- *Corresponding Author:
- Jin Li
Department of Laboratory Medicine,
Zhongnan Hospital of Wuhan University,
Wuhan, Hubei 430071,
China
E-mail: pearli1980@126.com
| This article was originally published in a special issue, “Trending Topics in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(1) Spl Issue “48-51” |
Abstract
Angiotensin II type 1 receptor activation has been shown to be associated with glomerular injury and glomerular disease. Therefore, the present study aims to investigate the effect of the angiotensin II type 1 receptor antagonist valsartan on angiotensin II type 1 receptor expression in cultured rat mesangial cells. Sprague-Dawley rat mesangial cell lines obtained from China Center for Type Culture Collection were used in the study. The viability of rat mesangial cells was assessed by 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide. The results showed that valsartan inhibited cell proliferation and downregulated messenger ribonucleic acid and protein expression of angiotensin II type 1 receptor in rat mesangial cells. These levels were significantly lower than control group (p<0.01). Because angiotensin II type 1 receptor has a central role in the development and progression of glomerular diseases. Valsartan down-regulation of angiotensin II type 1 receptor expression of mesangial cells may provide beneficial effects to the glomerular injury. These data will be beneficial for understanding the mechanism of valsartan on the glomerular protection.
Keywords
Valsartan, angiotensin II type 1 receptor, mesangial cells, 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide, sodium dodecyl sulfate
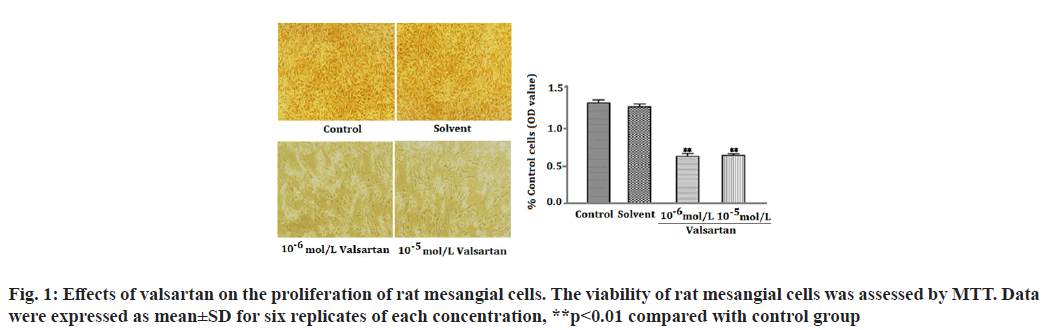
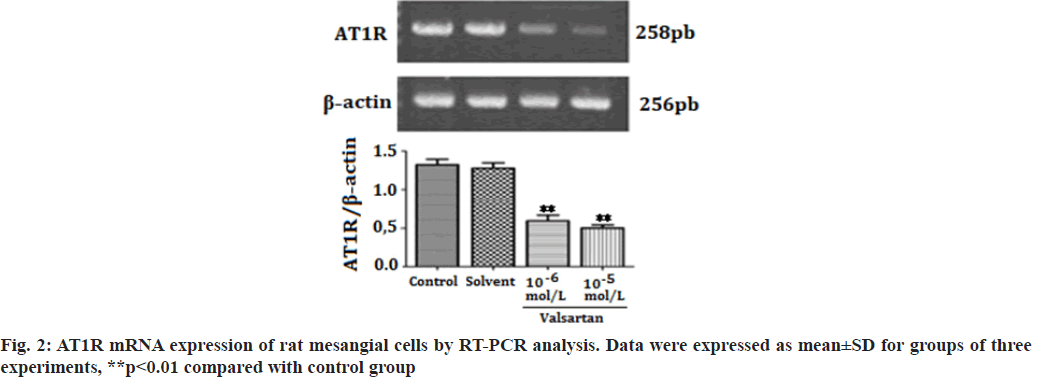
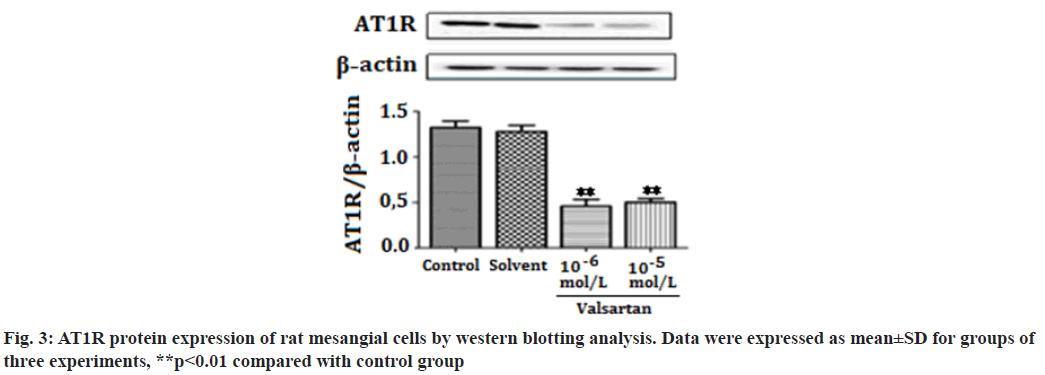
The Renin-Angiotensin System (RAS) has been recognized in playing an important role in the progression of renal diseases. Its primary effector hormone, Angiotensin II (Ang-II) plays a central role in renal tissue damage by activating Ang II type I Receptor (AT1R)[1,2]. Activation of RAS, renal inflammation and oxidative stress are characteristic findings in the pathogenesis of renal diseases. Ang II promotes inflammatory response and Reactive Oxygen Species (ROS) production via AT1R[3-5]. Currently, there is accumulating evidence that pharmacological interference with the chronic activation of Ang II represents an important therapeutic target, which may provide incremental end-organ protection. In particular, the use of AT1R blockers, solely or in combination with other regimens, may prove beneficial effect in opposing the detrimental effects of AT1R in the progression of renal diseases[6]. It has been reported that candesartan suppresses chronic renal inflammation by a novel antioxidant action independent of AT1R blockades[7]. Valsartan is an antihypertensive drug that blocks AT1R[8,9]. Previous studies have shown that valsartan alleviates cyclosporine A-induced tubular toxicity by upregulation of renal glutathione peroxidase expression and by altering oxidative stress in rats[10]. Our recent study demonstrated that valsartan significantly reduced ROS formation and protected kidneys from injury in doxorubicin-induced glomerular toxicity[11]. In order to further understand whether valsartan plays a role on proliferation and AT1R expression of mesangial cells, we examined the effects of valsartan on the proliferation and AT1R expression by utilizing cultured rat mesangial cells in the present study. To the best of our