicators in the Two Groups (x̄±s)
As shown in Table 2, the positive rate of H. pylori was reported at a higher rate in the GC group (68.09 %) compared to what was observed in the gastritis group (22.45 %) (p<0.05).
| Group | n | Positive | Negative |
|---|---|---|---|
| Gastritis | 49 | 11 (22.45) | 38 (77.55) |
| GC | 47 | 32 (68.09) | 15 (31.91) |
| χ2 | 20.204 | ||
| p | ≤0.001 | ||
Table 2: Comparison of the Positive Rate of H. pylori in the Two Groups n (%)
As shown in Table 3, the diagnostic results of H. pylori positive, PGI, and PGII for the early stage of GC showed a moderate consistency with the gold standard (p<0.05), and the diagnostic results of serum G-17 and PGR for the early stage of GC showed good consistency with the gold standard (p<0.05); the diagnostic results of the combination of various indicators showed a superior consistency with the gold standard (p<0.05) and the combined diagnosis exhibited the highest diagnostic efficacy, with a sensitivity of 89.36 %, a specificity of 95.92 %, and an accuracy of 92.71 %.
| Indicators | Sensitivity | Specificity | Accuracy | Positive predictive value | Negative predictive value | Kappa | p |
|---|---|---|---|---|---|---|---|
| PGR | 82.98 | 85.71 | 84.38 | 84.78 | 84 | 0.687 | ≤0.001 |
| Serum PGI | 80.85 | 81.63 | 81.25 | 80.85 | 81.63 | 0.625 | ≤0.001 |
| Serum PGII | 59.57 | 83.67 | 71.88 | 77.78 | 68.33 | 0.435 | ≤0.001 |
| Serum G-17 | 87.23 | 87.76 | 87.5 | 87.23 | 87.76 | 0.75 | ≤0.001 |
| H. pylori | 61.7 | 93.88 | 78.13 | 90.63 | 71.88 | 0.559 | ≤0.001 |
| Combination | 89.36 | 95.92 | 92.71 | 95.45 | 90.38 | 0.854 | ≤0.001 |
Table 3: Diagnostic Consistency of Various Indicators and Diagnostic Efficacy Indicators (%)
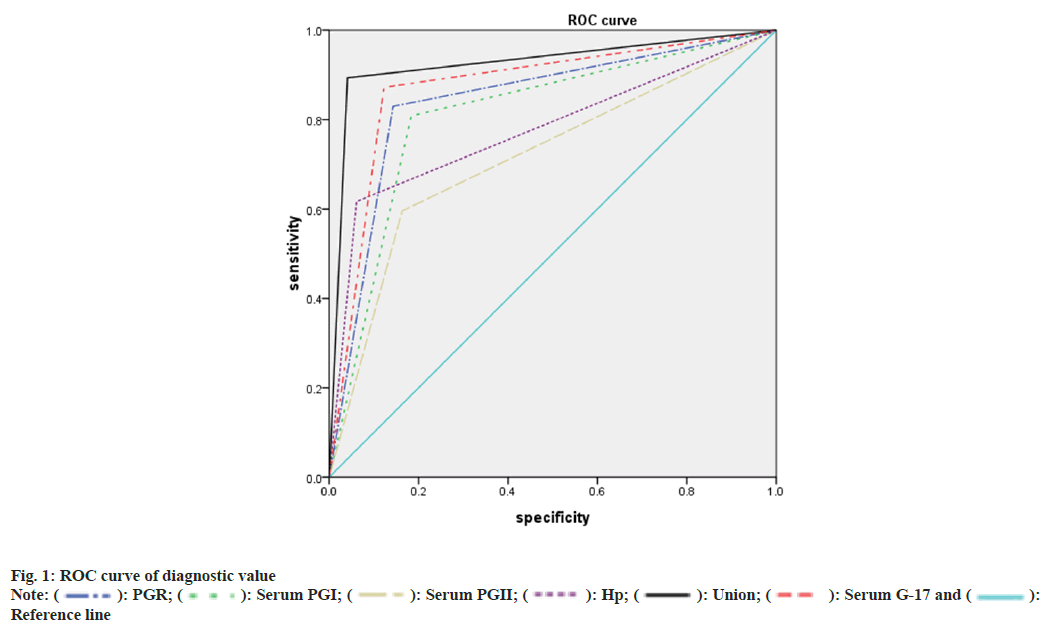
As shown in Table 4, the positive rate of H. pylori detected by serum G-17, PGI, PGII, and PGR levels, and C14 breath test showed a moderate diagnostic value for the early stage of GC (p<0.05), and the combination of various indicators embraced high diagnostic value (p<0.05). ROC curve is displayed in fig. 1.
