- *Corresponding Author:
- Songqi Tang
School of Pharmacy, Chengdu University of Traditional Chinese Medicine, China
E-mail: xiaoxiao_chen225@126.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “287-302” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Shenqi compound formula has been widely utilized to improve aging-related diabetic nephropathy. Nevertheless, the underlying active ingredients and their molecular mechanisms of action remain doubtful. Thus, we aimed to identify the Shenqi compound formula mechanism involved in diabetic nephropathy treatment using system pharmacology. Candidate constituents and targets of Shenqi compound formula were obtained from traditional Chinese medicine systems pharmacology database. DisGeNET and GeneCards were utilized to build a diabetic nephropathy target database. A interactive network diagram of “drug-active ingredient-target” was fabricated employing Cytoscape. Employing Search Tool for Retrieval of Interacting Genes database, a protein-protein interaction network was established, protein-protein interaction relationships were analyzed, and visual analysis was implemented. Afterwards, gene ontology and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis were implemented to identify the common targets. Finally, AutoDock Vina and PyMoL were utilized to confirm the molecular docking of key targets. Using electron microscopy, the morphological structure of MPC5 cells was observed. Western blotting was implemented to confirm the protein level in MPC5 cells. A total of 165 bioactive Shenqi formula compounds and 566 related target genes were obtained from traditional Chinese medicine systems pharmacology database. Furthermore, 521 diabetic nephropathy-related target genes were obtained from GeneCard and DisGeNet databases. We observed that Shenqi compound formula and diabetic nephropathy share 130 common target genes, providing a molecular basis to treat diabetic nephropathy with Shenqi compound formula. Protein-protein interaction visualization analysis showed that protein kinase 1, albumin, and insulin were key target genes. The key components apigenin, glycine, and quercetin were obtained using degree values. Enrichment analysis showed that Shenqi compound formula interfered with diabetic nephropathy related signaling. Nephrotic syndrome type 2 and synaptopodin levels were markedly inferior to normal in the high glucose state, but increased after Shenqi compound formula treatment, as determined by Western blotting. Shenqi compound formula treats diabetic nephropathy using multi-component, multi-target, and multi-channel mechanisms. It was demonstrated that Shenqi compound formula has a beneficial effect on diabetes.
Keywords
Shenqi compound, diabetic nephropathy, network pharmacology, molecular docking
Diabetic Nephropathy (DN), a Diabetic Complication (DC), is a major cause of death in patients with diabetes. A high incidence of DN was demonstrated in patients with diabetes and renal dysfunction[1]. There are many causes of DN, among which hypertensive nephropathy and acute renal failure are the main causes[2]. DN is associated with a history of diabetes and a pathologically altered structure and function of the kidney due to diabetes[1]. Clinical features of DN include persistently elevated albuminuria, glomerular hypertrophy, and increased thickness of the glomerular basement membrane[3]. It is estimated that by 2030, the number of diabetes patients will reach 366 million, and patients with DN will exceed 100 million[4]. DN severely affects the health of patients, yet the pathogenesis of DN remains unclear. Hence, it is crucial to investigate the pathogenesis and effective treatments of DN. Hence, it is crucial to establish the pathogenesis of DN and to explore effective therapeutics for DN.
Under the pathological conditions of hyperglycemia, insulin resistance, lipid metabolism disorders, etc., DN patients exhibit endocrine hormone disorders, vascular cell dysfunction, and other phenomena[2,5]. Improving the expression of adipose cytokines, insulin resistance, and oxidative stress is essential for slowing down or blocking the DN progression[5]. The current clinical treatment of DN involves the control of glucose, blood pressure, and blood lipid, combined with adoption of anti-inflammatory drugs, and has greatly improved renal function in DN patients. Based on Traditional Chinese Medicine (TCM), DN develops due to yin deficiency, dryness-heat, and qi-yin deficiency associated with diabetes and is a typical DC. The pathological changes in patients of DN mainly involve the lungs, stomach, spleen, and kidneys. As an empirical prescription to treat DN, Shenqi Compound (SQC) formula can effectively improve renal function and reduce the serum uric acid and creatinine levels and urinary Albumin (ALB) excretion rate in DN patients[6].
SQC contains a variety of herbal medicines[7], including ginseng, Astragalus, Salvia, radices Trichosanthes, yam, dogwood, rhubarb, and rehmannia. SQC has several pharmacological effects, and its main active constituents can impede DN progression. Ginseng, a natural plant food, is widely planted in China and enjoys the reputation of being the “king of herbs”. According to TCM, ginseng is one of the best supplements and has shown pharmacological effects such as tonifying the spleen, lungs, qi, and blood. Recent pharmacological studies suggested that ginseng contains abundant Active Components (ACs), including saponins, polysaccharides, and volatile oils[8]. Among these, ginsenosides, which have anti-inflammatory and antioxidant properties, are abundant in ginseng[9]. Ginseng polysaccharides exhibit several pharmacological activities, including immune regulation, anti-oxidation, hypoglycemia, antiinflammation, and anti-tumor effects[10]. Ginseng volatile oil is extracted from plants and may alleviate obesityrelated complications owing to anti-oxidative activity[11]. These main components have been shown to reduce blood lipid levels and improve renal hemorheology[12]. Astragalus polysaccharides are bioACs of Astragalus membranaceus (A. membranaceus) and pose great pharmacological impacts, such as regulating cell levels, and anti-inflammatory activities[13,14]. Recent studies have shown that A. membranaceus can regulate glucose metabolism, protect the glomerular basement membrane, and reduce proteinuria[12]. As a natural medicine in TCM, Salvia miltiorrhiza (S. miltiorrhiza) promotes circulation and removes stasis in blood. Tanshinone IIA (Tan IIA) is the main fat-soluble component of S. miltiorrhiza and plays a crucial role in immune regulation. Tan IIA can reduce the inflammatory mediators by regulating the function of immune cells and restoring abnormal signaling and has shown antitumor activity by inducing cell apoptosis and destroying tumor cells[15]. Radices Trichosanthes have been shown to lower blood sugar levels. The Chinese yam was one of the earliest plants to be eaten by humans. As a starchy food, Chinese yams are very popular among Chinese people. The main bioAC of Chinese yam is a non-starch polysaccharide. Studies have noted that Chinese yam polysaccharides have crucial biological activities, such as hypoglycemic, anti-inflammatory, anti-oxidation, and immune regulation[16]. Chinese yam improves microcirculation and reduces blood lipid and sugar levels through its anti-inflammatory activity in SQC[12]. Cornus officinalis is mainly grown in Shanxi and Gansu Provinces of China. As a TCM, fruit has long been adopted to treat kidney diseases and hypertension. The main ACs of Cornus officinalis were organic acids and iridoid terpenes. Its active ingredients have several pharmacological impacts, including antidiabetes, anti-oxidation, anti-tumor, anti-inflammatory, and immune-regulatory effects[17]. Rhubarb was first recorded in Classic of Shennong Materia Medica and has been utilized as a laxative in China[18]. The chemical composition of rhubarb is diversified. Anthraquinone, anthrone, stilbene, tannin, and polysaccharides are the main chemical components of rhubarb and are associated with anti-cancer, antibacterial, anti-inflammatory, and cardio-cerebrovascular activities[19]. As the main component of SQC, rhubarb can reduce urinary ALB excretion and improve glomerular hypertrophy[12]. Before clinical use, Rehmannia glutinosa must undergo various processing procedures. Its main chemical constituents include iridoid glycosides, phenylethanol glycosides, monosaccharides and oligosaccharides, and they play a synergistic role in anti-inflammatory and anti-thrombotic activities[20].
The main ACs of SQC have many advantages to treat DN. Nevertheless, the composition of SQC is complex, making it challenging to demonstrate its mechanism of action in DN therapy. Hence, network pharmacology was utilized to investigate mechanism and target of SQC in DN therapies, to provide theoretical support for clinical promotion of SQC.
Materials and Methods
Screening of active compounds and targets in SQC:
Active compounds of SQC were obtained from TCM Systems Pharmacology Database and Analysis Platform (TCMSP) (https://tcmspe.com). Absorption, Distribution, Metabolism, and Excretion (ADME) are key factors that determine the biological activity of drugs. Oral Bioavailability (OB) ≥30 % and Drug Similarity (DL) ≥0.18 were utilized for screening[21]. OB is the speed and extent of oral drug absorption from gastrointestinal tract that enters systemic circulation. DL is the similarity of a compound to a known drug[22]. After the ACs were decided, corresponding targets of known ACs were screened from TCMSP database. The official gene symbol and UniProt ID of the target protein were extracted from UniProtKB (https://www.uniprot.org/) database by restricting the conditions to “Homo sapiens”[21] and deleted duplicated data.
Target Genes (TGs) related to DN:
By searching the keyword “DN,” known targets related to insulin resistance were retrieved from GeneCards (http://www.genecards.org/) and DisGeNET (https://www.disgenet.org/). The targets acquired from Genecards and DisgeNET were screened based on their correlation score[12]. After merging the results from the two databases, duplicate values were excluded. The intersection of these targets regarded as the TGs for DN. The intersection of drug AC TGs and disease TGs was screened using Venny 2.1 (https://bioinfogp.cnb.csic.es/tools/venny/ index.html), and those results were considered as TGs to treat DN for subsequent analysis.
