- *Corresponding Author:
- Y. L. liu
Institute of materia medica, Chinese academy of medical sciences and peking union medical college, Beijing city key Laboratory of drug delivery technology and novel formulations, 1 xiannongtan street, beijing-100050, china
E-mail: ylliu@imm.ac.cn
| Date of Submission | 27 March 2013 |
| Date of Revision | 27 September 2013 |
| Date of Acceptance | 09 October 2013 |
| Indian J Pharm Sci 2013; 75(6): 672-679 |
Abstract
A high-performance liquid chromatography method was developed for the determination of related substances in an intravenous emulsion loaded with a paclitaxel–cholesterol complex. The separation was achieved using Agilent Eclipse XDB-C18 column (150×4.6 mm, 3.5 μm), which was kept at 40°. The gradient mobile phase consisted of acetonitrile and water with a flow rate of 1.2 ml/min. The ultraviolet detection wavelength was set at 227 nm. The preparation of the sample solution began with the addition of anhydrous sodium sulphate to break the emulsion. Then, methanol and ethyl ether were added to pick up the drug and remove the accessories of the emulsion by extraction and centrifugation. Finally, paclitaxel was enriched by a nitrogen blow method and resolved with a mixture of methanol:glacial acetic acid (200:1). The method was proven to be selective, sensitive, robust, linear, repeatable, accurate and suitable for the determination of paclitaxel-related substances in the emulsion formulations, and the major degradation products in the potential pharmaceutical product were 7-epipaclitaxel and 10-deacetylpaclitaxel.
Keywords
Paclitaxel, Highperformance liquid chromatography, Related substances, Determination, Emulsion
Paclitaxel (PAC) is a diterpenoid pseudoalkaloid that was isolated in early 1960s from the bark of Pacific Yew [1]. It has distinct antitumour activity and has been successfully used to treat a wide variety of cancers, including refractory ovarian cancer, metastatic breast cancer, nonsmall cell lung cancer, head and neck malignancies and AIDS-related Kaposi’s sarcoma [2,3]. Due to its low aqueous solubility, many generic PAC-based products besides the first patented PAC, named Taxol, are currently formulated in a 50/50 (v/v) mixture of Cremophor EL/absolute ethanol. Cremophor EL has been associated with serious sideeffects and leads to hypersensitivity [4,5]. According to AHFS Drug Information, it must have a premedicated regimen for steroids is usually started up to 12 h and for H-receptor antagonists 30-60 min before PAC to reduce the incidence of serious hypersensitivity. In addition, this formulation has been associated with a number of issues, such as stability, the possibility for drug precipitation upon dilution, filtering requirements and the use of nonplasticised containers and administration sets [6].
Over the past 15-20 years, many studies are focused on how to increase solubility of the drug, which is necessary for intravenous delivery. To achieve this aim, it can be by, for example, using cosolvents [6], modifying of the PAC molecule into prodrugs [7,8] or analogues [9,10] and preparing liposomes [11] or micelles [12,13]. However, none of these formulations has been introduced to clinical practice yet, due to the lack of sufficient biocompatibility to meet the requirements of intravenous preparations. The first product with a better clinical profile than Taxol is a nanosuspension of PAC conjugated with human albumin (Abraxane®) [14]. In spite of its better clinical profile, Abraxane is generally not replacing Taxol in oncological practice, mostly due to its high cost. Hence, alternative and cost-effective parenteral formulations of PAC are still needed.
Recently, a new kind of PAC o/w emulsion (PACE) loaded with a PAC–cholesterol complex has been developed in our laboratory [15]. According to the experiments, such PACE exhibits increased stability and it could survive completely thermal sterilisation and a long term storage. It also has a better biocompatibility and safety for intravenous infusion with regard to animal trials. Furthermore, since the PACE was fully composed of clinically acceptable excipients and was prepared by regular high-pressure homogenisation, it was suitable for industrial-scale production and clinical applications. To optimise the formulation, evaluate the stability and control the quality for the potential emulsion product, it was necessary to establish a determination method of the PAC-related substances in PACE.