| Indicators | AUC | Standard error | p | 95 % confidence interval | |
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| PGR | 0.843 | 0.043 | ≤0.001 | 0.759 | 0.928 |
| Serum PGI | 0.812 | 0.046 | ≤0.001 | 0.722 | 0.903 |
| Serum PGII | 0.716 | 0.054 | ≤0.001 | 0.611 | 0.821 |
| H. pylori | 0.778 | 0.05 | ≤0.001 | 0.681 | 0.875 |
| Serum G-17 | 0.875 | 0.039 | ≤0.001 | 0.798 | 0.952 |
| Combination | 0.926 | 0.031 | ≤0.001 | 0.866 | 0.987 |
Table 4: Statistics of ROC Curve of Diagnostic Value
An early stage of GC is a common malignancy in the digestive system[11,12]. Recently, the incidence of GC shows an upward trend gradually in China, and there is a tendency to progress towards younger. Abnormal changes in serum G-17, PGI, and PGII levels are observed during the formation and progression of GC. First discovered by Edkins, G-17 is amidated gastrin secreted by antral G-cells G-17[13,14], a polypeptide hormone, has the capability of promoting the proliferation and differentiation of gastric mucosal cells and stimulating gastric acid secretion physiologically. G-17 expression in humans accounts for >90 % of the total G-17[15,16], which is a sensitive index reflecting the secretory function of gastric antrum in clinical practice, is proven to be a mirror of the atrophy state of the gastric antrum and a promoter of the formation and progression of GC. An abnormal rise in serum G-17 is found in patients with early GC, and its rise degree is associated with the location and clinical stage of GC tissues. Increased serum G-17 is more prominent in GC patients occurring in the fundus and body. The detection of serum G-17 levels can thus provide crucial information for the early diagnosis of GC. Under the influence of gastric acid, PG, an inactive precursor, is transformed to pepsin. Through the capillaries of the gastrointestinal mucosa, about 1 % of PG enters the blood stream[17,18]. According to its immunocompetence and biochemical characteristics, PG is divided into two subtypes; PGI and PGII. PGI is mainly secreted by the mucous neck cells of chief cells in the fundic glands, and a few PGI are secreted by the pyloric glands of the antrum and Brunner’s glands of the proximal duodenum, and its content in the serum is relatively stable. Since it can reflect the capacity of the gastric body mucosa to secrete acid, PG is clinically known as "serological biopsy". PGI and PGII maintain dynamic balance in a normal state. After carcinogenesis, the PGI level reduces abnormally while the PGII level rises abnormally. As a result, the detection of the expression levels of two subtypes of PG in serum serves as an important reference for the early diagnosis of GC. As a common method, the C14 breath test shows high diagnostic accuracy for H. pylori infection. Currently, H. pylori infection is clinically recognized as an independent risk factor for GC, and H. pylori positivity indicated the presence of increased risk of GC[19,20].
The results of this study showed the patients in the GC group had a higher level of G-17 and PGII than the gastritis group, whereas the patients in the GC group had a lower level of PGI than the gastritis group; it was proven that patients with early GC may present with abnormal changes in serum G-17, PGI, PGII, and PGR levels. The diagnostic results of H. pylori positive, PGI, and PGII for the early stage of GC showed a moderate consistency with the gold standard (p<0.05), and the diagnostic results of serum G-17 and PGR for the early stage of GC showed good consistency with the gold standard (p<0.05); the diagnostic results of the combination of various indicators showed a superior consistency with the gold standard; it was obvious that apart from the combination of various indicators, various diagnostic efficacy indicators and consistency of serum G-17 were optimal, followed by PGR. It was suggested that the combination of various indicators could improve the consistency of serological indicators and H. pylori C14 detection for the early diagnosis of GC, with a sensitivity of 89.36 %, a specificity of 95.92 %, and an accuracy of 92.71 %. Moreover, among all indicators, the accuracy, sensitivity, and specificity of serum G-17 and serum C-14 alone and their consistency with the gold standard were far higher in the early diagnosis of GC. It was shown by the ROC curve that the serum levels of G-17, PGI, PGII, and the positive rate of H. pylori detected by the C14 breath test showed a moderate diagnostic value for the early stage of GC, whereas the combination of various indicators exhibited a relatively high value for screening the early GC; it was a reminder of us that the combination of serum G-17, PGI, PGII levels and the positive rate of H. pylori detected by C14 breath test has the potential to upgrade the diagnostic value for the early stage of GC, and serum G-17 alone among all indicators showed the highest value for screening early GC, followed by PGR.
In summary, serum G-17 combined with PGI, PGII, and H. pylori C14 shows a quite high screening value for the early stage of GC, and the combination of various indicators can play a complementary and mutually corrected role.
The combination of serum G-17, PGI, II, and H. pylori C14 has a high applicability value for early GC screening. Its benefits include ease of use, high reproducibility, and a wide range of applications. Furthermore, it can serve as a valuable resource for GC screening and early diagnosis. The results of this study revealed that patients with early GC had higher levels of G-17 and PGII than those with gastritis, but lower levels of PGI than those with gastritis; it was also shown that patients with early GC may have aberrant changes in serum G-17, PGI, PGII, and PGR levels. It served as a reminder to us that the combination of serum G-17, PGI, PGII levels, and the positive rate of H. pylori detected by the C14 breath test has the potential to improve the diagnostic value for early-stage GC, with serum G-17 alone showing the highest value for early-stage GC screening, followed by PGR. In conclusion, serum G-17 in combination with PGI, PGII, and H. pylori C14 has a relatively good screening value for early-stage GC, and the combination of diverse indicators can play a complementary and mutually corrected role.