Protein-Protein Interaction (PPI) network establishment and topology analysis:
Search Tool for the Retrieval of Interacting Genes (STRING) (https://cn.string-db.org/) was utilized to identify those interacting genes. The database contains interactions between 2031 species, 9.6 million proteins, and 13.8 million proteins. In addition to those available experimental data, it uses bioinformatics methods to predict the results. Core regulatory genes were identified by studying the interaction networks between the proteins. The SQC-DN overlapped targets were imported into STRING and each predicted protein was related to the association score (correlation score was 0-1) [23]. The score reflects the biological significance of the proteins and the confidence of the repeatability between both proteins. PPI network was fabricated by setting Homo sapiens and hiding independent nodes. Using the plug-in in Cytoscape 3.9.1, the network topology analysis was implemented according to the center degree, intermediate number center degree, and close center degree, and the hub TGs of the core of the PPI network were screened.
Enrichment analysis network establishment:
The PPI data obtained were analyzed to demonstrate Biological Process (BP) of SQC in DN therapies. The main targets (betweenness, closeness, and degree superior to the median) were identified as the major targets and imported into databases for annotation, visualization, and integrated discovery. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway Enrichment Analyses (EAs) were implemented[24]. GO contains three ontologies, describing gene Molecular Function (MF), Cellular Component (CC), and BP. As an internationally standardized functional classification system, it comprehensively describes gene attributes. Based on the KEGG database, KEGG enrichment was analyzed to identify SQC TG-related pathways. EA and related genes showed specific gene functions and pathways involved in TG therapy (p<0.05).
Determination of Key Components (KCs) and targets of SQC in DN therapies:
Using Cytoscape 3.9.1, drug component-targetpathway interaction network was fabricated, to identify the possible KCs and corresponding targets of SQC in DN therapy. Regarding the outcomes of the “network analysis,” the components with the first 5° values were deemed as KCs of SQC.
Molecular docking:
Molecular docking has a long history being applied in drug discovery. Molecular docking can be utilized to identify new compounds with therapeutic significance[25]. The ligand-target relationship between SQC and DN was confirmed using molecular docking experiments. Eleven key targets selected from the PPI network were sorted based on their degree value, followed by the selection of the first five key targets for molecular docking experiments. PubChem (https://pubchem.ncbi.nlm.nih.gov/) was utilized to determine the chemical structures of the bioactive components of the drug. Target protein structures were screened from Protein Data Base (PDB) (https://www.rcsb.org/). Finally, molecular docking was implemented using AutoDock Vina 1.2.0 and visualized using Discovery Studio 4.5.
Validation in vitro:
Preparation of Shenqi compound freeze-dried powder: SQC consists of various herbs[7], including Ginseng 15 g (lot no: 220319002, Beijing Qiancao Chinese Medicine; Beverage Co., Ltd.,); Astragalus 15 g (lot no: 220500951, Kangmei Pharmaceutical Co., Ltd.,); radix et Rhizoma 10 g (Batch No: 210802, Xiamen Yanlaifu Pharmaceutical Co., Ltd.,); smallpox powder 10 g (lot no: 220503941, Co. Ltd.,); Danshen 10 g (Batch No: 21123103, Anguo City Party Pharmaceutical Co., Ltd.,); Yam 10 g (Batch No: 20201201, Jiaozuo City Du Shengxing TCM Beverage Co., (Yonggang Beverage Tablets Factory Co.,).
Preparation of drug-containing serum of Sprague-Dawley (SD) rats: Sixty SD rats (male=30 and female=30) were acquired from a vendor. The animals were acquired from Hunan Slaughter Jingda Laboratory Animal Co. The license number for the production of laboratory animals is SCXK (Xiang) 2019-002, and the license number for the use of laboratory animals is SYXK (Chuan) 2020-225. All rats were 8 w old, and ate and drank freely for 5 d under standard conditions (22°-24° temperature, 50 %-60 % humidity and 12 h light-dark cycles). 30 SD rats were administered SQC freeze-dried powder via gavage (SQC group, 1.44 g/kg/d) and 30 SD rats were administered normal saline via gavage (control group, 5 ml/kg/d)[26]. After the final administration, the animals were anesthetized with 0.3 % pentobarbital sodium solution (15 ml/kg)[26], euthanized. The whole blood was collected and serum was separated. Experimental Animal Care and Ethics Committee of the Affiliated Hospital of Chengdu University of TCM approved the experimental protocol, which complied with National Institutes of Health (NIH) fuidelines for the care and use of experimental animals.
Cell culture: MPC5 cells were from Fenghui Biological and cultivated in low-sugar Dulbecco’s modified eagle’s medium (Item No: L170KJ, Shanghai Yuanpei Biotechnology Co., Ltd.,) with 10 % Fetal Bovine Serum (FBS) (Item No: 10099141C, Gibco, United states of America (USA)). MPC5 cells were grown at 33°C with 10 U/ml of Interferon-Gamma (IFN-γ). Next, cells were differentiated at 37° for 14 d without IFN-γ. MPC5 cells were stimulated for 24 h with high glucose (30 mM glucose) containing 1 % FBS and SQC (30 mM glucose+7.5 % ginseng compound-containing serum) for further research.
Electron microscope: Cell centrifuged at 1000 r/ min for 10 min were added with 3 % glutaraldehyde and fixed at 4° for 2 h, then fixed with 1 % osmic acid at 4° for 1 h after being rinsed twice with Phosphate Buffer Solution (PBS). After that, samples were dehydrated in acetone/isoamyl acetate (1:1)
Western blot: To extract cell protein fragments, Radioimmunoprecipitation analysis buffer (Beyotime, Haimen, China) was used. According to the supplier’s instructions, protein concentrations were measured after protein extraction. The sample (10 μg) was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Nephrotic Syndrome Type 2 (NPHS2) (Product Code: ab181143, USA) and synaptopodin (Product Code: ab259976, USA) were incubated overnight after the sealing of using 5 % skim milk for 1 h. Then, incubation lasted for 1 h with a secondary antibody (Item No: ab205718, USA). The band intensity was determined by a Chemiluminescence system (Bio-Rad, Hercules, California, USA). ImageJ (NIH, Bethesda, Maryland, USA) was utilized to quantitatively demonstrate β-actin protein bands.
Statistical analysis:
GraphPad Prism 9.0 was employed. Data were denoted as mean±standard error. Multiple groups were compared adopting a one-way Analysis of Variance (ANOVA), two groups were compared using Tukey’s test, and the Dunnett’s T3 test was utilized to examine the uneven variance. p<0.05 implied statistical significance.
Results and Discussion
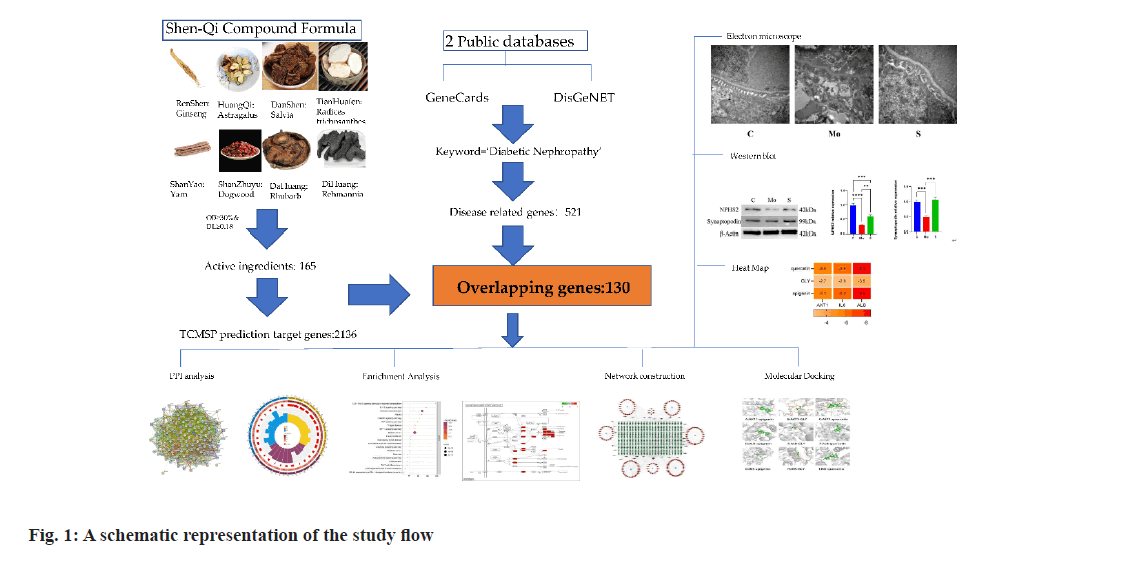
A schematic representation of the study flow is illustrated in fig. 1. In the TCMSP database, 8 Chinese herbs of SQC were screened for 163 active ingredients by fixing the OB and DL values (Table 1). Based on 163 active ingredients, the TCMSP database predicted the target proteins for SQC. Target proteins were transformed into TGs using the UniProt database. After removing repetitive TGs, 566 final TGs were identified.
