- *Corresponding Author:
- S. D. Jagtap
Department of Herbal Medicine, Interactive research School for Health Affairs (IRSHA), Bharati Vidyapeeth Deemed University, Pune-411 043, India
E-mail: chiritatml@rediffmail.com
| Date of Submission | 02 August 2016 |
| Date of Revision | 29 March 2017 |
| Date of Acceptance | 03 October 2017 |
| Indian J Pharm Sci 2017;79(6):965-973 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Amarkand plants are routinely used by Indian tribes for health and longevity. Taxonomically, Amarkand includes species from genera Eulophia and Dioscorea. Comparative immunomodulatory potential of Amarkand extracts were examined through biochemical, hematological, serological and histopathological analysis. A significant effect on the modulation of immune reactivity was observed in bulbils of Dioscorea bulbifera and tubers of Eulophia ochreata. A significant increase was also observed in relative organ weights, hematological parameters and total protein by E. ochreata (500 mg/kg) treatment compared to the negative control group. While aminotransferase enzyme levels have shown a significant decrease by the treatment of E. ochreata and D. bulbifera extracts, D. bulbifera (300 mg/kg) showed greater (61.7±3.62%) neutrophil adhesion and a significant increase in delayed type hypersensitivity response. E. ochreata treatment showed increased haemagglutination titre compared to negative control, thereby providing evidence that D. bulbifera and E. ochreata possessed immunomodulatory activity. Histopathology of rat organs treated with D. bulbifera and E. ochreata, showed normal cytoarchitecture and less neutrophil infiltration as compared to the negative control group, suggesting no evidence of pathological signs. Our findings demonstrated that E. ochreata and D. bulbifera have immunomodulatory potential and provide the basis for an alternative approach to investigate new traditional remedies.
Keywords
Amarkand, Eulophia, Dioscorea, immunomodulation
The immune system is a remarkably sophisticated defense system, which protects the body from invading pathogens. Lack of immunological balance and impaired immune system are often make the body prone to diseases including inflammatory and degenerative diseases. Modulation of immune system denotes alterations of immune responses by induction, expression and amplification of part or phase of the reaction [1]. Thus, immunomodulators are often employed, targeting an effect on the immune system.
Immunomodulatory agents have immunostimulating or immunosuppressive effects depending on the target site, including both innate and adaptive arms of the immune system. This includes a large number of phytocompounds. Their effects are of three types namely stimulation, suppression and restoration of the immune system [2]. Immunomodulators may play a role in maintaining the immune system by increasing T cell adhesion, blocking the suppressor activity, stimulating the interferon production, natural killer (NK) cells as well as it activates target cells by inducing specific cytokine production [3-5]. Immunomodulators isolated and purified from natural sources such as plants and microorganisms are the most promising alternatives to conventional therapy in a variety of diseases [6].
India is blessed with rich medicinal plant diversity, which would be the best source to obtain a variety of drugs and natural products and some are being used by several tribal communities since ages [7]. An example of one of the groups of medicinal plants, credited with innumerable medicinal qualities used since ancient times is Amarkand [8]. Tribal people have extensively used members of genus Eulophia and Dioscorea bulbifera L. as an important constituent in their diet and called them Amarkand. But, a nagging ambiguity between the authentic species among these species remained unaddressed [9]. Amarkand is praised in Ayurveda for having good health promoting ability as well as rasayana properties [10]. Eulophia species comprises a perennial terrestrial orchids (herb) with one to three feet height having fleshy tubers in the form of chain, generally grows along the water channel on deep soil slopes, distributed worldwide [11] and mostly tropical Himalaya and Deccan peninsular region in India [12], while D. bulbifera (family: Dioscoraceae) is a tuberous large perennial wine with broad leaves. It also produces bulbils at the base of its leaves, generally grows at moist places also found as planted in kitchen garden of villagers [13]. It is distributed in tropics and subtropics of world and found throughout the warmer part of India [14].
From ethnopharmacological point of view, multiple uses of Amarkand is an advantage, it is an indicator of medicinal value. Previously, Eulophia ochreata species have shown antiglycation [15], antioxidant [16], antiinflammatory properties [17]. Phenanthrene compounds isolated from E. nuda [12] have antiproliferative [18] as well as antiinflammatory potential [19]. Furthermore, studies on D. bulbifera demonstrated antitumor activity of tubers [20] and analgesic and antiinflammatory activity of bulbils [21]. In our previous work, out of 21 Amarkand plants, methanol extract of bulbils of D. bulbifera (DBB), tubers of E. ochreata (EO), E. leghapannsis and D. bulbifera have found a significant antioxidant potential [16] as well as antifatigue potential [9]. There are however, no scientific data available to substantiate their immunomodulatory effect. Thus, the present study was undertaken to explore the immunomodulatory potential of these four extracts on humoral and cellular immune responses to the antigenic challenge by sheep red blood cells (SRBC) and efficacy was checked on serological, haematological parameters as well as on vital organs of the body as these are the primary signs to diagnose any disease.