Most of the analytical methods found in the literature for the separation of PAC involved highperformance liquid chromatography (HPLC) and were aimed at determining related substances in plant extracts, raw material and taxol preparations [16-22,23]. Few methods were reported in detail for the PACE. The major problem encountered during HPLC method development was the presence of multiple excipients in the emulsion formulations, such as plant oil, emulsifier, coemulsifier and osmotic regulator. For this reason, the key to the quantitative analysis of emulsions is the preparation of emulsion sample solutions. Previous studies have reported an easy method, which involves directly diluting the sample with organic solvent [15,24,25], thus requiring large amounts of organic solvent. However, since PACE samples were loaded at a PAC concentration of 0.8-1.2 mg/ml, it is impossible for the solvent dilution method to get a high enough concentration of PAC to perform a determination of related substances. In addition, multiple excipients in the emulsions often absorb the ultraviolet (UV) light, which might exert interferences in the determination of drug and its related substances. Further, if emulsion samples are not preprocessed, it is easy to damage the column because of the accumulation of oils and the effects of surfactants on the column when the sample is injected into the HPLC.
A demulsification method has proven efficient to remove the oils. Demulsification can achieve by adding chemical demulsifiers, such as acids, bases and salts, and by centrifugation [24,26,27]. However, the addition of demulsifiers is not suitable in all cases, and it can even destroy certain drugs. So far, there is no report on how to ensure an unchangeable ratio of related substances to drug during the emulsion breaking process. In addition, after breaking the emulsion, it is difficult to extract lipophilic drugs from the oils. Generally, the drug can only be separated from the oils when the solubility of the drug in oils was significantly different than in the organic solvents. Therefore, the selection of suitable organic solvents and extraction procedures is important for sample preparation.
The objective of the present study was to establish a sample solution preparation method and develop a validated HPLC method for the determination of related substances in PACE. All of the validation parameters were performed, including specificity, robustness, linearity, accuracy, precision, limit of detection (LOD) and limit of quantification (LOQ). Chromatographic separation was performed on an Agilent Eclipse XDB-C18 column with a gradient mobile phase consisting of acetonitrile and water. This method should be suitable for the determination of related substances of PAC in the new pharmaceutical product.
Materials and Methods
PAC was purchased from Beijing Union Pharmaceutical Factory, Beijing, China. Cephalomannine (PAC-related compound A), 10-deacetyl-7-epipaclitaxel (PAC-related compound B) and 7-epipaclitaxel reference substance were purchased from National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China. Baccatin III reference substance was purchased from Sigma-Aldrich China Inc., Shanghai, China, and the 10-deacetylpaclitaxel reference substance was purchased from ChromaDex Inc., CA, USA. Acetonitrile and methanol (HPLC grade) were purchased from Fisher Scientific, USA. All other materials were of analytical grade. Double-distilled deionised water was used.
HPLC instrumentation and conditions
The HPLC unit was an Agilent 1100 series apparatus equipped with a quaternary pump, a vacuum degasser, a column oven, a diode array UV detector, and HP workstation software. An Agilent Eclipse XDB-C18 column (150×4.6 mm, 3.5 μm) was used. The mobile phase was composed of mobile phase A (water) and mobile phase B (acetonitrile) at a flow rate of 1.2 ml/min. All the samples were measured at a wavelength of 227 nm at 40°. The injection volume was set as 10 μl, and the chromatograph was set to the program shown in the Table 1.
| Time (min) | Mobile phase A (%) | Mobile phase B (%) |
|---|---|---|
| 0 | 60 | 40 |
| 25 | 60 | 40 |
| 41 | 40 | 60 |
| 41.1 | 0 | 100 |
| 50 | 0 | 100 |
| 50.1 | 60 | 40 |
| 55 | 60 | 40 |
Table 1: Optimised gradient programme
Preparation of system suitability solution
Dissolved accurately weighed quantities of PAC and PAC-related compound B reference substance in a mixture of methanol:glacial acetic acid (200:1) and diluted to obtain a system suitability solution with known PAC and PAC-related compound B concentrations of 800 and 4 μg/ml, respectively
Preparation of mixed standard solution of PAC and its 5 related compounds
Dissolved accurately weighed quantities of PAC and its five related compound reference substance (including baccatin III, 10-deacetylpaclitaxel, PACrelated compound A, PAC-related compound B and 7-epipaclitaxel) in a mixture of methanol:glacial acetic acid (200:1) and diluted it to obtain a mixed standard solution with known PAC and its five related compound concentrations of 800 and 4 μg/ml, respectively.