| Molecule ID | Molecule name | MW | OB (%) | DL |
|---|---|---|---|---|
| MOL002879 | Diop | 390.62 | 43.59 | 0.39 |
| MOL000449 | Stigmasterol | 412.77 | 43.83 | 0.76 |
| MOL000358 | Beta-sitosterol | 414.79 | 36.91 | 0.75 |
| MOL003648 | Inermin | 284.28 | 65.83 | 0.54 |
| MOL000422 | Kaempferol | 286.25 | 41.88 | 0.24 |
| MOL004492 | Chrysanthemaxanthin | 584.96 | 38.72 | 0.58 |
| MOL005308 | Aposiopolamine | 271.34 | 66.65 | 0.22 |
| MOL005314 | Celabenzine | 379.55 | 101.88 | 0.49 |
| MOL005317 | Deoxyharringtonine | 515.66 | 39.27 | 0.81 |
| MOL005318 | Dianthramine | 289.26 | 40.45 | 0.2 |
| MOL005320 | Arachidonate | 304.52 | 45.57 | 0.2 |
| MOL005321 | Frutinone A | 264.24 | 65.9 | 0.34 |
| MOL005344 | Ginsenoside rh2 | 622.98 | 36.32 | 0.56 |
| MOL005348 | Ginsenoside-Rh4_qt | 458.8 | 31.11 | 0.78 |
| MOL005356 | Girinimbin | 263.36 | 61.22 | 0.31 |
| MOL005357 | Gomisin B | 514.62 | 31.99 | 0.83 |
| MOL003648 | Inermin | 284.28 | 65.83 | 0.54 |
| MOL000422 | Kaempferol | 286.25 | 41.88 | 0.24 |
| MOL005360 | Malkangunin | 432.56 | 57.71 | 0.63 |
| MOL005376 | Panaxadiol | 460.82 | 33.09 | 0.79 |
| MOL000449 | Stigmasterol | 412.77 | 43.83 | 0.76 |
| MOL005384 | suchilactone | 368.41 | 57.52 | 0.56 |
| MOL000211 | Mairin | 456.78 | 55.38 | 0.78 |
| MOL000239 | Jaranol | 314.31 | 50.83 | 0.29 |
| MOL000296 | Hederagenin | 414.79 | 36.91 | 0.75 |
| MOL000033 | (3S, 8S, 9S, 10R, 13R, 14S, 17R)-10, 13-dimethyl-17-(2R,5S)-5-propan-2-yloctan-2-yl]-2, 3, 4, 7, 8, 9, 11, 12, 14, 15, 16, 17-dodecahydro-1H-cyclopenta (a) phenanthren-3-ol | 428.82 | 36.23 | 0.78 |
| MOL000354 | Isorhamnetin | 316.28 | 49.6 | 0.31 |
| MOL000371 | 3,9-di-O-methylnissolin | 314.36 | 53.74 | 0.48 |
Table 1: ACs in SQF
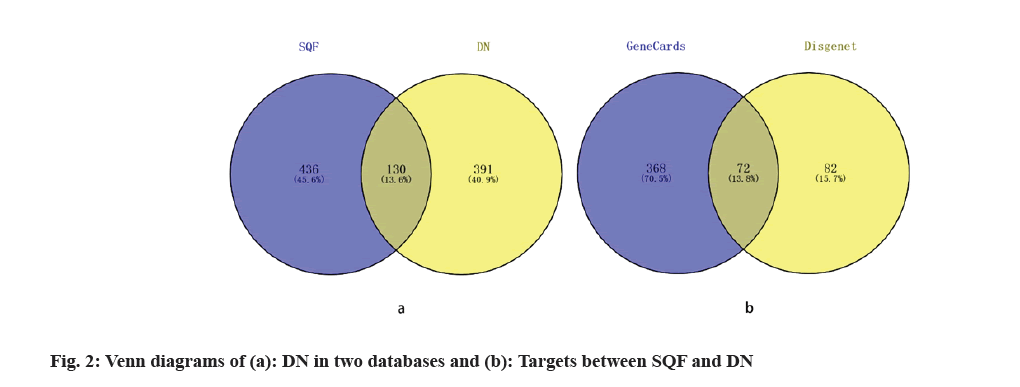
A total of 521 DN-associated genes were obtained after excluding duplicate values (fig. 2a). A total of 130 identical SQC and DN-related TGs were screened using Venny 2.1 (fig. 2b). The crossover genes were referred to as the genes associated with DN for SQC treatment.
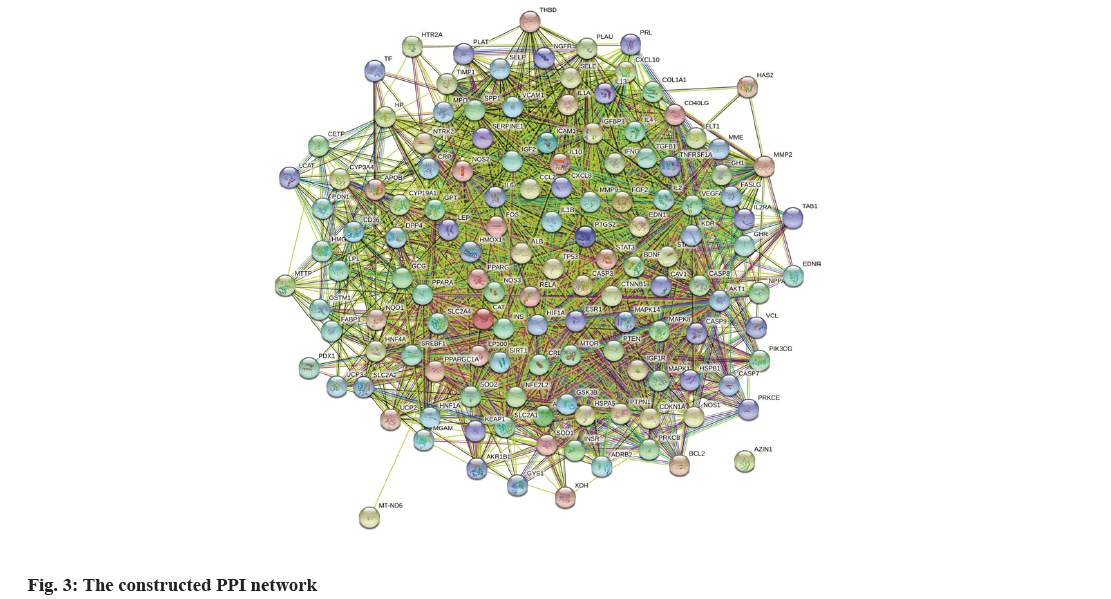
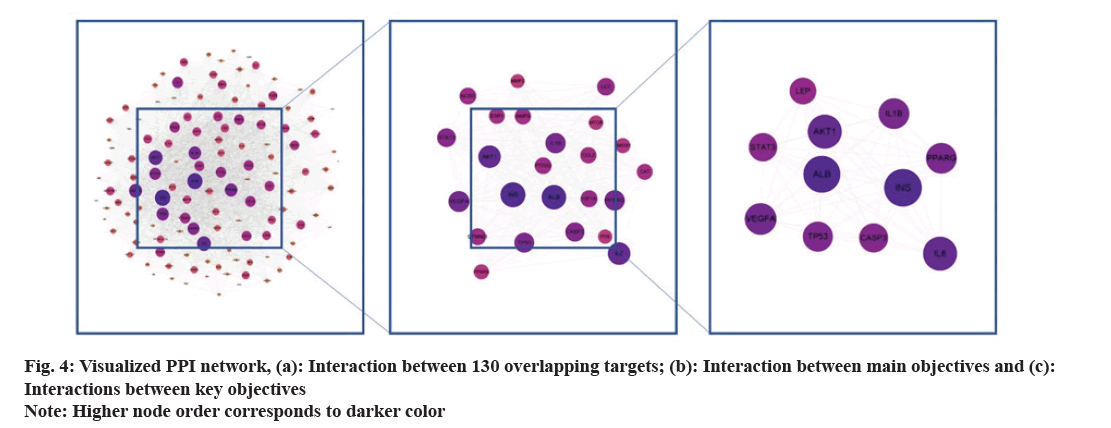
The 130 common goals were imported into STRING. Homo sapiens were set in this database, followed by the confidence threshold set at 0.4. Finally, we obtained network relationship data for the target interaction (fig. 3). Each edge represents a PPI. The presence of more lines, the higher the degree of association and ranking of the targets in the PPI network. PPI network data were then transferred to Cytoscape to generate a network of 129 nodes with 5900 edges. After screening the three key values of organism, confidence, and node association to the median, 11 nodes were identified as primary targets: ALB, insulin, AKT1, Interleukin (IL)-6, Peroxisome Proliferator-Activated Receptor Gamma (PPARG), Vascular Endothelial Growth Factor A (VEGFA), Tumor Protein 53 (TP53), IL-1 alpha (α), Signal Transducer And Activator of Transcription 3 (STAT3), Leptosin (LEP), and Caspase-3 (CASP3) (Table 2 and fig. 4). Finally, 11 nodal targets were considered key targets to treat DN by SQC.