Materials and Methods
Fresh tubers of EO and E. leghapanensis (Noven) (EL) were collected in early monsoon. While tubers of D. bulbifera L. (DBT) and DBB, respectively, were collected in late monsoon. All these plants were identified and collected from Leghapani (Nandurbar) and Bhimashankar (Pune) area of Maharashtra, India. Plants were authenticated at Medicinal Plants Conservation Centre (MPCC), Pune with voucher specimen numbers, MPCC3125 for E. ochreata, MPCC3468 for EL and MPCC906 for D. bulbifera. Pulverization of dried material was carried out using an electronic blender and stored in an airtight polythene bags for further use. Extracts were prepared in methanol for all the samples using hot extraction method and concentrated to dryness under reduced pressure using a rotary evaporator. Yeild obtained for EO, EL, DBB and DBT was 70.2, 44.4, 64.6 and 47.6 mg/ml, respectively.
Experimental animals
The study was carried out on female Wistar rats (150- 250 g, 6 w old). Animals were maintained under standard husbandry conditions 25±2° and fed with standard pellet diet and tap water ad libitum. All animals were procured from National Institute of Biosciences, Pune and handled according to the international guidelines for the care and use of laboratory animals of national research council. This study was carried out in accordance with Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines. The study was approved by the animal ethical committee (CPCSEA/7/2679/2012-2013) of Bharati Vidyapeeth Deemed University, Medical College, Dhankawadi, Pune, India.
Preparation of antigen
Sheep blood was aseptically collected from a city slaughter house in sterile Alsever’s solution (1:1), to avoid coagulation of blood. RBCs were washed repeatedly by thoroughly mixing and centrifuging at 2000 rpm for 10 min. Supernatant was discarded and centrifugation was repeated for 4-5 times until the supernatant fluid became colourless. Obtained SRBCs were resuspended in saline and adjusted to the required concentration for immunization and to challenge as an antigen [22].
Treatment
Animals were randomly divided into 15 groups and each plant extract has three groups as per its dosage viz., 100, 300, 500, including healthy, negative and a positive control group having six (n=6) rats per group. Healthy and negative control groups were fed orally with saline by gavage, the positive control group received prednisolone (10 mg/kg) standard and another 12 treated groups were force fed with different doses of EO, EL, DBT and DBB (100, 300 and 500 mg/kg) by gavage once a day for seven days. SRBCs were injected on day zero by intraperitoneal administration of 0.4 ml of 5×109 SRBC/ml/rat [23]. On the 8th d, animals were sacrificed, vital organs viz., thymus, spleen, liver and kidney were dissected, cleaned, dabbed with filter paper to remove moisture and then weighed separately on a digital balance.
Haematological parameters
Blood samples were collected by cardiac puncture in ethylenediamine tetra acetic acid (EDTA) coated heparinized vials to estimate haematological parameters using an auto analyser (Abacus-3, Diatron, Austria). Haematological parameters included hemoglobin content; total RBC count, total white blood cells (WBCs) count, differential WBC count and lymphocyte count were estimated. Whole blood was used to analyse hemoglobin, which is expressed in gm %.
Serum biochemical parameters
Serum was separated by centrifugation at 3000 rpm at 4° for 10 min. Aminotransferase enzyme levels were analysed by the references given in the kit literature mentioning the basis of the methods on which test procedures were carried out. Total protein and albumin to globulin (A/G) ratios were analysed according to Biuret method [24].
Neutrophil adhesion test
The method of Fulzele et al. [25] was used for this test. SRBCs were injected on day 0 by intraperitoneal administration of 0.4 ml of 5×109 SRBC/ml/rat. On 8th d of the experiment, blood samples were collected by heart puncture and analysed for total leukocyte count (TLC) and differential leukocyte count (DLC). After original counts, all blood samples were incubated for 15 min with 80 mg/ml of nylon fibres at 37°. These incubated samples were again analysed for TLC and DLC. Neutrophil index (NI) of a blood sample is obtained from TLC of sample and express as a percentage of neutrophil. Percent neutrophil adherence was calculated using the formula, percent neutrophil adhesion= [NIu–NIt/NIu], where, NIu is a NI of untreated blood sample and Nit is NI of blood sample incubated with nylon fiber.
Delayed type of hypersensitivity (DTH) response
On the 7 d, treated rats were again sensitized with 0.1 ml of 1.25×109 cells SRBC/ml subcutaneously in right hind foot pad [23]. Whereas, the healthy control group was administered with equal volume of saline. Swelling edema in foot pad thickness was measured 24 h after sensitization using plethysmometer (Orchid Scientific, India). Difference in foot pad thickness pre and post challenge, was taken as a measure of DTH response.
Humoral immune response
Haemagglutination antibody (HA) titre assay was used to measure the humoral immune response using animal serum by the procedure of Ismail and Asad [26]. The microtiter plate was filled with 0.1 ml sterile normal saline and serial two fold dilutions were made with 0.1 ml of the serum in sterile saline solution. Titre value was obtained by titrating each microtitre well with 0.1 ml of 3× saline washed SRBCs having 1.25×109 cells. The plate was allowed to stand overnight at RT until control wells showed a negative pattern (small button formation). A minimum volume of serum required to produce hemagglitination was taken as antibody titre and converted to log 2 values for easy comparison.
Histopathology analysis
Liver, thymus, spleen and kidneys were dissected at the end of the experiment and transferred to 10 % phosphate buffered formalin for histopathological studies. Slides were stained with haematoxylin and observed at various magnifications to note changes in cytoarchitecture of tissue studied.