Ethical approval:
This study has been reviewed and approved by the Medical Ethics Committee of West China Fourth Hospital, Sichuan University. All the patients were informed about this study and signed the informed consent form.
Conflict of interests:
The authors declared no conflict of interest.
References
- Zhu C, Zhao J, Shen X, Qian W, Ma Y, Zhang S, et al. A multi-center clinical research of diagnostic value of serum gastrin-17 combined with pepsinogen for gastric cancer. Chin J Dig Endosc 2017;34(1):19-23.
- Zhu Qi, Yu W, Xue L, Ai Z. Clinical study on serum pepsinogen and gastrin-17 levels of gastric cancer and their correlation with Helicobacter pylori infection in xinjiang uygur and han. Gastroenterology 2016;21(6):348-52.
- Ma Y, Sun J, Liu Li. Correlation between serum gastrin 17 level and gastric cancer and precancerous lesion. Chin J Gastroenterol Hepatol 2017;26(8):915-7.
- Yang Lu, Shen J, Zhang W. Clinical significance of serum pepsinogen I/II and gastrin-17 in the diagnosis and prognosis of gastric cancer. Zhejiang Clin Med J 2017;19(2):329-30.
- Zhou Y, Ren Y. Value of G17, PGI, PGII and H. pylori antibodies in the diagnosis of early gastric cancer. Clin Med 2019;39(12):30-1.
- Zhang S, Li Y, Xu H. Diagnostic and therapeutic value of 14C urea breath test for H. pylori infection in upper gastrointestinal diseases. Label Immunoassay Clin Med 2017;24(3):359-60.
- Dong Q, Chen X, Ding J. Changes and significance of serum gastrin-17, carbohydrate antigen-125 and pepsinogen levels in patients with early gastric cancer before and after endoscopic submucosal dissection. Chin J Endosc 2020;26(2):37-42.
- Yao F, Lu L, Gao F. Value of combined detection of serum total bile acid, gastrin-17 and pepsinogen in the diagnosis of gastric cancer. Chin J Clin Gastroenterol 2017;29(5):317-8.
- Association of digestive endoscopy of Chinese society of medicine, cancer endoscopy Committee of China anti-cancer association. Chinese consensus opinions on early gastric cancer screening and endoscopic diagnosis and treatment (2014, Changsha). Chin J Dig Endoscopy 2014;34(7):361-77.
- Lai S, Ding K, Fan C. Effect of combined detection of serum pepsinogen, gastrin-17, and Helicobacter pylori 14 carbon breathe test in the diagnosis of gastric cancer. J Contemp Med 2019;25(18):81-3.
- Jiang X, Zhang H, Lu L. Clinical Study on the correlation between serum gastrin-17, pepsinogen levels and OLGA system. Chin Mod Doctor 2019;57(2):25-8.
- Ke Y, Liang C, Ji W. Correlation analysis of serum pepsinogen, gastrin-17 and Helicobacter pylori infection in patients with atrophic gastritis and gastric cancer among different ethnic groups in Xinjiang. Lab Med Clin 2017;14(16):2355-8.
- Fang J, Yan L, Wang Y. Clinical significance of serum pepsinogen combined with gastrin-17 in gastric cancer screening. Lingnan J Emerg Med 2017;22(4):353-5.
- Yang W, Ding Yu, Yuan N. Clinical value of combined detection of serum pepsinogen, gastrin-17 and Helicobacter pylori IgG antibody in the diagnosis of gastric cancer. Zhejiang Clin Med J 2017;19(10):1930-1.
- Xie J, Wu X, Xie N. Application of serum pepsinogen, gastrin-17 combined with endoscopic optimized screening protocol in early gastric cancer. China Mod Med 2017;24(13):81-3.
- Wu G, Zhao C, Jiang Qi. Clinical significance and research progress of serum markers of gastric cancer. Chin Mod Med 2016;23(14):10-2.
- Tan Y, Chen H, Liu F. Diagnostic value of peripheral blood pepsinogen I, gastrin-17 and soluble human leukocyte antigen-g in gastric cancer. Chin J Clin Pharmacol 2017;33(10):945-7.
- Yuan M, Liu H, Ren J. Value of Helicobacter pylori, pepsinogen and serum gastrin-17 in diagnosis and differential diagnosis of senile gastric cancer and precancerous lesion. J Clin Exp Med 2019;18(4):376-9.
- Wang JX, Cao YP, Su P, He W, Li XP, Zhu YM. Serum gastrin-17 concentration for prediction of upper gastrointestinal tract bleeding risk among peptic ulcer patients. World J Clin Cases 2021;9(35):10948-55.
- Xu Y, Tan Y, Wang Y, Gao J, Wu D, Xu X. A gratifying step forward for the application of artificial intelligence in the field of endoscopy: A narrative review. Surg Laparos Endosc Percutan Tech 2021;31(2):254-63.
[Crossref] [Google Scholar] [PubMed]
 Reference line
Reference line 