knowledge this is the first study of its kind to observe the effects of valsartan on the proliferation and AT1R expression of culture mesangial cells. Sprague-Dawley rat mesangial cell lines (Chc Collection (CCTCC)), Wuhan, China) were maintained at 37° in a humidified incubator with 5 % Carbon Dioxide (CO2)/95 % air and propagated in Dulbecco’s Modified Eagle Medium (DMEM) containing 100 mg/dl d-glucose, 10 % fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mmol/l supplemental glutamine. Cell lines were passaged once per week after treatment with trypsin-Ethylenediaminetetraacetic acid (EDTA). Cells used in the experiments were of 5-15 passages. The viability of rat mesangial cells was assessed by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). In brief, cells were plated in 96-well tissue culture plates at a density of 4×104 cell/ml. Cell cultures were washed twice with Phosphate Buffered Saline (PBS), followed by a final incubation in serumdeprived medium. During this final incubation, mesangial cells were treated with one of the following: Control; solvent (methanol solution); valsartan (10-6 mol/l, 10-5 mol/l) (St. Louis, Missouri, USA) for 14 h. Each concentration was tested for at least six replicates. At the end of each treatment, 10 μl MTT (5 mg/ml) was added to each well and the plates were incubated at 37° for another 3 h. The purple formazan crystals were dissolved in 50 μl Sodium Dodecyl Sulfate (SDS) (10 %) and absorbance was determined at 565 nm using a Stat Fax 2100 microplate reader. Cell viability was calculated as follows: Inhibition rate (%)=(Absorbance of control wells-Absorbance of treated wells)/ Absorbance of treated wells×100. Rat mesangial cells were treated with one of the following: Control; solvent; valsartan (10-6 mol/l, 10-5 mol/l) for 14 h. Each concentration was tested for at least three experiments. Ribonucleic Acid (RNA) was extracted with the One Step TRIzol kit. Primer sequences were as follows: AT1R (sense: 50′-CCCGGATGCGTACCTAAGGA-30′, antisense: 50′-CGGACGTTGCTTCGCTGT-30') and Beta (β)-actin (sense: 50′-AGCCAACTCTCACTGAAGCC-30′, antisense: 50′-GCCAACACGTGGATGCTC-30′). β-actin was used as an internal control for sample normalization. Complementary Deoxyribonucleic acid (cDNA) steps were omitted. Polymerase Chain Reaction (PCR) system was the total system of 25 μl including SYBR Green mix 12.5 μl, primer (5 pmol/μl) 2 μl, cDNA (10- fold dilution) 2.5 μl, Double Distilled Water (ddH2O) 8 μl; the conditions were as follosolvent (methanol solution); valsartanws: 50° for 2 min, 95° for 2 min, 95° for 15 s, annealing for 15 s, 72° for 45 s, 72° for 10 min, 40 cycles. Cell groups and treatments were the same as in real time Reverse Transcription- Polymerase Chain Reaction (RT-PCR) analysis. Cells were washed in PBS and then lysed in 1 ml of 1 % Non- Ionic Detergent (Nonidet) P-40, 25 mM Tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl), 150 mM Sodium Chloride (NaCl), 10 mM EDTA, pH 8.0, containing a 1:50 dilution of a protease inhibitor cocktail for 30 min on ice. Samples were centrifuged at 14 000 g for 5 min to pellet cell debris. Samples (20 μg) were mixed with Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDSPAGE) sample buffer, boiled for 5 min, electrophoresed on a 10 % SDS polyacrylamide gel and electro-blotted onto HybondTM Enhanced chemiluminescence (ECL) nitrocellulose membrane. The membrane was blocked in PBS containing 5 % skimmed milk powder and 0.02 % Tween 20. To detect AT1R, the membrane was