Statistical analysis
The values are presented as a mean±SD. One way ANOVA was used to determine significant differences between groups and negative control group. All analyses were performed using graph pad prism version 7.0 followed by Dunnett’s multiple comparison test. The level of significance was set at p<0.05.
Results and Discussion
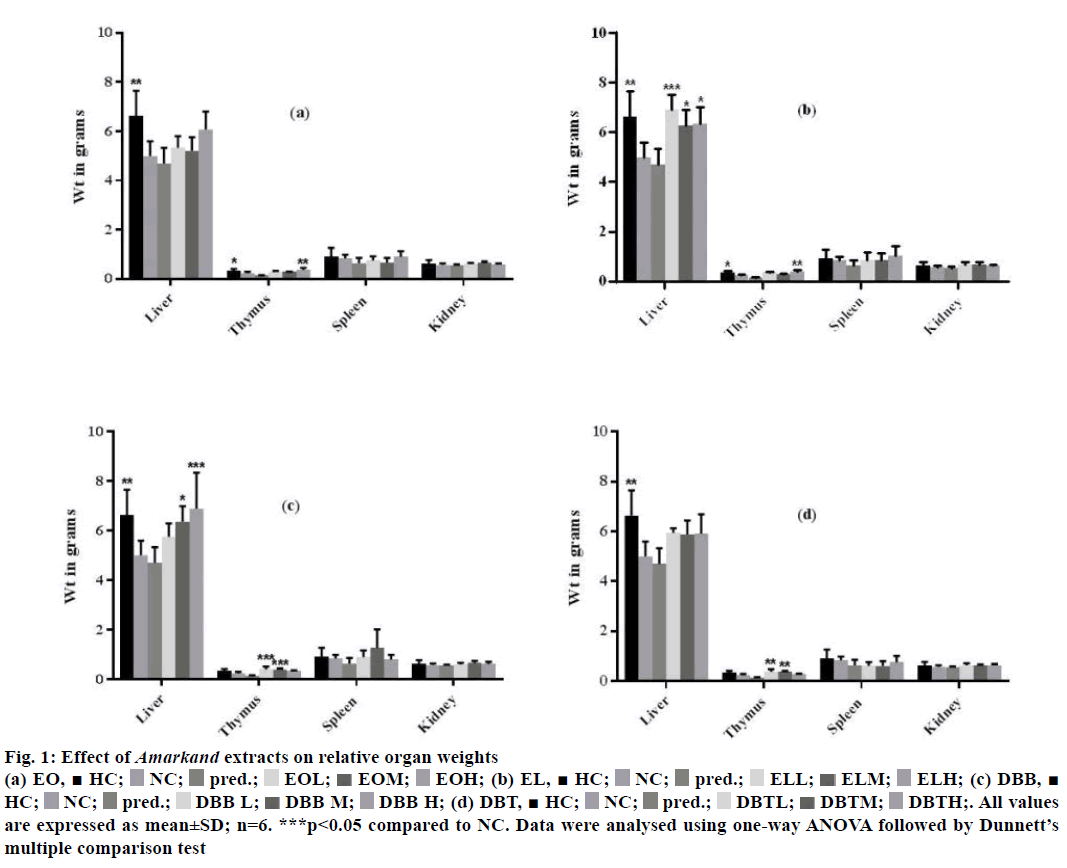
Effect of Amarkand species on relative organ weight is shown in Figure 1. It was observed that, treatment with all the Amarkand extracts did not alter the relative organ weights. Among the four extracts, EL100 treatment followed by DBB500, have shown a significant increase in the weight of the liver (p<0.05) as compared to the negative control group. Additionally, DBB100 and DBB300 treatment significantly increased the weight of thymus (p<0.05) as that of healthy group, compared to negative control group. EL and DBB apparently attenuated the weight loss in the spleen and kidney as compared to the negative control group.
Figure 1: Effect of Amarkand extracts on relative organ weights
(a) EO,  HC;
HC;  NC;
NC;  pred.;
pred.;  EOL;
EOL;  EOM;
EOM;  EOH; (b) EL,
EOH; (b) EL,  HC;
HC;  NC;
NC;  pred.;
pred.;  ELL;
ELL;  ELM;
ELM; ELH; (c) DBB,
ELH; (c) DBB,  HC;
HC; NC;
NC;  pred.;
pred.; DBB L;
DBB L; DBB M; DBB H; (d) DBT,
DBB M; DBB H; (d) DBT,  HC;
HC;  NC;
NC;  pred.;
pred.;  DBTL;
DBTL;  DBTM;
DBTM;  DBTH;. All values
are expressed as mean±SD; n=6. ***p<0.05 compared to NC. Data were analysed using one-way ANOVA followed by Dunnett’s
multiple comparison test
DBTH;. All values
are expressed as mean±SD; n=6. ***p<0.05 compared to NC. Data were analysed using one-way ANOVA followed by Dunnett’s
multiple comparison test
Alteration in immunity would lead to a significant decrease in the counts of total WBC, lymphocytes, RBC and hemoglobin content in prednisolone treated and negative control group. While EL100 treatment had shown no significant effect in the total WBC count, EO500 significantly increased the RBC count as compared to the negative control group (Table 1). Among the four extracts, EO treatment failed to produce any significant increase in lymphocyte count and hemoglobin content as compared to negative control group.