| Gene | UniProt ID | Degree | Gene | UniProt ID | Degree |
|---|---|---|---|---|---|
| ALB | P02768 | 228 | IL1B | P01584 | 190 |
| INS | P01308 | 232 | STAT3 | P40763 | 176 |
| AKT1 | P31749 | 210 | TP53 | P04637 | 186 |
| IL6 | P05231 | 210 | CASP3 | P42574 | 180 |
| PPARG | P37231 | 190 | LEP | P41159 | 166 |
| VEGFA | P15692 | 196 |
Table 2: Key tgs and their Uniprot Ids
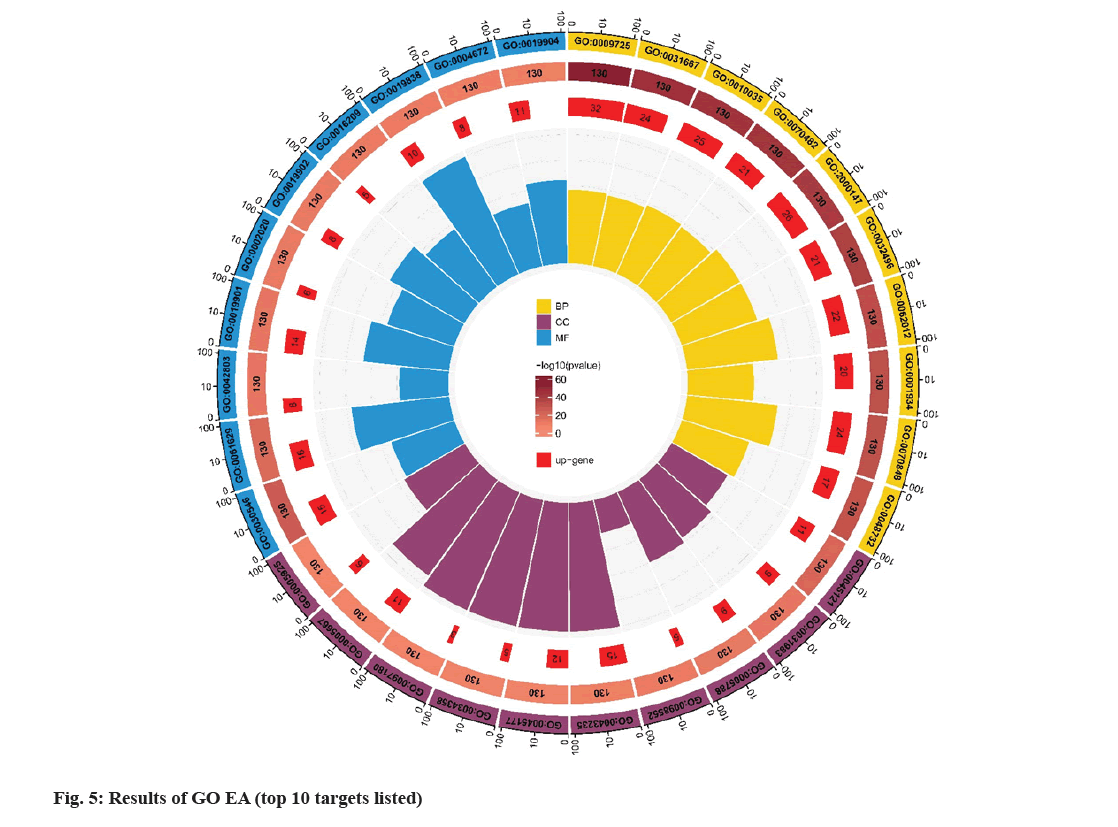
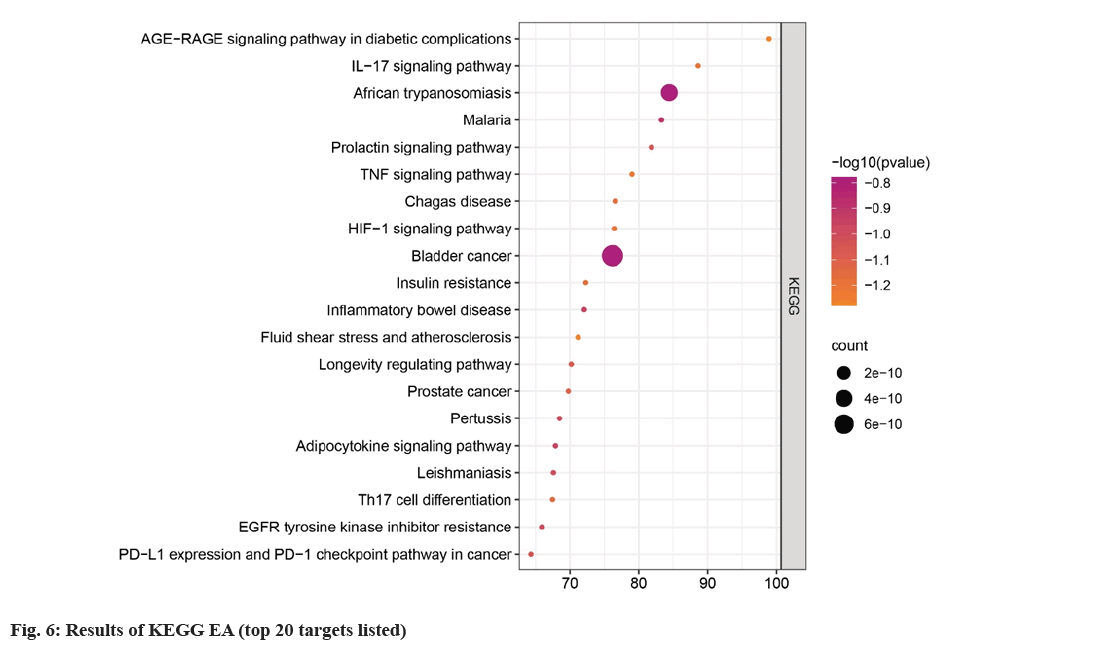
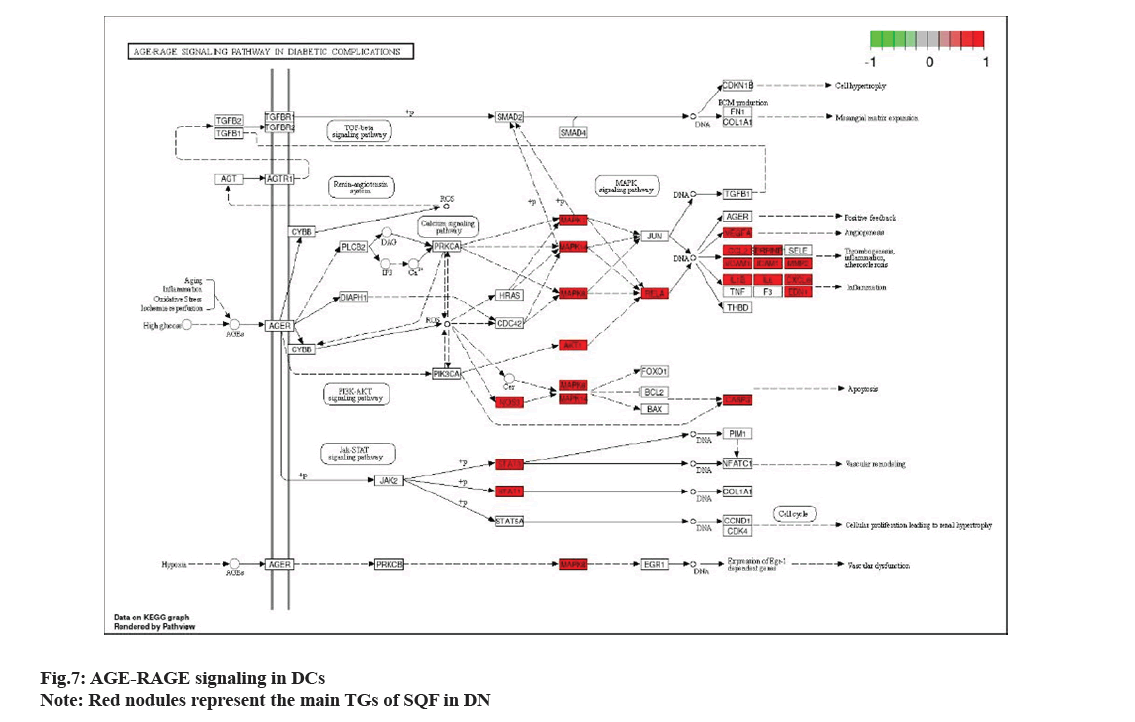
Metascape database (https://metascape.org/gp/ index.html#/main/step1), which can conduct EA of biological pathways, display PPI network structure analysis, and rich gene annotation functions, was utilized to perform GO function and KEGG pathway EA. To confirm the therapeutic mechanism of SQC, 130 main targets were imported into Metascape database, and Homo sapiens was set upto perform GO and KEGG, EA. The screening thresholds were p<0.05 and q<0.05. Through EA, 1478 GO items were obtained, including 1363 BP, 43 CC, and 72 MF items (fig. 5). Studies have shown that in DN, the SQC targets are mainly located in the chromatin and plasma membrane. BP are involved in the negative regulation of smooth muscle adaptation, epithelial cell differentiation in kidney development, and the positive regulation of transcription of the Ribonucleic acid (RNA) polymerase II promoter in the hypoxia response. MF is mainly involved in the activation of Mitogen-Activated Protein (MAP) kinase activity, transcriptional coactivator binding, etc. A total of 167 pathways were identified based on KEGG pathway EA. Based on p value, the first 20 signaling were determined (fig. 6). These include the role of Advanced Glycation Endproducts-Receptor for Advanced Glycation Endproducts (AGE-RAGE) signaling in DCs, IL-17 signaling, African trypanosomiasis, malaria, prolactin signaling, Tumor Necrosis Factor (TNF) signaling, Chagas disease, Hypoxia Inducible Factor-1 (HIF-1) signaling, bladder cancer, insulin resistance, inflammatory bowel disease, fluid shear stress, atherosclerosis, longevity regulating pathway, prostate cancer, pertussis, adipocytokine signaling, leishmaniasis, Th17 cell differentiation, Epidermal Growth Factor Receptor (EGFR) tyrosine kinase inhibitor resistance, Programmed Death-Ligand 1 (PD-L1) level, and PD-1 checkpoint pathway in cancer. Among these, AGE-RAGE signaling was the most abundantly expressed in DCs (fig. 7).