incubated for 1 h with goat polyclonal antibody (Santa Cruz, California, USA) to AT1R. After washing, the membrane was incubated with a 1:20 000 dilution of peroxidase-conjugated goat anti-mouse Immunoglobulin G (IgG) (Santa Cruz, California, USA) in PBS containing 1 % normal goat serum and 1 % fetal calf serum. The blotting was then developed using the ECL detection kit to produce a chemiluminescence signal, which was captured on x-ray films. Equal loading of proteins was confirmed based on immunoblotting with an antibody against β-actin (Santa Cruz, California, USA). The results are presented as mean±Standard Error of Mean (SEM). One-way Analysis of Variance (ANOVA) and Tukey’s tests were used to analysis the data, p<0.05 was considered statistically significant. As shown in fig. 1, valsartan at both concentrations (10-6, 10-5 mol/l) significantly suppressed cell proliferation in rat mesangial cells as compared with the control group (p<0.01). Fig. 2 showed that AT1R messenger RNA (mRNA) expression was significantly decreased in rat mesangial cells after treatment for 14 h with valsartan at both concentrations (10-6, 10-5 mol/l) (p<0.01). The level of protein expression of AT1R in valsartan group was also significantly lower in comparison with control group (p<0.01) (fig. 3). The kidney glomerulus has three intrinsic cell types: Mesangial cells, endothelial cells and splanchnic wall epithelial cells. Mesangial cells are the most active cells among the three types of cells. They have many functions, including maintenance of glomerular capillary architecture and permeability of the glomerular filtration membrane, phagocytosis of macromolecules and immune complexes, production of bioactive substances, synthesis of an extracellular matrix, and secretion of variety of cytokines. In pathological conditions, abnormal proliferation, accumulation of extracellular matrix and collagen of mesangial cells are leading causes of glomerular injury[12]. Ang II was previously reported as a stimulator of collagen and extracellular matrix synthesis in many cell lines such as mesangial cells and rat, porcine and human vascular smooth muscle cells[13]. Ang II signaling through AT1R has a critical role in the pathogenesis of nephropathy. Previous studies have shown that overexpression of AT1R in the cell surface of Human Embryonic Kidney 293 (HEK293) cells can cause the cardiomyocyte hypertrophy and overexpression of AT1R in mesangial cells can induce mesangial cell proliferation[14,15]. The blocking of AT1R by losartan decreases collagen expression at mRNA and protein levels, which corroborates clinical investigations indicating a beneficial effect of losartan in patients with vascular diseases and in experimental models of aortic pathology, since it reduces aortic hypertrophy and collagen accumulation in rat[13,16]. Every known component of RAS is contained within mesangial cells, Ang II/AT1R can induce extracellular matrix accumulation and cell proliferation in rat renal mesangial cells[17,18]. Previous studies showed that chronic activation of Ang II/AT1R is critical in the development of chronic kidney disease. Ang II/AT1R activates mesangial cells and stimulates the synthesis of collagen and extracellular matrix[19]. Additional research has also shown that Ang II induces extracellular matrix metalloproteinase inducer expression via an AT1R dependent pathway in aortic atherosclerotic plaque in apolipoprotein E knockout mice. Valsartan could inhibit the effect of Ang II[20].