| Groups | Leukocyte count | RBCs (106/µl)a | Lymphocytes (%)a | Hb (g%)a |
|---|---|---|---|---|
| WBC(10 E3/mm3)a | ||||
| HC | 15.3±1.2 | 8.8±0.9 | 73.7±4.3 | 13.2±1.6 |
| NC | 8.7±4.3 | 8.2±0.9 | 70.7±9.4 | 12.7±1.6 |
| Pred | 5.7±2.0 | 9.0±0.2 | 53.8±14.3 | 13.3±0.4 |
| EO100 | 13.7±3.6 | 8.6±0.3 | 79.2±5.1 | 12.8±0.5 |
| EO300 | 14.3±0.7 | 8.5±0.5 | 80.0±8.3 | 13.0±0.6 |
| EO500 | 7.3±0.8 | 9.6±0.8 | 75.7±13.0 | 13.9±1.3 |
| EL100 | 14.5±2.8 | 8.1±0.5 | 66.5±20.8 | 12.3±0.6 |
| EL300 | 14.0±3.7 | 8.3±0.9 | 80.2±6.4 | 12.3±1.2 |
| EL500 | 11.0±3.5 | 7.9±0.5 | 78.2±5.5 | 12.0±0.4 |
| DBB100 | 11.1±4.5 | 9.0±0.7 | 74.8±10.3 | 12.2±2.2 |
| DBB300 | 10.7±1.4 | 8.8±0.4 | 70.3±14.3 | 13.1±0.3 |
| DBB500 | 13.5±3.4 | 8.8±0.8 | 67.7±14.5 | 13.4±0.7 |
| DBT100 | 10.2±1.5 | 7.4±1.0 | 72.5±10.0 | 12.6±0.8 |
| DBT300 | 14.5±6.5 | 8.1±0.7 | 75.5±15.1 | 13.4±0.8 |
| DBT500 | 12.5±1.7 | 8.3±0.7 | 79.2±7.6 | 13.2±0.41 |
aAll values are expressed as mean±SD; n=4 for WBC and n=6 for other. Data were analysed using one-way ANOVA followed by Dunnett’s Multiple Comparison test
Table 1: Effect of Amarkand extracts on WBC, RBC, lymphocytes and hemoglobin
Administration of EO and DBB extracts significantly attenuated liver enzymes (Table 2). DBB500 and EO100 (p<0.05) effectively reduced serum glutamic pyruvic transaminase (SGPT) levels while, serum glutamic-oxaloacetic transaminase (SGOT) levels were significantly maintained within range by EO100 (p<0.05) and EL500 (p<0.05) treatment as compared to the negative control group. Table 2 depicts the level of serum protein and A/G ratio where, EO at higher dose showed a marked increase in serum protein (p<0.05) as compared to SRBC treated group. DBB treatment also shown increase in A/G ratio comparatively, but this was not statistically significant relative to the negative control group.
| Groups | SGPT (U/ml)a | SGOT (U/ml)a | Totprotein (g/dl)a | A/G ratioa |
|---|---|---|---|---|
| HC | 76.3±8.3 | 147.8±9.6 | 7.1±1.0 | 6.2±0.3 |
| NC | 90.8±14.5 | 177.4±22.6 | 5.4±0.3 | 3.5±0.4 |
| Pred | 61.0±5.4*** | 161.4±26.8 | 7.4±1.5 | 3.9±0.3 |
| EO100 | 61.8±8.3*** | 140±17.7 | 4.6±1.0 | 5.5±2.4 |
| EO300 | 70.3±16.6 | 161.4±29.6 | 7.2±0.9 | 4.5±0.2 |
| EO500 | 77.5±5.3 | 170±5.6 | 7.6±1.9 | 5.4±0.3 |
| EL100 | 89.8±4.0 | 159.2±17.1 | 6.5±0.7 | 7.4±1.1 |
| EL300 | 60.0±2.8 | 151.4±6.3 | 7.4±0.8 | 4.1±1.6 |
| EL500 | 59.5±2.5 | 139.6±31.3 | 7.1±0.9 | 3.3±1.9 |
| DBB100 | 76.8±5.4 | 164.6±21.3 | 5.3±1.3 | 5.6±2.0 |
| DBB300 | 68.5±11.0 | 152.8±20.1 | 6.1±2.0 | 3.9±1.0 |
| DBB500 | 62.5±6.5*** | 149.4±6.5 | 7.3±0.7 | 3.9±2.5 |
| DBT100 | 85.3±2.9 | 160.6±27.5 | 5.8±1.1 | 3.6±1.5 |
| DBT300 | 73.0±4.7 | 155.8±17.7 | 7.0±0.6 | 5.2±0.2 |
| DBT500 | 70.3±10.5* | 172±17.5 | 7.1±1.7 | 3.5±1.7 |
aAll values are expressed as mean ± SD; n=4, 5, 6, 3 respectively.