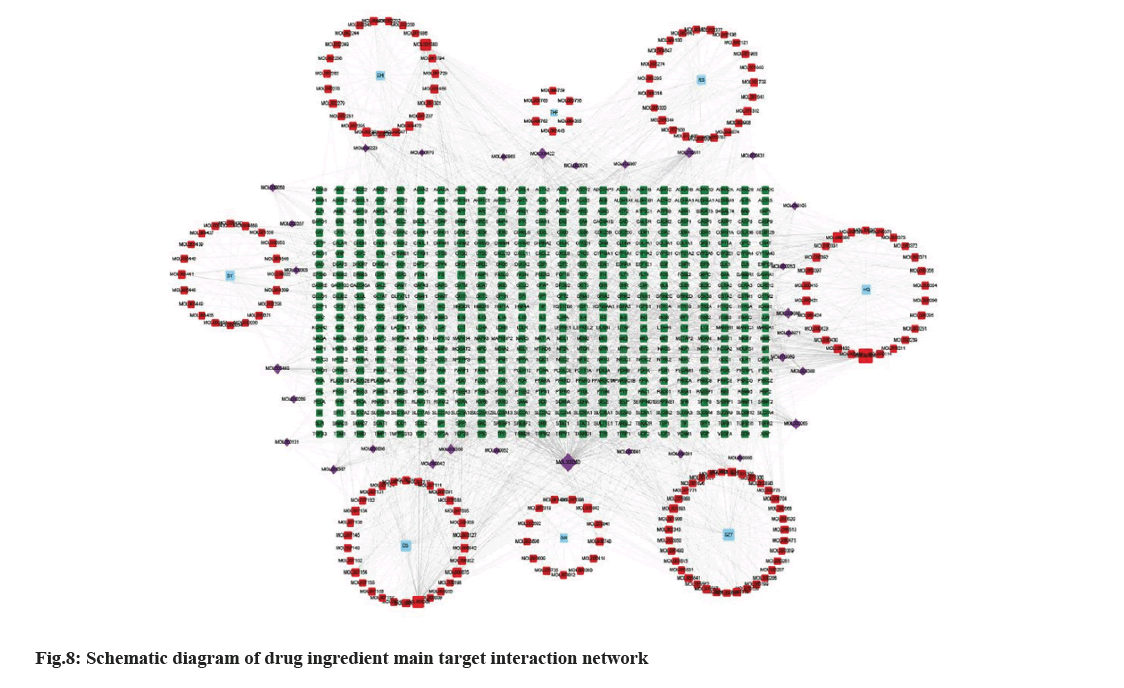
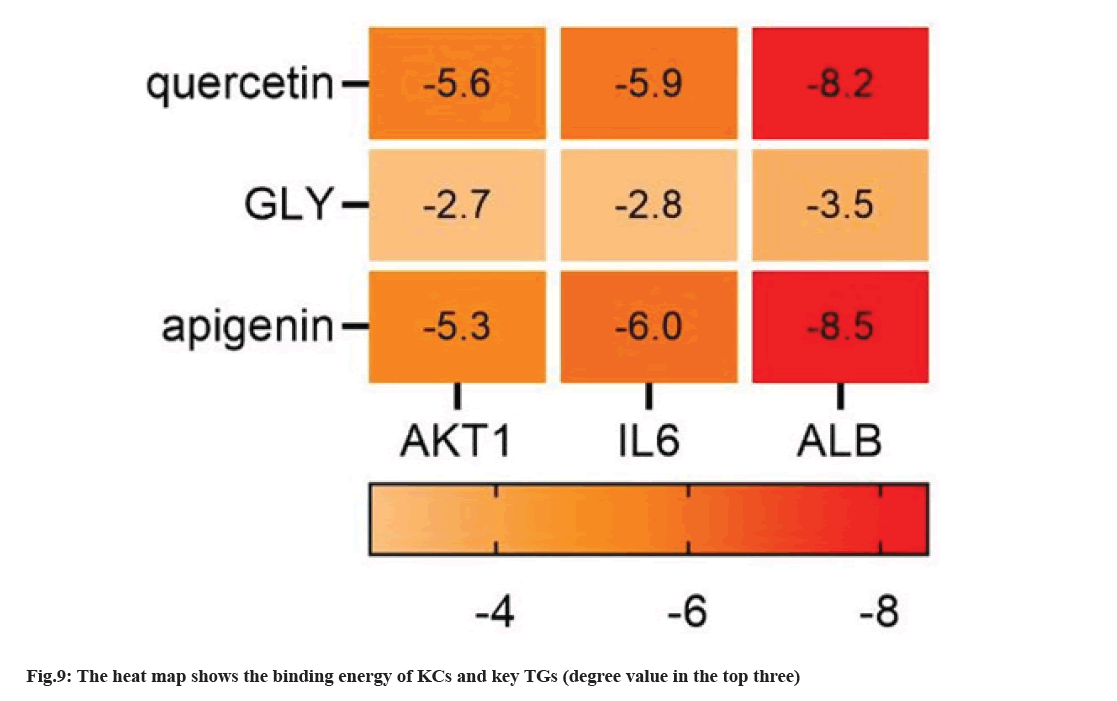
The active compounds of SQC and TGs were imported into Cytoscape 3.9.1 to build network structure. Consequently, an interactive network of 766 nodes and 1928 edges was formed (fig. 8). Different colors were utilized to classify the analysis results; blue for drug name acronyms, red for active compounds, purple for active compounds common to multiple drugs, and green for primary TGs. Relationship between active compounds and TGs is indicated by connecting lines. This shows that SQC has multi components, targets, and pathway characteristics to treat DN. Three compounds were screened based on the degree values; apigenin (MOL00008), GLY (MOL000050), and quercetin (MOL000098). These compounds were considered key compounds.
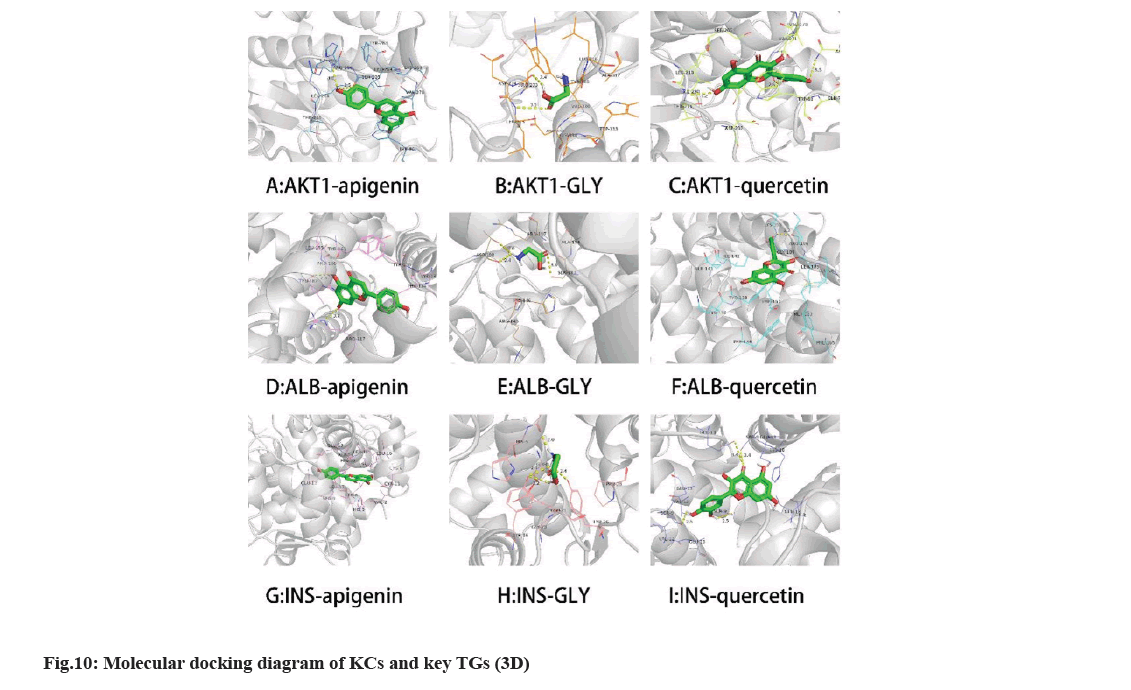
We selected three compounds with the top three degrees as key compounds (apigenin, GLY, and quercetin) through screening. The key compounds were docked with the key TGs (AKT1, ALB, and insulin) using AutoDock Vina. Visualization was performed using PyMOL 2.5.3 at the same time. The absolute value of docking fraction indicates the affinity between the component and the TG, in which an absolute value >4.25, >5.0, and >7.0 implies a certain, favorable, and strong binding activity, respectively[27]. All three components showed strong binding with AKT1, and binding energy of each AC was markedly superior to INS (fig. 9). Notably, the absolute values of binding energies of GLY with the three key TGs were <4.25, suggesting poor binding of GLY with the three key TGs. The specific binding mode between the key compounds and key TGs was optimized using PyMoL 2.3.0. The Three- Dimensional (3D) visualization diagram shows the connection between KCs and the key targets under different forces (fig. 10).