Several previous reports indicate that valsartan plays a pivotal role in protecting against progressing renal tubule injury[21,22]. Our recent study demonstrated that valsartan significantly reduced ROS formation and protected kidneys from injury in doxorubicin-induced glomerular toxicity[11]. The results of our current study indicated that valsartan inhibited cell proliferation and down-regulated mRNA and protein expression of AT1R in rat mesangial cells. Valsartan inhibited cell proliferation by downregulating AT1R expression in mesangial cells and that may provide beneficial effects by protecting the kidneys from glomerular injury. The results of the present study show that in combination with results that we reported earlier suggest that valsartan may play a renoprotective role in glomerular injury and that the mechanisms of this effect may involve down-regulation of AT1R expression in mesangial cells. Whether this mechanism also related to its antioxidant properties or not needs further investigation.
Conflict of interests:
The authors declare no conflicts of interest.
References
- Tamura K, Tanaka Y, Tsurumi Y, Azuma K, Shigenaga AI, Wakui H, et al. The role of angiotensin AT1 receptor-associated protein in renin-angiotensin system regulation and function. Curr Hypertens Rep 2007;9(2):121-7.
[Crossref] [Google Scholar] [PubMed]
- Sharma N, Anders HJ, Gaikwad AB. Fiend and friend in the renin angiotensin system: An insight on acute kidney injury. Biomed Pharmacother 2019;110:764-74.
[Crossref] [Google Scholar] [PubMed]
- Burns KD. Angiotensin II and its receptors in the diabetic kidney. Am J Kidney Dis 2000;36(3):449-67.
[Crossref] [Google Scholar] [PubMed]
- Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol 2007;18(9):2439-46.
[Crossref] [Google Scholar] [PubMed]
- Rincón J, Correia D, Arcaya JL, Finol E, Fernández A, Pérez M, et al. Role of Angiotensin II type 1 receptor on renal NAD (P) H oxidase, oxidative stress and inflammation in nitric oxide inhibition induced-hypertension. Life Sci 2015;124:81-90.
[Crossref] [Google Scholar] [PubMed]
- Susantitaphong P, Sewaralthahab K, Balk EM, Eiam-ong S, Madias NE, Jaber BL. Efficacy and safety of combined vs. single renin–angiotensin–aldosterone system blockade in chronic kidney disease: A meta-analysis. Am J Hypertens 2013;26(3):424-41.
[Crossref] [Google Scholar] [PubMed]
- Chen S, Ge Y, Si J, Rifai A, Dworkin LD, Gong R. Candesartan suppresses chronic renal inflammation by a novel antioxidant action independent of AT1R blockade. Kidney Int 2008;74(9):1128-38.
[Crossref] [Google Scholar] [PubMed]
- Criscione L, de Gasparo M, Bühlmayer P, Whitebread S, Ramjoué HP, Wood J. Pharmacological profile of valsartan: A potent, orally active, nonpeptide antagonist of the angiotensin II AT1‐receptor subtype. Br J Pharmacol 1993;110(2):761-71.
[Crossref] [Google Scholar] [PubMed]
- Müller P, Cohen T, de Gasparo M, Sioufi A, Bacine-Poon A, Howald H. Angiotensin II receptor blockade with single doses of valsartan in healthy, normotensive subjects. Eur J Clin Pharmacol 1994;47(3):231-45.