***p<0.05 compared to NC. Data were analysed using one-way
ANOVA followed by Dunnett’s Multiple Comparison test
Table 2: Effect of Amarkand extracts on aminotransferases and total protein
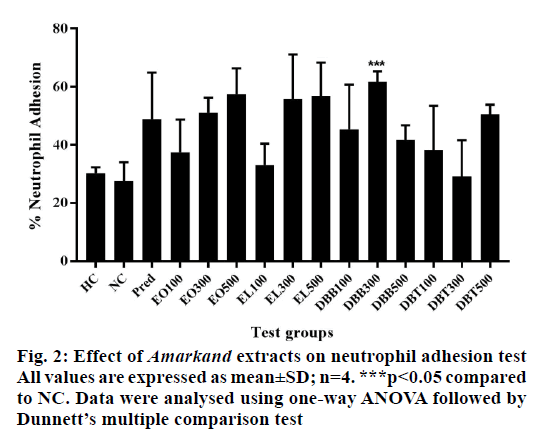
Neutrophils incubated with nylon fibres showed decrease in neutrophil percentage due to their adhesion to fibres. DBB300 (p<0.05), EO500 (p<0.05) followed by EL500 (p<0.05) extracts have shown significantly more neutrophil adhesion as compared to the negative control group (Figure 2). Hence it was inferred that DBB, EL and EO treatment caused stimulation of neutrophils towards the site of inflammation.
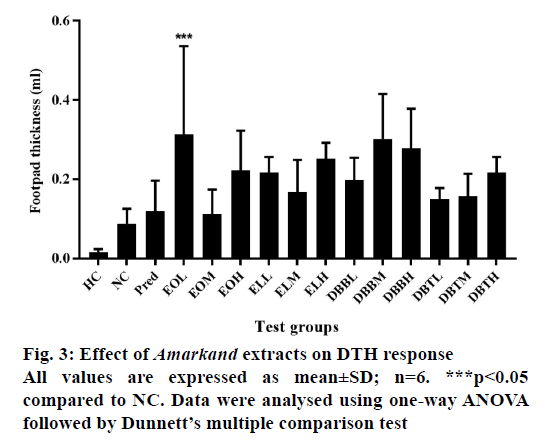
Increased DTH response indicates the stimulatory effect of the extract, which has occurred on the lymphocytes and accessory cell types required for the expression of the reaction. Figure 3 demonstrates a time course alterations in DTH response for negative and standard drug treated group. DBB extract at the dose of 300 and 500 mg/kg (p<0.05) and EO at the dose of 100 and 500 mg/kg (p<0.05) elicited significantly DTH response compared to negative control animals.
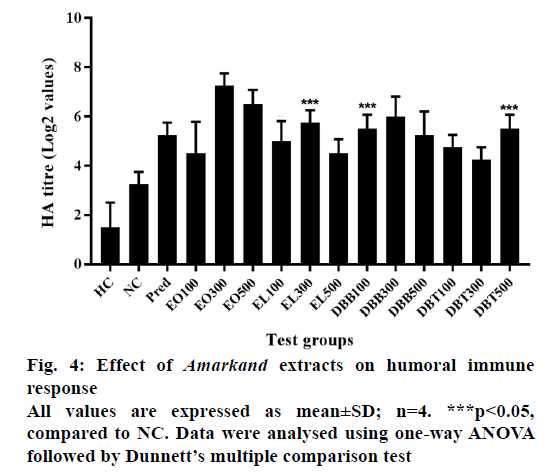
The indirect haemagglutination titre value was considered to confirm the effect of drug treatment on humoral arm of the immune system. As shown in Figure 4, a significant increase in titre values of circulating antibodies by all the doses of EO and DBB treatment was observed. EO300 and EO500 followed by DBB300 significantly raised (p<0.05) antibody formation as compared to negative control group.
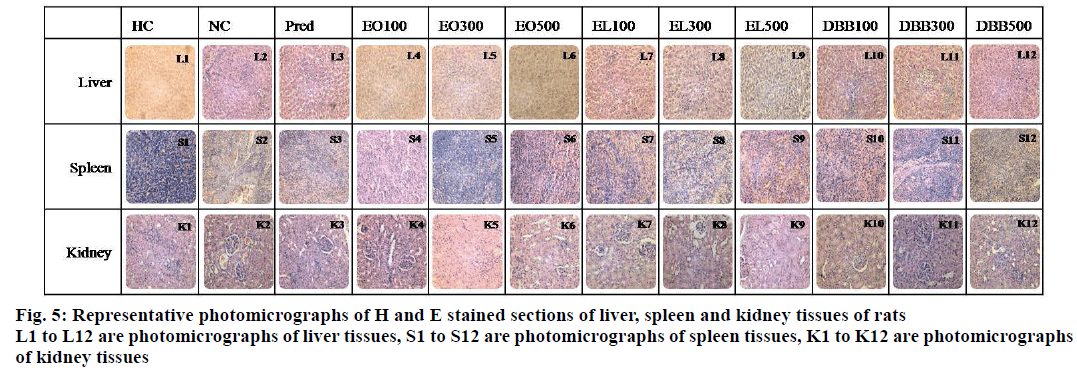
Histopathological examination of various organs was performed for healthy control, negative control and treated groups which are given in Figure 5. Microscopic examination of liver section studied for negative control animals have shown inflammatory infiltrations having marginalization of lymphocytes as compared to healthy control animal. EO and DBB treated group animal showed well maintained cytoarchitecture, while EL and DBT treatment showed mild inflammation in portal tracts and sinusoids. No pathological changes were observed in the thymus of HC, NC and drugtreated animals. Spleen tissue section of NC group animal showed congestion of red pulp and blood vessels compared to HC. DBT-treated animals showed mild congestion of red pulp and rest, i.e. EO, DBB and EL-treated animals showed normal appearing red and white pulp. Kidney histology of spleen in the NC group showed focal areas of inflammation consisting of lymphocytes and congestion. Among drug treated groups EL group showed some congested blood vessels and DBT group showed moderate infiltration. On the other hand, DBB and EO treatment effectively protected the normal cytoarchitecture compared with negative control.