Preparation of standard solution
Dissolved accurately weighed quantities of PAC reference substance in a mixture of methanol: glacial acetic acid (200:1) and diluted it to obtain a standard solution with a concentration of 800 μg/ml.
Preparation of sample solutions
Sample solutions were prepared at a concentration of 800 μg/ml. First, 2 ml of emulsion sample was placed in a centrifuge tube. Then 400 mg of anhydrous sodium sulphate was added. After shaking and ultrasonicating for 3 and 5 min, respectively, 2 ml of methanol was added to extract PAC for 5 min, and the sample was centrifuged at 10 000 rpm for 10 min. Next, 1 ml of supernatant was placed in a centrifuge tube, and 4 ml of ethyl ether (a distillate of ethyl ether redistillated at 35-40°) was added to extract PAC for 3 min. The sample was then centrifuged at 10 000 rpm for 5 min. After centrifugation, 2.5 ml of the supernatant was transferred to a tube and dried under nitrogen. Finally, 0.5 ml of methanol: glacial acetic acid (200:1) was added, then the sample was shaken for 3 min to dissolve the residue and filtered. The filtrate was used as the sample solution.
Preparation of forced degradation sample solutions
About 5 ml of PACE sample was exposed to 100 μl of aqueous hydrochloric acid solution for 5 h, 50 μl of aqueous 1 N sodium hydroxide for 5 min and 100 μl of 30% hydrogen peroxide solution for 12 h to study the formation of degradation impurities under acidic, basic and oxidative conditions, respectively. To study degradation impurities under thermal and photolytic degradations, PACE samples were kept at 100° for 5 h or exposed to UV light for 12 h, respectively. After the forced degradation, the samples were prepared according to the previous section to obtain forced degradation sample solutions with a concentration of about 800 μg/ml.
Stability of sample solutions
The stability of sample solutions was investigated at room temperature at different times. The related substances of PACE were determined.
Method validation
The methods were validated according to International Conference on Harmonisation Guideline on Validation of Analytical Procedures [28]. Analysis of variance (ANOVA) was used to verify the validity of the methods. A mixed standard solution of PAC and its five related compounds was used to determine the relative retention times of baccatin III, 10-deacetylpaclitaxel, PAC-related compound A, PAC-related compound B and 7-epipaclitaxal to PAC. A system suitability solution [20] was used to evaluate the selectivity of the method. The two major degradation products of PACE, 10-deacetylpaclitaxel and 7-epipaclitaxel, were used as representatives of related substances to validate the method.
An emulsion placebo (without drug) sample prepared by the same method described for the emulsions [15] was used to investigate the interferences resulted from emulsion excipients. Another study to check the selectivity was the degradation test, which tested the samples under different stress conditions. Photodiode array detection was also used as evidence of the specificity of the method to evaluate the homogeneity of the peak. The samples exposed to acidic, basic, oxidative, thermal and UV stress conditions were subjected to photodiode array analysis to determine the peak purity of PACE.
Robustness was evaluated by analysing a 12 mo degraded PACE sample under a variety of HPLC conditions. The changes of conditions included the ratio of the mobile phase by ±2% absolute, the flow rate by ±0.2 ml/min, the column temperature by ±5° and the wavelength by ±2 nm.
Linearity was investigated by evaluating the response at the level of 0.1, 0.2, 1.0, 5, 20, 50 and 125% of 800 μg/ml for PAC, and at the level of 0.1, 0.2, 0.5, 1, 1.5, 3 and 5% of 800 μg/ml for 10-deacetylpaclitaxel and 7-epipaclitaxel. The values of slope, intercept and coefficient correlation for PAC and the two related compounds were calculated. The repeatability of peak areas was evaluated by consecutively injecting PAC at the level of 100% of 800 μg/ml, 10-deacetylpaclitaxel and 7-epipaclitaxel at the level of 0.5% of 800 μg/ml, respectively, for six times.