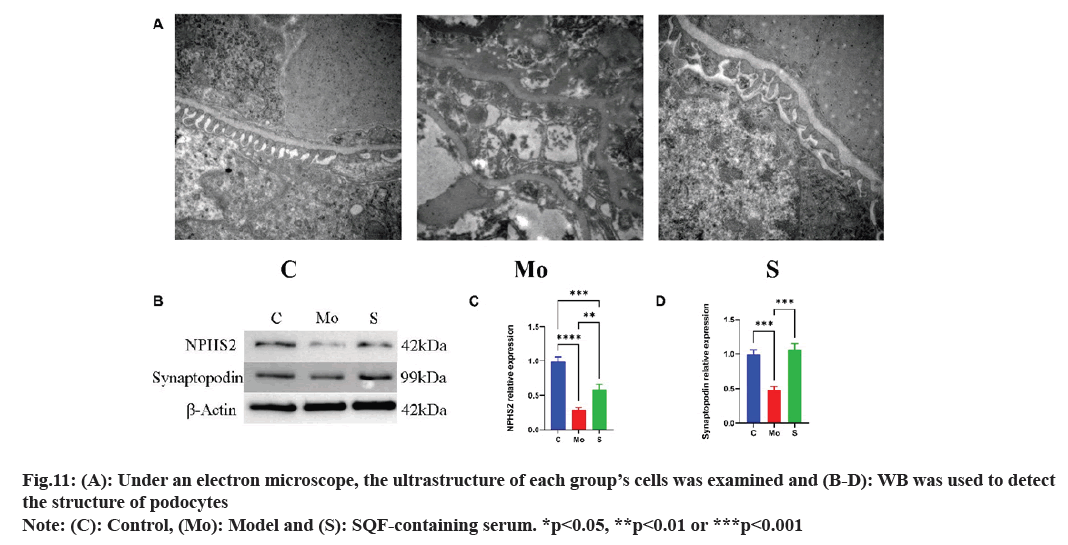
Structure of the capillary endothelium was evident in normal control group, podocyte protrusions surrounded the basement membrane of the capillary endothelium, and the cytoplasm contained numerous auto phagosomes. Relative to normal control group, the endothelial cells in model group were more disorganized, and the epithelial cells contained more vacuoles. The number of autophagy bodies in the SQC drug-containing serum group increased vs. control group, and the microvilli were arranged more systematically, indicating that SQC protected podocytes from high glucose-induced damage (fig. 11A). Western blotting demonstrated that vs. the model, NPHS2 and synaptopodin levels in the podocyte structure of the SQC drug-containing serum group was markedly increased (fig. 11B-fig. 11D).
Hence, SQC has a protective effect on podocyte structure.
A diabetic environment can lead to the abnormal expression of advanced glycosylation products and increased blood pressure and blood lipids, which leads to the development of DN[28]. The average kidney weight of patients with DN increases by lb compared to that of normal kidneys. Studies have shown that the renal function of patients with DN gradually declines[29], but the kidney volume increases. At present, blood pressure, glucose and lipid levels in the blood of DN patients are strictly controlled in the clinic using different interventions[30]. Nevertheless, the reported interventions fail to prevent DN progression in most patients. Hence, it is crucial to identify novel mechanisms and drugs for DN treatment.
Recently, extensive research has focused on identifying mechanism of TCM to treat DN has been increased. TCM believes that the causes of DN are mostly related to phlegm dampness, blood stasis, and deficiency syndromes. After years of clinical studies, SQC has become an empirical prescription for the clinical therapy of DN[31]. Several preclinical studies noted that SQC can enhance renal function and pathological damage, and reduce the thickness of glomerular capillary basement membrane, blood glucose, and blood lipid levels in animal models of DN. As observed, ginseng extract effectively controlled blood glucose levels in DN induced disease animal model[32]. A. membranaceus reduced renal injury by regulating apoptosis in the streptozotocin-induced DN rat model[33]. Furthermore, S. miltiorrhiza extract was shown to improve renal function and reduce proteinuria by mediating antioxidant stress factors in a streptozotocin-induced DN mouse model[34]. Nevertheless, the specific mechanism of action responsible for SQC efficacy in DN treatment is poorly understood. Hence, the possible mechanism of SQC to treat DN was elucidated.
In database, 165 ACs were screened from SQC and 566 related targets were identified. The captured targets were coincident with DN-related targets, and 130 overlapping targets were obtained, implying the potential molecular basis of SQC in DN therapy. By building a network for the interaction of drugs, components, and main targets, it was observed that SQC acts via multiple targets and pathways in the prevention and therapy of DN.
We analyzed the PPI network data, visually analyzed 129 nodes, and identified 11 key targets (ALB, INS, AKT1, IL6, PPARG, VEGFA, TP53, IL1B, STAT3, LEP, and CASP3). The first three key targets were determined regarding degree values for the analysis (AKT1, ALB, and INS). Serum ALB can regulate the osmotic pressure of blood colloids[35]. Proteinuria is one of the main clinical symptoms of DN, and its level is positively correlated with renal injury degree[36], indicating that ALB is related to DN progression. Insulin (INS) reduces the concentration of blood glucose, increases the permeability of cells to monosaccharides, amino acids, and fatty acids, and accelerates glycolysis, pentose phosphate circulation, and glycogen synthesis in the liver.
Studies have shown that INS can protect the kidneys by lowering blood glucose levels and preventing disease progression and renal dysfunction in DN induced rats[37]. INS has the potential to improve renal function in DN patients. AKT1 regulates many diseases[38]. Several studies noted that AKT1 regulates glucose uptake by mediating insulin production[39], regulates cell survival through apoptosis signalrelated kinases[40], and plays an crucial role in gene transcription. The mechanism of AKT1 might be associated with regulation of apoptosis and glucose levels. As reported, SQC lowers blood glucose and lipid levels, improves glucose metabolism, promotes insulin secretion, and improves insulin resistance and hemodynamics[2]. The aforementioned studies on these KCs of SQC provide strong preclinical evidence to treat DN.
Regarding KEGG pathway enrichment results, main targets can interfere with DN-related signaling, such as the AGE-RAGE signaling in DCs, IL-17 signaling, malaria, prolactin signaling, and TNF signaling. The expression of AGE-RAGE signaling in DCs is in abundance. AGEs play a crucial role in DN progression. Additionally, binding of the RAGE and its ligand causes oxidative stress and chronic inflammation in renal tissue, which eventually leads to loss of renal function. Hence, blocking the AGERAGE signaling in DCs may aid DN treatment[41]. One key target of AKT1 affects AGE-RAGE signaling expression in DCs, indicating that it may antagonize DN progression through this pathway. Through the KEGG map, we observed that IL-6 might also treat DN through this signaling. Simultaneously, IL-6 appears to be the main target, as observed during visual analysis. Clinical trials have shown that SQC regulates the expression of IL-6, reduces blood glucose levels, and protects insulin cells in DN patients[42].
Notably, two key targets of ALB and INS do not appear in AGE-RAGE signaling in DCs, suggesting that key targets may not play a role through the AGE-RAGE pathway. ALB is the most crucial protein in human plasma and has the functions of free fatty acids, anti-inflammation, anti-oxidation, and apoptosis regulation. The function of ALB is to maintain colloidal osmotic pressure and regulate the permeability of glomerular capillaries, protect microvessels through anti-inflammatory and anti-oxidative effects, and affect structure and function of podocytes by apoptosis regulation[43,44]. Hyperalbuminuria and decreased glomerular filtration rate are the key diagnostic criteria for DN. The rising urinary ALB excretion rate can reduce glomerular filtration rate and atrophy of the glomeruli and renal tubules[45-47]. Insulin is a protein hormone secreted by β-cells that regulates metabolism[48]. Insulin can promote the synthesis of glycogen, fatty acids, and proteins as the only hormone to reduce blood sugar in the body[37]. Insulin is considered as the greatest scientific and technological advancement in the 20th century because it changes the process of diabetes[49]. DN is one of the main DCs, suggesting that insulin acts in DN progression. Hence, even though the two key targets of ALB and INS do not appear in the highly expressed signaling, we cannot deny the influence of these two key targets on DN. The key target comes from the screening of the median of betweenness centrality, closeness centrality, and degree, while AGE-RAGE signaling in DCs is the most abundant one, which was identified through EA. Their screening indicators are single, and promoting the diversification of screening methods is a direction of attention in the future.
The three KCs of apigenin, GLY, and quercetin have strong binding energy with three key TGs, suggesting that these three components are key compounds to treat DN. The three KCs showed strong potential affinities for ALB but weak potential affinities for INS. Ursolic acid and ALB showed the strongest binding energy (11.5 kcal/mol), which suggested the reliability of the docking result.
As a natural flavonoid, apigenin exists widely in herbs, vegetables, and fruits in nature[50]. Based on public reports, apigenin induces apoptosis[51] and has anti-inflammatory[52], anti-oxidative[50], immune regulatory[53], and anti-cancer activities[54]. Preclinical and clinical studies indicated that apigenin delays DN progression by inhibiting glomerular mesangial cell proliferation[55,56]. As a KC of SQC, apigenin may be a KC to treat DN. GLY is an amino acid mainly found in liver and kidney that exhibits anti-inflammatory and antioxidant effects but also participates in the regulation of apoptosis[57,58]. Appropriate GLY supplementation can regulate metabolic disorders in patients with obesity, cancer, or diabetes. Clinical studies have shown that renal injury can lead to the accumulation of GLY[59]. This phenomenon is not only observed in patients with DN[60]. Quercetin is a polypeptide found in plants that exhibits anti-cancer, anti-inflammatory, anti-viral, and increased capillary permeability[61]. Appropriate supplementation with quercetin can improve immunity in the body[62]. A study showed that quercetin reduced blood glucose levels, 24 urine protein contents, and the glomerular mesangial matrix expansion index in streptozotocin induced DN rats[63]. Quercetin was found to protect the kidneys in the DN mouse model[64]. These preclinical studies lay a reliable theoretical basis to treat DN using quercetin. Apigenin, GLY, and quercetin, as the KCs of SQC, have shown pharmacological activities in treating DN in different studies, which provided more possibilities to explain mechanism of SQC in treating DN.