Immunomodulators are agents that activate or suppress the immune system function by altering immune responses. Immunomodulators of plant origin increase the immune responsiveness of the body against pathogens by activating the non-specific immune system [27]. Presently, plant derived compounds having immunomodulatory effects plays a major role in many pharmaceutical interventions and has lead to rigorous research to determine efficacy and safety of the treatment [28]. Additionally, immunomodulatory regimes offer a striking approach as these compounds have fewer side effects than existing synthetic drugs [6]. From our earlier study, qualitative phytochemical screening of these selected species from Amarkand group revealed presence of bioactive compounds, which include alkaloids, anthraquinones, cardiac glycosides, flavonoids, steroids, tannins and terpenoids. Additionally, they contain high amount of polyphenols like phenols, flavonoids and proanthocynidins [16]. These may be the principal constituents to produce immunomodulatory activity. In the present study, these four methanolic extracts were studied for immunomodulatory potential using rat model where SRBCs were given intra-peritonealy. SRBCs were used both for sensitization and to determine antibody titre [29].
Thymus is one of the important secondary lymphoid organs while liver being the main detoxification organ in mammals. Spleen is responsible for purifying blood as well as storing blood cells while kidney is concerned with excretion of foreign substances. All these organs are susceptible to the toxicities of drugs [30]. Organ to body weight ratios are useful indicator to investigate the toxic effect of drug treatment on the vital organs as well as to determine disease state and progression [31]. In the present study, the observed protection in thymus by DBB treatment may be due to stimulation of the gland activity. While, EL and DBB treatment, maintained normal weight of liver as decreased liver weight leads to diseased conditions. Significant results by the DBB and EL treatment may be attributed to its beneficial effect on the body and its immunostimulant properties, which was also seen in the histopathological results.
Neutrophils including WBC and lymphocytes play a major role in the immune response. They participate in host defence, both as the first line of innate immune defence and as effectors of adaptive immunity. Normal functioning of these cells would ensure disease free status of the body [32]. Prednisolon treated group have shown decrease in the neutrophils count, showing adverse effect of steroids on the immune system. While, compared to DBB and DBT, treatment with EO and EL increase blood leucocytes as well as hemoglobin content suggesting improved immune status.
SGPT is an enzyme normally present in liver and heart cells. Measurement of these enzyme activities in tissue or body fluid serves as a marker for liver damage [33]. Oral administration of extracts has a lowering effect on these enzyme levels, confirming their role in immunomodulation. Likewise, circulatory proteins, especially albumin and globulins are an integral part of immune system. They are involved in immunity or synthesized as the need arises. Albumin and globulins help to carry certain medicines/bioactive molecules through the blood. While, certain globulins made by the immune system may transport metal ions and help fight infection [34]. Treatment by DBB and EO extracts, prevented cellular tissue damage of vital organs like liver (Figure 5) and heart compared to that of EL and DBB treatment.
Neutrophils are important immune cells and are markers of healthy immune system, which can efficiently carry inflammation followed by adhesion; they use different defence strategies [32]. Among all plant extracts, DBB treatment showed a significant increase in adhesion of neutrophils to nylon fibres, which correlates with margination process of cells in blood vessels towards the site of inflammation. This activity may be due to bioactive compounds like steroidal saponins, phenanthrene, flavonoids and diterpenoids found in earlier studies in the methanol extract of DBB [21]. DBB extracts may cause up-regulation of β2-integrins on neutrophils, which help them to adhere firmly to nylon fibres.
DTH response is a type IV hypersensitivity reaction used to assess the effect of the fraction on cell-mediated immunity. It is characterized by influx of immune cells at the site of injection [35]. DTH reaction elicited by SRBCs requires specific recognition of antigen by activated T-lymphocytes, which subsequently proliferate and release pro-inflammatory lymphokines. Thus, DTH reaction is important in host defence against parasites and bacteria that can live and proliferate intracellularly [36]. Methanol extracts of DBB and EO showed immunostimulatory effect by enhancing DTH reaction comparatively, which is reflected from increased foot-pad thickness compared to negative control group (Figure 3). These overall effects suggest the effect of Amarkand extracts through intense infiltration of macrophages to the inflammatory site assisting the cell-mediated immunity response.
Humoral immunity is mediated by circulating antibodies. It involves transformation of B cells into plasma cells that produce and secrete antibodies specific to an antigen. These antibodies bind to antigens and neutralize it or facilitate its elimination by more ready ingestion by phagocytic cells [37]. The indirect haemagglutination test was carried out to evaluate the effect of plant extracts on the humoral immunity. In the present study, antiSRBC antibody titre was significantly raised by methanolic extract of EO over to negative control as well as other plant extract groups, which indicates enhanced responsiveness of T and B lymphocyte subsets, macrophages involved in antibody synthesis. This in turn signifies its immunostimulatory effect on circulating antibodies. Out of the four extract treatments, positive effect of EO on humoral immunity was observed which may be due to two bioactive phenanthrenes obtained found in earlier studies [12].