Accuracy was evaluated by the determination of absolute recovery, which was calculated by comparing the content of 10-deacetylpaclitaxel and 7-epipaclitaxel spiked into PAC standard solution with those obtained by extracting the two related compounds spiked into the emulsion placebo (with PAC) at the 0.5, 1 and 1.5% level of 800 μg/ml.
The precision of the determination of 10-deacetylpaclitaxel and 7-epipaclitaxel in a PACE solution was studied with respect to both repeatability and intermediate precision. Repeatability was demonstrated by analysing a PACE sample five times. Intermediate precision was demonstrated by two chemists analysing the same PACE sample on two different days on two different instruments. Variations of the two related compounds of PACE are expressed in terms of % RSD values.
LOD and LOQ were separately determined at a signal-to-noise ratio (S/N) of 3 and 10, respectively. LOD and LOQ were experimentally verified by diluting known concentrations of PAC, 10-deacetylpaclitaxel and 7-epipaclitaxel until the average responses were approximately 3 or 10 times the standard deviation of the responses
Analysis of emulsions
The related substances of PACE samples were determined by injecting sample solution into the chromatograph, recording the chromatograms and measuring the areas of all the peaks. All peaks before baccatin III (the relative retention time was about 0.18) and after 7-epipaclitaxel (the relative retention time was about 1.46) were discarded and calculated the percentage of each impurity with the following formula: percent impurity=100 (Fri/ru), where, F was the relative response factor for each impurity peak (F was 1.29, 1.26 and 1.00 for baccatin III, PAC-related compound A (the relative retention time was about 0.81) and any other related substances, respectively), ri was the peak area for each individual impurity obtained from the sample solution and ru was the peak area for PAC obtained from the sample solution. The sum of the percentage of all the related substances was calculated for total impurity.
Results and Discussion
The preparation of sample solution was important for the determination of the related substances in PACE because of the presence of multiple excipients in the emulsions. According to the request that at least 0.1% of related substances can be detected [22], the solvent dilution method was unsuitable for the determination of the related substances in PACE. The volume of solvents needed to dilute 1 ml of PACE (containing 1.2 mg of PAC) to a clear solution was about 10, 15, 25, 125 and at least 200 ml for tetrahydrofuran, isopropanol, ethanol, methanol and acetonitrile, respectively. Moreover, column damage from the accumulation of oils and surfactants and the interferences in the determination of the related substances could not be eliminated with the solvent dilution method.
The demulsification method seemed to be a more effective sample solution preparation method for the determination of the related substances in the emulsion. Through extraction with a suitable organic solvent and centrifugation, oils could be removed and the drug could be enriched. Because of the instability of PAC in acid and alkali conditions, anhydrous sodium sulphate was chosen as the demulsifier. Numerous experimental variables, such as the amount of demulsifier, demulsification time, the type and amount of organic solvent, extraction time, extraction procedure and pretreatment method of solvents were investigated. The results showed that methanol was a good solvent, and it could successfully pick up the drug from the oil layer after breaking the emulsion.
Moreover, the addition of ethyl ether, which is a volatile solvent, was essential to further extract the drug from the methanol layer. A nitrogen drying method was adopted for drug enrichment because of the instability of PAC in thermal conditions.
The HPLC conditions for the determination of the related substances in PAC and its Cremophorbased injection have been described in the pharmacopoeias [20,22]. An optimisation based on the USP HPLC condition was performed. A 9 min acetonitrile elution procedure (from 41.1 to 50 min) was adopted to quicken the elution of the residual excipients with a long elution time and ensure uninterrupted sample injection. Under the final conditions, the sample preparation procedure allowed the PACE sample to be available for HPLC analysis in approximately 1 h and the HPLC determination of related substances could be completed in less than 1 h. The sample solution was stable for at least 72 h at room temperature.