In summary, we predicted the possible mechanism of SQC in DN therapies by cyber-pharmacology and confirmed it with molecular docking and experimental verification. Through the construction of the network, it was observed that one AC binds to multiple targets and that one target can act on multiple pathways, which are the characteristics of SQC in treatment of DN. It is worth noting that some of the KCs screened by PPI visual analysis did not play a role in expressing the richest signal pathways, suggesting that EA is not the only criterion for evaluating DN signaling in SQC therapy. Next, molecular docking was utilized to verify the direct interaction between KCs and TGs to reveal the potential mechanism of SQC in treating DN. Under an electron microscope, we discovered that SQC enhanced the sorting of microvilli in cells and promoted the release of autophagy bodies. WB confirmed that SQC protected podocytes from damage caused by high glucose levels. The mechanism by which SQC protects podocytes may involve apigenin, GLY, and quercetin. This will be the focus of our future research.
We are the first to systematically explore the molecular mechanisms of SQC in DN treatment. SQC is an effective treatment alternative for DN by acting on multiple components, targets, and pathways. Apigenin, GLY, quercetin, and other KCs may target AKT1, ALB, INS, and other key TGs during DN treatment. In addition to AGE-RAGE signaling, several other signaling may play a crucial role in DCs. SQC has shown efficacy against DN through its anti-inflammatory effects, anti-oxidation of free fatty acids, and promotion of glycogen synthesis. Nevertheless, the exact mechanism of SQC in DN therapy is unclear and requires preclinical and clinical studies. Our study provides a basis for elucidating the mechanism of action of SQC in DN treatment and paves the way for future research.
Author contributions:
The conceptualization, methodology, and writingoriginal draft by Xiaoxiao Chen; methodology, software, and visualization by Shiyun Tang; software and validation by Li Song; investigation was done by Dehui Yin and Ye Zhu; writing-review and editing by Yingqi Chen and writing-review, editing and project administration by Songqi Tang. All authors have read and agreed to the published version of the manuscript.
Ethical approval:
Ethical approval to report this case series was obtained from Chengdu University of TCM Application for Laboratory Animal Welfare and Ethical Review Board (Approval Id: 2021-0027).
Funding:
This research was funded by the National Natural Science Foundation of China (grant number: 82004351).
Conflict of interests:
The authors declared no conflict of interests.
References
- Umanath K, Lewis JB. Update on diabetic nephropathy: Core curriculum 2018. Am J Kidney Dis 2018;71(6):884-95.
[Crossref] [Google Scholar] [PubMed]
- Zhong M, Song X, Zhang X, Chen J, Wang L, Xia J, et al. Treatment of microcirculation dysfunction in type 2 diabetic mellitus with shenqi compound prescription: A protocol of systematic review and meta-analysis of randomized clinical trials. Medicine 2020;99(41):e22347.
[Crossref] [Google Scholar] [PubMed]
- Flyvbjerg A. The role of the complement system in diabetic nephropathy. Nat Rev Nephrol 2017;13(5):311-8.
[Crossref] [Google Scholar] [PubMed]
- Tu Q, Li Y, Jin J, Jiang X, Ren Y, He Q. Curcumin alleviates diabetic nephropathy via inhibiting podocyte mesenchymal transdifferentiation and inducing autophagy in rats and MPC5 cells. Pharm Biol 2019;57(1):778-86.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Yao H, Li C, Sun W, Chen X, Cao Y, et al. Gandi capsule improved podocyte lipid metabolism of diabetic nephropathy mice through SIRT1/AMPK/HNF4A pathway. Oxid Med Cell Longev 2022;2022:6275505.
[Crossref] [Google Scholar] [PubMed]
- Yang Y, Fu X, Zhong W, Hu Z, Tian Y, Zhou H, et al. Effect of Shenqi compound on inflammatory markers and glycemic measures among diabetes mellitus: A protocol for systematic review and meta-analysis. Medicine 2020;99(27):e20736.
[Crossref] [Google Scholar] [PubMed]
- Hu Z, Yang M, Xie C, Gao H, Fu X, Xie H, et al. Efficacy and safety of Shenqi compound for the treatment of diabetic macroangiopathy: A protocol for systematic review and meta-analysis. Medicine 2020;99(15):e19682.
[Crossref] [Google Scholar] [PubMed]
- Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng CA Meyer. Acta Pharmacol Sin 2008;29(9):1109-18.
[Crossref] [Google Scholar] [PubMed]
- Liu H, Lu X, Hu Y, Fan X. Chemical constituents of Panax ginseng and Panax notoginseng explain why they differ in therapeutic efficacy. Pharmacol Res 2020;161:105263.
[Crossref] [Google Scholar] [PubMed]
- Guo M, Shao S, Wang D, Zhao D, Wang M. Recent progress in polysaccharides from Panax ginseng CA Meyer. Food Function 2021;12(2):494-518.
- Kim HJ, Kang HJ, Seo JY, Lee CH, Kim YS, Kim JS. Antiobesity effect of oil extract of ginseng. J Med Food 2011;14(6):573-83.
[Crossref] [Google Scholar] [PubMed]
- Hu Z, Yang M, Yang L, Xie C, Gao H, Fu X, et al. Network pharmacology-based identification of the mechanisms of Shenqi compound formula in treating diabetes mellitus. Evid Based Complement Alternat Med 2020;2020:5798764.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Yu P, Fu W, Cai L, Yu Y, Feng Z, et al. Ginseng-Astragalus-oxymatrine injection ameliorates cyclophosphamide-induced immunosuppression in mice and enhances the immune activity of RAW264. 7 cells. J Ethnopharmacol 2021;279:114387.
[Crossref] [Google Scholar] [PubMed]
- Liu F, Sun L, You G, Liu H, Ren X, Wang M. Effects of Astragalus polysaccharide on the solubility and stability of 15 flavonoids. Int J Biol Macromol 2020;143:873-80.
[Crossref] [Google Scholar] [PubMed]
- Guo R, Li L, Su J, Li S, Duncan SE, Liu Z, et al. Pharmacological activity and mechanism of tanshinone IIA in related diseases. Drug Des Devel Ther 2020;14:4735-48.
[Crossref] [Google Scholar] [PubMed]
- Huang R, Xie J, Yu Y, Shen M. Recent progress in the research of yam mucilage polysaccharides: Isolation, structure and bioactivities. Int J Biol Macromol 2020;155:1262-9.
[Crossref] [Google Scholar] [PubMed]
- Huang J, Zhang Y, Dong L, Gao Q, Yin L, Quan H, et al. Ethnopharmacology, phytochemistry, and pharmacology of Cornus officinalis Sieb. et Zucc. J Ethnopharmacol 2018;213:280-301.
[Crossref] [Google Scholar] [PubMed]
- Barceloux DG. Rhubarb and oxalosis (Rheum species). Dis Month 2009;6(55):403-11.
[Crossref] [Google Scholar] [PubMed]
- Cao YJ, Pu ZJ, Tang YP, Shen J, Chen YY, Kang A, et al. Advances in bio-active constituents, pharmacology and clinical applications of rhubarb. Chin Med 2017;12:36.
[Crossref] [Google Scholar] [PubMed]
- Gong PY, Guo YJ, Tian YS, Gu LF, Qi J, Yu BY. Reverse tracing anti-thrombotic active ingredients from dried Rehmannia Radix based on multidimensional spectrum-effect relationship analysis of steaming and drying for nine cycles. J Ethnopharmacol 2021;276:114177.
[Crossref] [Google Scholar] [PubMed]
- Shi S, Sun M, Liu Y, Jiang J, Li F. Insight into Shenqi Jiangtang Granule on the improved insulin sensitivity by integrating in silico and in vivo approaches. J Ethnopharmacol 2022;282:114672.
[Crossref] [Google Scholar] [PubMed]
- Jian GH, Su BZ, Zhou WJ, Xiong H. Application of network pharmacology and molecular docking to elucidate the potential mechanism of Eucommia ulmoides-Radix Achyranthis bidentatae against osteoarthritis. BioData Min 2020;13:12.
[Crossref] [Google Scholar] [PubMed]
- Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucl Acids Res 2016:gkw937.
[Crossref] [Google Scholar] [PubMed]
- Dong Y, Hao L, Fang K, Han XX, Yu H, Zhang JJ, et al. A network pharmacology perspective for deciphering potential mechanisms of action of Solanum nigrum L. in bladder cancer. BMC Complement Med Ther 2021;2(1):45.
[Crossref] [Google Scholar] [PubMed]
- Pinzi L, Rastelli G. Molecular docking: Shifting paradigms in drug discovery. Int J Mol Sci 2019;20(18):4331.
[Crossref] [Google Scholar] [PubMed]
- Ya L, Xiyu Z, Heting W, Jundong W, Chunguang X, Liu Y et al. Effect of Shenqi compound on pancreatic islets of GK rats with type 2 diabetes mellitus β effect of cell function. Chin J Exp Prescriptions 2020;26(22):34-9.
- Hsin KY, Ghosh S, Kitano H. Combining machine learning systems and multiple docking simulation packages to improve docking prediction reliability for network pharmacology. PloS One 2013;8(12):e83922.
- Rayego-Mateos S, Morgado-Pascual JL, Opazo-Ríos L, Guerrero-Hue M, Garcia-Caballero C, Vázquez-Carballo C, et al. Pathogenic pathways and therapeutic approaches targeting inflammation in diabetic nephropathy. Int J Mol Sci 2020;21(11):3798.