Among the four extracts, DBB and EO treatments showed pronounced recovery and near-normal cytoarchitecture than negative control group followed by EL and DBT treatment. Therefore, DBB and EO treatment proved to be immunoprotective and effective as an immunomodulator, which corroborates the results obtained in biochemical parameters. Previous studies also support the action of DBB [38] and EO [39], as they are rich in minerals like Na, K, Ca, Fe and Zn which play an important role in proper immune functions whereas deficiencies leads to irregular immunological profiles [40].
Folklore knowledge regarding the therapeutic use of these plants is the important sources for drug discovery. Results obtained from the present study depict that D. bulbifera bulbils and E. ochreata tubers (Amarkand) have shown appreciable immunomodulatory activity by potentiating humoral as well as cellular immunity. This may be due to secondary metabolites present in it. Phytochemical content present in these two plants, especially, phenols, flavonoids and proanthocyanidin seem to be most likely candidates eliciting immunostimulatory effect. The immunomodulatory effect of these species warrants further investigation to know their possible mechanism of action or isolation of bioactive compounds.
Acknowledgments
Authors thank the Department of Science and grant to perform the experiments. We are also thankful to Prof. Sharma G. D., Principal Rajiv Gandhi Institute of Biotechnology and Director, IRSHA for their support and encouragement.
Conflict of interest
The authors declare that they have no conflict of interest.
Financial support and sponsorship
Nil.
References
- Saroj P, Verma M, Jha KK, Pal M. An overview on immunomodulation. J Adv Scient Res 2012;3:7-12.
- Yeap SK, Rahman MB, Alitheen NB, Ho WY, Omar AR, Beh BK, et al. Evaluation of immunomodulatory effect: selection of the correct targets for immunostimulation study. Am J Immunol 2011;7:17-23.
- Gabius HJ. Probing the cons and pros of lectin induced immunomodulation: Case studies for the mistletoe lectin and galectin-1. Biochemie 2003;83:659-66.
- Stanilove SA, Dobreva ZG, Slavov S, Mitera LD. C3 binding glycoprotein from Cuscuta europea induced different cytokine profiles from human PBMC compared to other plant and bacterial immunomodulators. Int Immunopharmacol 2005;5:723-34.
- Lam HY, Yeap SK, Alitheen NB, Ho WY, Omar AR, Yusoff K, et al. Understand the role of natural killer. Am J Immunol 2010;6:54-61.
- Nagarathna PKM, Reena K, Reddy S, Wesley J. Review on Immunomodulation and Immunomodulatory activity of some herbal plants. Int J Pharm Sci Rev Res 2013;22:223-30.
- Singh K, Verm B. The concept of Vyadhikshamatva (Immunity) in Ayurveda. Ayurpharm Int J Ayur Alli Sci 2012;1:99-108.
- Narkhede AN, Kasote DM, Kuvalekar AA, Harsulkar AH, Jagtap SD. Amarkand: A comprehensive review on its ethnopharmacology, nutritional aspects, and taxonomy. J Intercult Ethnopharmacol 2016;5:198-204.
- Narkhede AN, Jagtap SD, Nirmal PS, Giramkar SA, Nagarkar BE, Kulkarni OP, et al. Anti-fatigue effect of Amarkand on endurance exercise capacity in rats. BMC Compl Altern Med 2016;16:23-7.
- Vaidya B. Nighantu Adarsha. Varanasi: Chaukhambha Bharati Academy: 2004.
- Patil MC, Mahajan RT. Ethnobotanical potential of Eulophia species for their possible biological activity. Int J Pharm Sci Rev Res 2013;21(2):297-307.
- Kshirsagar RD, Kanekar YB, Jagtap SD, Upadhyay SN, Rao R, Bhujbal SP, et al. Phenanthrenes of Eulophia ochreata Lindl. Int J Green Pharm 2014;4:147-52.
- Suriyavathana M, Sivanarayan V. Anti-inflammatory activity of Delonix elata on collagen induced paw edema in swiss albino mice. Indo Am J Pharm Res 2014;4(2):1792-8.
- Ghosh S, Ahire M, Patil S, Jabgunde A, BhatDusane M, Joshi B, et al. Antidiabetic Activity of Gnidia glauca and Dioscorea bulbifera: Potent Amylase and Glucosidase Inhibitors. Evid Based Complementary Altern Med 2012;2012.
- Narkhede AN, Nirmal PS, Tupe R, Kulkarni OP, Harsulkar AM, Jagtap SD. In vitro antioxidant, antiglycation and alpha amylase inhibitory potential of Eulophia ochreata L. J Pharm Res 2012;5:2532-37.
- Narkhede AN, Jagtap SD. Screening of Amarkand species with respect to their polyphenolic content and free radical quenching potential. Int J Pharm Bio Sci 2015;6:1122-33.
- Jagtap SD, Gilda S, Bhondave P, Paradkar A, Pawar P, Harsulkar A. Validation of the potential of Eulophia ochreata tubers for anti-inflammatory and antioxidant activity. Pharmacol Online 2009;2:307-16.