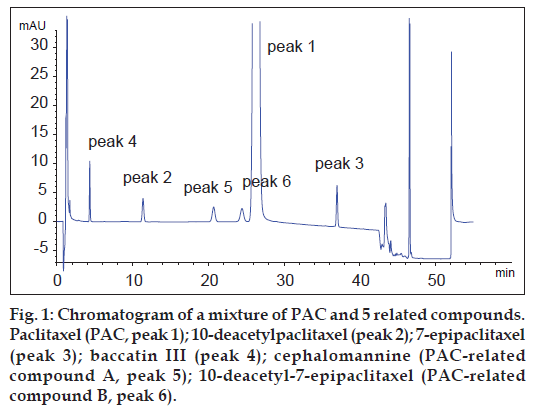
A chromatogram of a mixture of PAC and five related compounds is depicted in fig. 1. The results showed that PAC eluted at a retention time of 24.9 min and the relative retention times (relative to the retention time for PAC) were about 0.18 for baccatin III, 0.45 for 10-deacetylpaclitaxel, 0.81 for PAC-related compound A, 0.94 for PAC-related compound B and 1.46 for 7-epipaclitaxel. System suitability test shows that the resolution between PAC and PAC-related compound B was 1.74.
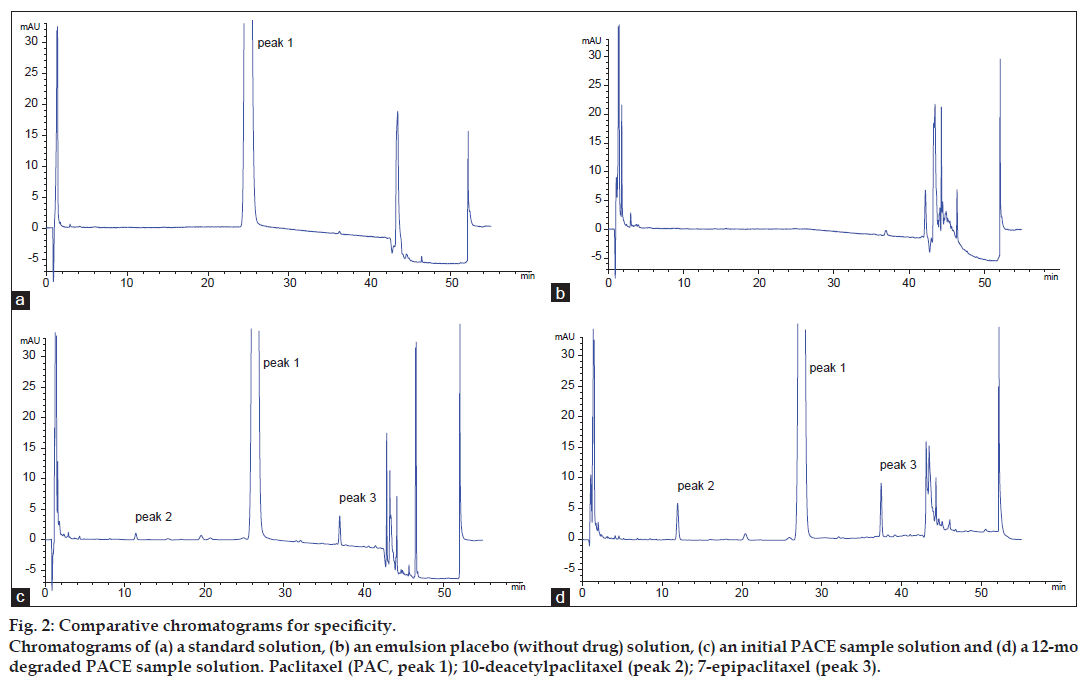
The chromatograms of PAC standard solution, emulsion placebo solution and PACE sample solutions are shown in fig. 2. The study of the peak purity of PAC obtained from standard solution showed that the spectrum was within the established threshold for this peak. No peaks were detected in the migration range of PAC or its related substances in the emulsion placebo. Moreover, the PAC peak of PACE samples gave a spectrum that was identical to the spectrum of the pure standard. Due to the high price and availability of other known reference compounds described in the literature, a degradation test to evaluate the selectivity of the method was developed. Degraded PACE samples included samples that had been stored at 6° for 12 mo and the forced degradation samples described earlier.
Figure 2: Comparative chromatograms for specificity. Chromatograms of (a) a standard solution, (b) an emulsion placebo (without drug) solution, (c) an initial PACE sample solution and (d) a 12-mo degraded PACE sample solution. Paclitaxel (PAC, peak 1); 10-deacetylpaclitaxel (peak 2); 7-epipaclitaxel (peak 3).