[Crossref] [Google Scholar] [PubMed]
- Raeisi S, Ghorbanihaghjo A, Argani H, Dastmalchi S, Ghasemi B, Ghazizadeh T, et al. The effects of valsartan on renal glutathione peroxidase expression in alleviation of cyclosporine nephrotoxicity in rats. Bioimpacts 2016;6(3):119-4.
[Crossref] [Google Scholar] [PubMed]
- Liu HX, Li J, Li QX. Therapeutic effect of valsartan against doxorubicin-induced renal toxicity in rats. Iran J Basic Med Sci 2019;22(3):251-54.
[Crossref] [Google Scholar] [PubMed]
- Suzuki K, Han GD, Miyauchi N, Hashimoto T, Nakatsue T, Fujioka Y, et al. Angiotensin II type 1 and type 2 receptors play opposite roles in regulating the barrier function of kidney glomerular capillary wall. Am J Pathol 2007;170(6):1841-53.
[Crossref] [Google Scholar] [PubMed]
- Dab H, Hachani R, Dhaouadi N, Sakly M, Hodroj W, Randon J, et al. Regulation of aortic extracellular matrix synthesis via noradrenergic system and angiotensin II in juvenile rats. Pharm Biol 2012;50(10):1219-25.
[Crossref] [Google Scholar] [PubMed]
- Filipeanu CM, Zhou F, Claycomb WC, Wu G. Regulation of the cell surface expression and function of angiotensin II type 1 receptor by Rab1-mediated endoplasmic reticulum-to-Golgi transport in cardiac myocytes. J Biol Chem 2004;279(39):41077-84.
[Crossref] [Google Scholar] [PubMed]
- Michel MC, Brunner HR, Foster C, Huo Y. Angiotensin II type 1 receptor antagonists in animal models of vascular, cardiac, metabolic and renal disease. Pharmacol Ther 2016;164:1-81.
[Crossref] [Google Scholar] [PubMed]
- Reddy MA, Sumanth P, Lanting L, Yuan H, Wang M, Mar D, et al. Losartan reverses permissive epigenetic changes in renal glomeruli of diabetic db/db mice. Kidney Int 2014;85(2):362-73.
[Crossref] [Google Scholar] [PubMed]
- Xu JL, Gan XX, Ni J, Shao DC, Shen Y, Miao NJ, et al. SND p102 promotes extracellular matrix accumulation and cell proliferation in rat glomerular mesangial cells via the AT1R/ERK/Smad3 pathway. Acta Pharmacol Sin 2018;39(9):1513-21.
[Crossref] [Google Scholar] [PubMed]
- Xue H, Zhou L, Yuan P, Wang Z, Ni J, Yao T, et al. Counteraction between angiotensin II and angiotensin-(1–7) via activating angiotensin type I and Mas receptor on rat renal mesangial cells. Regul Pept 2012;177(1-3):12-20.
[Crossref] [Google Scholar] [PubMed]
- Yano N, Suzuki D, Endoh M, Zhao TC, Padbury JF, Tseng YT. A novel phosphoinositide 3-kinase-dependent pathway for angiotensin II/AT-1 receptor-mediated induction of collagen synthesis in MES-13 mesangial cells. J Biol Chem 2007;282(26):18819-30.
[Crossref] [Google Scholar] [PubMed]
- Yang LX, Yang ZH, Guo RW, Ye JS, Liu H. Angiotensin II induces extracellular matrix metalloproteinase inducer expression via an AT1R dependent pathway in aortic atherosclerotic plaque in apolipoprotein E knockout mice. J Renin Angiotensin Aldosterone Syst 2012;13(1):67-75.
- Sun Y, Peng PA, Ma Y, Liu XL, Yu Y, Jia S, et al. Valsartan protects against contrast-induced acute kidney injury in rats by inhibiting endoplasmic reticulum stress-induced apoptosis. Curr Vasc Pharmacol 2017;15(2):174-83.
[Crossref] [Google Scholar] [PubMed]
- Wu K, Zhou T, Sun G, Wang W, Zhang Y, Zhang Y, et al. Valsartan inhibited the accumulation of dendritic cells in rat fibrotic renal tissue. Cell Mol Immunol 2006;3(3):213-20.
[Google Scholar] [PubMed]