- Shriram V, Kumar V, Kavi Kishor PB, Suryawanshi SB, Upadhyay AK, Bhat MK. Cytotoxic activity of 9, 10-dihydro-2, 5-dimethoxyphenanthrene-1,7-diol from Eulophia nuda against human cancer cells. J Ethnopharmacol 2010;128:251-53.
- Datla P, Kalluri MD, Basha K, Bellary A, Kshirsagar R, Kanekar Y, et al. 9, 10-Dihydro-2, 5-dimethoxyphenanthrene-1,7-diol, from Eulophia ochreata, inhibits inflammatory signalling mediated by Toll-like receptors. Br J Pharmacol 2010;160:1158-70.
- Gao H, Hou B, Kuroyanagi M, Wu L. Constituents from anti-tumor-promoting active part of Dioscorea bulbifera L. in JB6 mouse epidermal cells. Asian J Tradit Med 2007;2:104-9.
- Mbiantcha M, Kamanyi A, Teponno RB, Tapondjou AL, Watcho P, Nguelefack TB. Analgesic and anti-inflammatory properties of extracts from the bulbils of Dioscorea bulbifera L. var sativa (Dioscoreaceae) in mice and rats. Evid Based Complement Alternat Med 2011;2011:1-9.
- Patel S, Banji D, Banji O, Patel MM, Shah KK. Scrutinizing the role of aqueous extract of Trapa bispinosa as an immunomodulator in experimental animals. Int J Res Pharm Sci 2010;1(1):13-9.
- Gaur K, Kori ML, Nema RK. Comparative screening of immunomodulatory activity of hydro-alcoholic extract of Hibiscus rosa sinensis Linn. and ethanolic extract of Cleome gynandra Linn. Global J Pharmacol 2009;3:85-9.
- Chawla R. Practical Clinical Biochemistry: Methods and Interpretations. 3rd ed. New Delhi: Jaypee brothers Medical Publishers; 2003. p. 136-41.
- Fulzele SV, Satturwar PM, Joshi SB, Dorle AK. Study of the immunomodulatory activity of Haridradi ghrita in rats. Indian J Pharmacol 2003;35:51-4.
- Ismail S, Asad M. Immunomodulatory activity of Acacia catechu. Indian J Physiol Pharmacol 2009;53:25.
- Akekar SA, Ponkshe CA, Indap MM. Evaluation of immunomodulatory activity of extracts from marine animals. Indian J Mar Sci 2009;38:22-7.
- Mukharjee PK, Nema NK, Bhandra S, Mukharjee D, Braga FC. Immunomodulatory leads from medicinal plants. Indian J Tradit Know 2014;13:235-56.
- Manjuladevi K, Reddy GP, Kothai AR, Thenmozhi M, Dhanalakshmi M, Sarumathy S. Evaluation of immunomodulatory activity of aqueous extract of a polyherbal formulation by in vivo method. Asian J Pharm Clin Res 2013;6:129-33.
- Bigoniya P, Rana AC. Subacute Effect of Euphorbia neriifolia Linn. on hematological, biochemical and antioxidant enzyme parameters of rat. Acad J Plant Sci 2009;2:252-9.
- Rajani J, Ashok BK, Patgiri BJ, Prajapati PK, Ravishankar B. Immunomodulatory activity of Amalaki Rasayana: An experimental evaluation. Anc Sci Life 2012;32:93-8.
- Mocsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med 2013;210:1283-99.
- Sharififar F, Pournourmohammadi S, Arabnejad M. Immunomodulatory activity of aqueous extract of Achillea wilhelmsii C. Koch in mice. Indian J Exp Biol 2009;47:668-71.
- Mishra P, Gupta S. Modulatory effect of Eclipta alba on biochemical parameters of catfish, Clarias batrachus. World J Pharm Res 2014;3:1223-33.
- Narkhede AN, Jagtap SD, Kasote DM, Kulkarni OP, Harsulkar AM. Comparative immunomodulation potential of Tinospora cordifolia (Willd.) Miers ex Hook. F., Tinospora sinensis (Lour.) Merrill and Tinospora cordifolia growing on Azadirachta indica A. Juss. Indian J Exp Biol 2014;52:808-13.
- Vinothapooshan G, Sundar K. Immunomodulatory activity of various extracts of Adhatoda vasica Linn. in experimental rats. Afr J Pharm Pharmacol 2011;5:306-10.
- Dhumal JS, Yele SU, Ghodekar SN. Evaluation of immunomodulatory activity of Vigna mungo (L) hepper. J Pharm Phytother 2013;1:9-14.
- Ogbuagu MN. Nutritive and anti-nutritive composition of the wild (inedible) species of Dioscorea bulbifera (potato yam) and Dioscorea dumentorum (bitter yam). Pac J Sci Technol 2008;9:224-26.
- Aberoumand A, Deokule SS. Proximate and mineral composition of wild coco (Eulophia ochreata L.) tubers in Iran. As J Food Ag-Ind 2009;2:203-9.
- Meglia GE, Johannisson A, Petersson L, Waller KP. Changes in some blood micronutrients, leukocytes and neutrophil expression of adhesion molecules in periparturient dairy cows. Acta Vet Scand 2001;42:139-50.