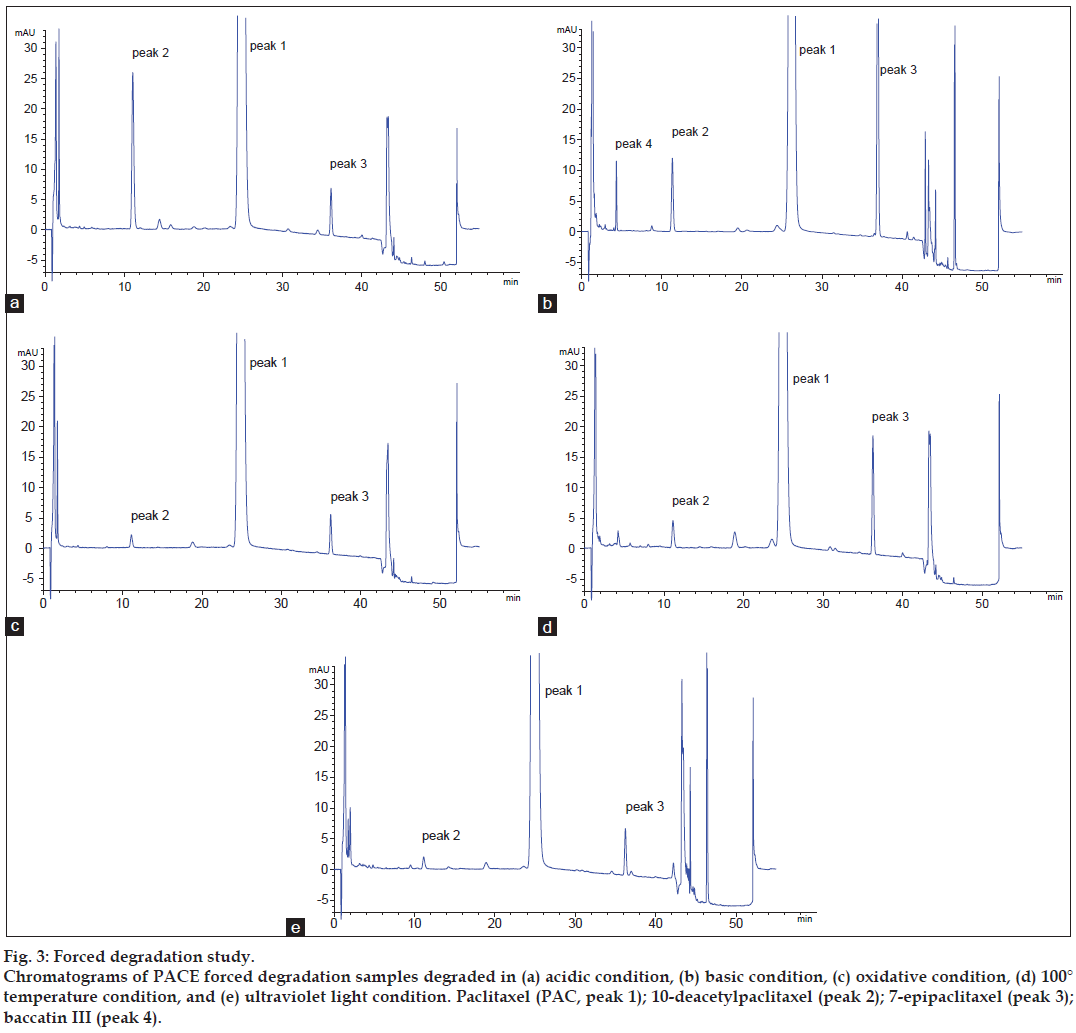
The chromatograms of forced degradation sample solutions did not show any differences between a fresh sample and a sample in oxidative conditions. Thus, it could be concluded that PACE was stable in an oxidative condition. However, sample degradation was observed in acidic, basic, 100° temperature and UV light conditions. The typical chromatograms are shown in fig. 3. The plots with flat tops in all instances showed that the PAC peak had no detectable impurity peaks embedded in it and was free of coeluting degradation compounds. Taken together, these results clearly demonstrated that the method was selective and suitable for routine work.