[Crossref] [Google Scholar] [PubMed]
- Tsai YC, Kuo MC, Hung WW, Wu LY, Wu PH, Chang WA, et al. High glucose induces mesangial cell apoptosis through miR-15b-5p and promotes diabetic nephropathy by extracellular vesicle delivery. Mol Ther 2020;28(3):963-74.
[Crossref] [Google Scholar] [PubMed]
- Wang G, Ouyang J, Li S, Wang H, Lian B, Liu Z, et al. The analysis of risk factors for diabetic nephropathy progression and the construction of a prognostic database for chronic kidney diseases. J Transl Med 2019;17:264.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Kang J, Gao H, Zhang X, Chao J, Gong G, et al. Exploration of the effect and mechanism of Shenqi compound in a spontaneous diabetic rat model. Endocr Metab Immune Disord Drug Targets 2019;19(5):622-31.
[Crossref] [Google Scholar] [PubMed]
- Su WY, Li Y, Chen X, Li X, Wei H, Liu Z, et al. Ginsenoside Rh1 improves type 2 diabetic nephropathy through AMPK/PI3K/Akt-mediated inflammation and apoptosis signaling pathway. Am J Chin Med 2021;49(5):1215-33.
[Crossref] [Google Scholar] [PubMed]
- Zhai R, Jian G, Chen T, Xie L, Xue R, Gao C, et al. Astragalus membranaceus and Panax notoginseng, the novel renoprotective compound, synergistically protect against podocyte injury in streptozotocin-induced diabetic rats. J Diabetes Res 2019;2019:1602892.
[Crossref] [Google Scholar] [PubMed]
- An L, Zhou M, Marikar FM, Hu XW, Miao QY, Li P, et al. Salvia miltiorrhiza lipophilic fraction attenuates oxidative stress in diabetic nephropathy through activation of nuclear factor erythroid 2-related factor 2. Am J Chin Med 2017;45(7):1441-57.
[Crossref] [Google Scholar] [PubMed]
- Zeng M, Liu J, Yang W, Zhang S, Liu F, Dong Z, et al. Multiple-microarray analysis for identification of hub genes involved in tubulointerstial injury in diabetic nephropathy. J Cell Physiol 2019;234(9):16447-62.
[Crossref] [Google Scholar] [PubMed]
- Hashemi E, Dehghanbanadaki H, Baharanchi AA, Forouzanfar K, Kakaei A, Mohammadi SM, et al. WT1 and ACE mRNAs of blood extracellular vesicle as biomarkers of diabetic nephropathy. J Transl Med 2021;19:1-9.
[Crossref] [Google Scholar] [PubMed]
- Tong MQ, Luo LZ, Xue PP, Han YH, Wang LF, Zhuge DL, et al. Glucose-responsive hydrogel enhances the preventive effect of insulin and liraglutide on diabetic nephropathy of rats. Acta Biomater 2021;122:111-32.
[Crossref] [Google Scholar] [PubMed]
- Rönnstrand L. Signal transduction via the stem cell factor receptor/c-kit. Cell Mol Life Sci 2004;61(19-20):2535-48.
[Crossref] [Google Scholar] [PubMed]
- Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, et al. A method to identify serine kinase substrates: Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem 2002;277(25):22115-8.
[Crossref] [Google Scholar] [PubMed]
- Kim AH, Khursigara GU, Sun X, Franke TF, Chao MV. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol 2001;21(3):893-901.
[Crossref] [Google Scholar] [PubMed]
- Sanajou D, Haghjo AG, Argani H, Aslani S. AGE-RAGE axis blockade in diabetic nephropathy: Current status and future directions. Eur J Pharmacol 2018;833:158-64.
[Crossref] [Google Scholar] [PubMed]
- Zhu Q, Kang J, Xu G, Li J, Zhou H, Liu Y. Traditional Chinese medicine Shenqi compound to improve lower extremity atherosclerosis of patients with type 2 diabetes by affecting blood glucose fluctuation: Study protocol for a randomized controlled multicenter trial. Medicine 2020;99(11):e19501.
[Crossref] [Google Scholar] [PubMed]
- Vincent JL. Relevance of albumin in modern critical care medicine. Best Pract Res Clin Anaesthesiol 2009;23(2):183-91.
[Crossref] [Google Scholar] [PubMed]
- Vincent JL, Russell JA, Jacob M, Martin G, Guidet B, Wernerman J, et al. Albumin administration in the acutely ill: What is new and where next? Crit Care 2014;18(4):1-10.
[Crossref] [Google Scholar] [PubMed]
- Van JA, Scholey JW, Konvalinka A. Insights into diabetic kidney disease using urinary proteomics and bioinformatics. J Am Soc Nephrol 2017;28(4):1050-61.
[Crossref] [Google Scholar] [PubMed]
- Oshima M, Shimizu M, Yamanouchi M, Toyama T, Hara A, Furuichi K, et al. Trajectories of kidney function in diabetes: A clinicopathological update. Nat Rev Nephrol 2021;17(11):740-50.
[Crossref] [Google Scholar] [PubMed]
- Radcliffe NJ, Seah JM, Clarke M, MacIsaac RJ, Jerums G, Ekinci EI. Clinical predictive factors in diabetic kidney disease progression. J Diabetes Invest 2017;8(1):6-18.
[Crossref] [Google Scholar] [PubMed]
- Du P, Fan B, Han H, Zhen J, Shang J, Wang X, et al. NOD2 promotes renal injury by exacerbating inflammation and podocyte insulin resistance in diabetic nephropathy. Kidney Int 2013;84(2):265-76.
- Lewis GF, Brubaker PL. The discovery of insulin revisited: Lessons for the modern era. J Clin Invest 2021;131(1).
[Crossref] [Google Scholar] [PubMed]
- Lefort EC, Blay J. Apigenin and its impact on gastrointestinal cancers. Mol Nutr Food Res 2013;57(1):126-44.
[Crossref] [Google Scholar] [PubMed]
- Zhao G, Han X, Cheng W, Ni J, Zhang Y, Lin J, et al. Apigenin inhibits proliferation and invasion, and induces apoptosis and cell cycle arrest in human melanoma cells. Oncol Rep 2017;37(4):2277-85.
[Crossref] [Google Scholar] [PubMed]
- Ginwala R, McTish E, Raman C, Singh N, Nagarkatti M, Nagarkatti P, et al. Apigenin, a natural flavonoid, attenuates EAE severity through the modulation of dendritic cell and other immune cell functions. J Neuroimmune Pharmacol 2016;11(1):36-47.
[Crossref] [Google Scholar] [PubMed]
- Xu L, Zhang Y, Tian K, Chen X, Zhang R, Mu X, et al. Apigenin suppresses PD-L1 expression in melanoma and host dendritic cells to elicit synergistic therapeutic effects. J Exp Clin Cancer Res 2018;37(1):1-5.
[Crossref] [Google Scholar] [PubMed]
- Madunic J, Madunic IV, Gajski G, Popic J, Garaj-Vrhovac V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett 2018;413:11-22.
[Crossref] [Google Scholar] [PubMed]
- Kim CS, Kim J, Kim YS, Jo K, Lee YM, Jung DH, et al. Improvement in diabetic retinopathy through protection against retinal apoptosis in spontaneously diabetic torii rats mediated by ethanol extract of Osteomeles schwerinae CK Schneid. Nutrients 2019;11(3):546.
[Crossref] [Google Scholar] [PubMed]
- Li P, Bukhari SN, Khan T, Chitti R, Bevoor DB, Hiremath AR, et al. Apigenin-loaded solid lipid nanoparticle attenuates diabetic nephropathy induced by streptozotocin nicotinamide through Nrf2/HO-1/NF-kB signalling pathway. Int J Nanomed 2020;15:9115.
[Crossref] [Google Scholar] [PubMed]
- Imenshahidi M, Hossenzadeh H. Effects of glycine on metabolic syndrome components: A review. J Endocrinol Invest 2022;45(5):927-39.
[Crossref] [Google Scholar] [PubMed]
- Weinberg JM, Bienholz A, Venkatachalam MA. The role of glycine in regulated cell death. Cell Mol Life Sci 2016;73:2285-308.
[Crossref] [Google Scholar] [PubMed]
- Razak MA, Begum PS, Viswanath B, Rajagopal S. Multifarious beneficial effect of nonessential amino acid, glycine: A review. Oxid Med Cell Longev 2017;2017.
[Crossref] [Google Scholar] [PubMed]
- Barrios C, Zierer J, Wurtz P, Haller T, Metspalu A, Gieger C, et al. Circulating metabolic biomarkers of renal function in diabetic and non-diabetic populations. Sci Rep 2018;8(1):15249.
- Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, et al. Quercetin, inflammation and immunity. Nutrients 2016;8(3):167.
[Crossref] [Google Scholar] [PubMed]
- Davis JM, Murphy EA, Carmichael MD. Effects of the dietary flavonoid quercetin upon performance and health. Curr Sports Med Rep 2009;8(4):206-13.
[Crossref] [Google Scholar] [PubMed]
- Tang L, Li K, Zhang Y, Li H, Li A, Xu Y, et al. Quercetin liposomes ameliorate streptozotocin-induced diabetic nephropathy in diabetic rats. Sci Rep 2020;10(1):2440.
- Gomes IB, Porto ML, Campagnaro BP, Gava AL, Meyrelles SS, Pereira TM, et al. The protective effects of oral low-dose quercetin on diabetic nephropathy in hypercholesterolemic mice. Front Physiol 2015;6:158936.
[Crossref] [Google Scholar] [PubMed]