Figure 3: Forced degradation study. Chromatograms of PACE forced degradation samples degraded in (a) acidic condition, (b) basic condition, (c) oxidative condition, (d) 100° temperature condition, and (e) ultraviolet light condition. Paclitaxel (PAC, peak 1); 10-deacetylpaclitaxel (peak 2); 7-epipaclitaxel (peak 3); baccatin III (peak 4).
Analysis based on the degradation test showed that the first and last peaks of the degraded PACE samples were baccatin III and 7-epipaclitaxel, respectively. The two major degradation products of PACE were 10-deacetylpaclitaxel and 7-epipaclitaxel. Accordingly, 10-deacetylpaclitaxel and 7-epipaclitaxel were chosen as representatives of related substances to validate the method, and during the analysis of PAC-related substances, all peaks before baccatin III and after 7-epipaclitaxel were discarded.
Robustness tests showed that the method was demonstrated to be robust over an acceptable working range of its HPLC operational parameters. Values of slope, intercept and coefficient correlation of linearity and the relative standard deviation (RSD) of repeatability for PAC and the two related compounds are shown in Table 2. Absolute recoveries of the 7-epipaclitaxel and 10-deacetylpaclitaxel ranged from 95.24% to 100.00% as shown in Table 3. Repeatability and intermediate precision for the 7-epipaclitaxel and 10-deacetylpaclitaxel in PACE were found to be <6.0% RSD. The LOD and LOQ values were found to be 0.08 and 0.24 μg/ml, respectively, for PAC and its two related compounds.
| Chemical name | Repeatability | Linearity | ||
|---|---|---|---|---|
| RSD (%) | Slope | Intercept | Correlation | |
| (n=6) | co-efficient | |||
| Paclitaxel | 0.51 | 16.967 | -19.642 | 0.9999 |
| 10-Deacetylpaclitaxel | 0.66 | 15.586 | -0.4518 | 0.9999 |
| 7-Epipaclitaxel | 0.57 | 16.024 | -0.3580 | 0.9999 |
Table 2: Linearity and repeatability data
| Related compound | Spikelevel(%) | Added(µg) | Recovered(µg) | Recovery(%) |
|---|---|---|---|---|
| 10-Deacetylpaclitaxel | 0.5 | 10.56 | 10.56±0.00 | 100.00±0.00 |
| 1 | 21.60 | 21.04±0.14 | 97.41±0.64 | |
| 1.5 | 31.68 | 31.68±0.24 | 100.00±0.76 | |
| 7-Epipaclitaxel | 0.5 | 11.76 | 11.20±0.14 | 95.24±1.18 |
| 1 | 22.32 | 21.76±0.14 | 97.49±0.62 | |
| 1.5 | 32.64 | 31.12±0.50 | 95.34±1.53 |
Table 3: Recovery of two related compounds from pace formulation
The results of the determination of related substances of an initial (0 mo) PACE sample and a 12 mo degraded PACE sample are shown in Table 4 and fig. 2. The major degraded products in PACE were 10-deacetylpaclitaxel and 7-epipaclitaxel, and the maximum degraded product was 7-epipaclitaxel. The percentage of the maximum individual related substance for the initial sample and the 12 mo degraded sample were below 0.5 and 1.0%, respectively, and the total related substances were below 1.0 and 2.0%, respectively.
| Sample | Related substances (%) | |||
|---|---|---|---|---|
| 10-Deacetylpaclitaxel | 7-Epipaclitaxel | Total | ||
| Initial | 0.12±0.00 | 0.43±0.00 | 0.68±0.00 | |
| Stored for | 0.67±0.00 | 0.79±0.01 | 1.65±0.01 | |
| 12 mo | ||||
Table 4: Analysis of pace samples
The developed method was found to be selective, sensitive, precise and accurate, and it can be used for the determinations of PAC-related substances in PACE.References
- Wani M, Taylor H, Wall M. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxusbrevifolia. J Am ChemSoc 1971;93:2325-7.
- Rowinsky E, Donehower R. The clinical pharmacology of paclitaxel (Taxol). SeminOncol 1993;20Suppl 3:S16-25.
- Rowinsky E, Donehower R. Paclitaxel (taxol). N Engl J Med 1995;332:1004-14.
- Dorr R. Pharmacology and toxicology of Cremophor EL diluent. Ann Pharmacother 1994;28Suppl 5:S11-4.
- Weiss R, Donehower R, Wiernik P. Hypersensitivity reactions from taxol. J ClinOncol 1990;8:1263-8.
- Singla A, Garg A, Aggarwal D. Paclitaxel and its formulations. Int J Pharm 2002;235:179-92.
- Li C. Poly(L-glutamic acid)-anticancer drug conjugates. Adv Drug Deliv Rev 2002;54:695-713.
- Wolff A, Donehower R, Carducci M. Phase I study of docosahexaenoicacid-paclitaxel: a taxane-fatty acid conjugate with a unique pharmacology and toxicity profile. Clin Cancer Res 2003;9:3589-97.
- Plummer R, Ghielmini M, Calvert P. Phase I and pharmacokinetic study of the new taxane analog BMS-184476 given weekly in patients with advanced malignancies. Clin Cancer Res 2002;8:2788-97.
- Sessa C, Cuvier C, Caldiera S. Phase I clinical and pharmacokinetic studies of the taxoid derivative RPR 109881A administered as a 1-hour or a 3-hour infusion in patients with advanced solid tumors. Ann Oncol
- Hennenfent K, Govindan R. Novel formulations of taxanes: A review. Old wine in a new bottle? Ann Oncol 2006;17:735-49.
- Kim T, Kim D, Chung J. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res
- Sznitowska M, Klunder M, Placzek M. Paclitaxel solubility in aqueous dispersions and mixed micellar solutions of lecithin. Chem Pharm Bull (Tokyo) 2008;56:70-4.
- Miele E, Spinelli G, Miele E. Albumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancer. Int J Nanomedicine 2009;4:99-105.
- Xia X, Guo R, Liu Y. Formulation, Characterization and hypersensitivity evaluation of an intravenous emulsion loaded with a paclitaxel-cholesterol complex. Chem Pharm Bull (Tokyo) 2011;59:321-6.
- Shao L’Locke D. Determination of paclitaxel and related taxanes in bulk drug and injectable dosage forms by reversed phase liquid chromatography. Anal Chem 1997;69:2008-16.
- Badea I, Ciutaru D, Lazar L. Rapid HPLC method for the determination of paclitaxel in pharmaceutical forms without separation.
- Ciutaru D, Badea I, Lazara L. A HPLC validated assay of paclitaxel’s related impurities in pharmaceutical forms containing Cremophor EL. J Pharm Biomed Anal 2004;34:493-9.
- United States Pharmacopoeia. United States Pharmacopoeia Convention. Rockville, MD, 2004. p. 1394-6.
- Sun P, Wang X, Alquier L. Determination of relative response factors of impurities in paclitaxel with high performance liquid chromatography equipped with ultraviolet and charged aerosol detectors. J Chromatogr A 2008;1177:87-91.
- Chinese Pharmacopoeia (Parta). Beijing, China: Chinese Pharmacopoeia Commission; 2010, p. 1007-8.
- Volka K, Hill S, Kerns E. Profiling degradants of paclitaxel using liquid chromatography–mass spectrometry and liquid chromatography–tandem mass spectrometry substructural techniques. J Chromatogr B Biomed SciAppl 1997;696:99-115.
- Corvari S, Hollenbeck R, Leslie J, Plaisance KI, Young D, Absorption and disposition of colloidal drug delivery systems. I. High-performance liquid chromatographic (HPLC) analysis of a cyclosporin emulsion. Pharm Res 1991;8:40-2.
- Lu Y, Zhang Y, Yang Z. Formulation of an intravenous emulsion loaded with a clarithromycin-phospholipid complex and its pharmacokinetics in rats. Int J Pharm 2009;366:160-9.
- Wu J, Xu Y, Dabros T. Effect of demulsifier properties on destabilization of water-in-oil emulsion. Energy Fuels 2003;17:1554-9.
- Fortuny M, Oliveira C, Melo R. Effect of salinity, temperature, water content, and pH on the microwave demulsification of crude oil emulsions. Energy Fuels 2007;21:1358-64.
- ICH Q2 (R1), International Conference on Harmonisation Guideline on Validation of Analytical Procedures: Text and Methodology, 2005.