- *Corresponding Author:
- Pathak K
Department of Pharmaceutics, Rajiv Academy for Pharmacy, NH No. 2, Mathura-281 001, India
E-mail: kamla_rap@yahoo.co.in
| Date of Received : | 06 October 2007 |
| Date of Revised : | 26 June 2008 |
| Date of Accepted : | 25 September 2008 |
| Indian J. Pharm. Sci., 2008, 70 (5): 609-613 |
Abstract
One of the important methods to improve the solubility of a less water-soluble drug is by the use of co solvents. The solubility enhancement produced by two binary blends with a common co solvent (water-propylene glycol and propylene glycol-ethyl acetate) was studied against the solubility parameter of solvent blends (δ1 ) to evaluate the solubility parameter of drug (δ2 ). The binary blend water:propylene glycol (20:80) gave maximum solubility with an experimental δ2 value of 16.52 (Cal/cm 3 ) 0.5 that was comparable to the theoretical value of 16.52 (Cal/cm 3 ) 0.5 determined by molar volume method and 16.35 (Cal/cm 3 ) 0.5 when determined by method proposed by Lin and Nash. The solvent blend water:propylene glycol (20:80) in which the drug exhibited maximum solubility was used as the reconstituting medium for formulation of dry suspension of cefaclor. The percentage cumulative drug release of cefaclor from the formulation F7 was compared to the marketed formulation by calculating the f 1 (dissimilarity factor) and f 2 (similarity factor) factors. A higher f 1 value and f 2 value below 50 indicates difference between the two dissolution profiles.
Keywords
Solubility parameter, Cefaclor, Solubility, Solvent blends
Cefaclor, an orally active cephalosporin in clinical practice, belongs to the group of β-lactam antibiotics. It is a slightly water soluble drug and its antibacterial activity is dependent on the presence of β-lactam functionality that can be hydrolyzed under aqueous conditions [1]. Solubility and stability problems of cefaclor can be overcome by selection of a suitable co-solvent. One important concept of solubilization by co-solvents is the polarity scale, which includes surface tension, solubility parameter, dielectric constant and partition coefficient to express the polarity of the solvents [2]. The choice of an appropriate co-solvent is important to obtain maximum solubility of the drug and solubility parameter serves as a guide in the selection of appropriate co-solvent [3,4].
The use of single mixture limits the polarity range while the binary mixture with a common co solvent allows to expand the polarity range and to test the influence of the co solvent on drug solubility [5]. Evaluation of solubility parameter in different solvent blends of various polarities provides an important insight into the solubility of drug. The aim of the present study is to determine the solubility parameter of cefaclor by evaluating the solubility of cefaclor in different blends of water:propylene glycol (PG) to overcome the problem of solubility and hydrolytic instability, and to design and formulate dry suspension for reconstitution of cefaclor as a dual pack system and compare with marketed formulation.
Materials and Methods
Cefaclor was obtained as gift sample from Siemens Laboratories, Gurgaon, India and Himedia dialysis membrane-50 was procured from Himedia Lab. Pvt. Ltd., Mumbai. Polyplasdone XL was a gift sample from ISP Technologies Inc., NJ. Methyl cellulose, microcrystalline cellulose, acacia gum and sodium citrate were obtained from Ranbaxy Fine Chemicals Ltd., New Delhi, and sodium benzoate, sodium starch glycolate, sucrose from Qualigens Fine Chemicals, Mumbai. The binary mixtures were prepared (by volume) with glycerin or propylene glycol (Ranbaxy Fine Chemicals Ltd., New Delhi) and all glass double distilled water.
Solubility measurements
Sealed flasks containing an excess of cefaclor in the pure solvents and solvent blends were shaken at 37±0.50 in a temperature controlled water bath (Hicon, India). When the saturation concentration was attained (after 72 h), the solid phase was removed by fi ltration through nylon fi lter disk (0.45 μ). The clear solutions were diluted with double distilled water and assayed in a double-beam spectrophotometer (Shimadzu Pharmaspec, UV-1700, Japan). The spectrophotometric measurements were performed at 264 nm. The densities of the solutions were determined at 37±0.50 in 10 ml pycnometer to convert molar solubility into mole fraction units6. All the experimental results are the average of at least three replicated experiments. The coefficient of variation (SD/meanΧ100) was within 2% among replicated samples for the solubility measurements.
Solubility parameter determination
Solubility parameter determination of cefaclor (δ2) was done by solubility measurement method (experimental method) and by theoretical methods namely molar volume method and by method proposed by Lin and Nash6. In solubility measurement method, the solubility parameter of cefaclor is assumed to be similar to that of the solubility parameter of the solvent (δ1) in which the drug exhibits maximum solubility7. Hence, the solubility data (Table 1) obtained by the method described in preceding section was used to determine δ2.
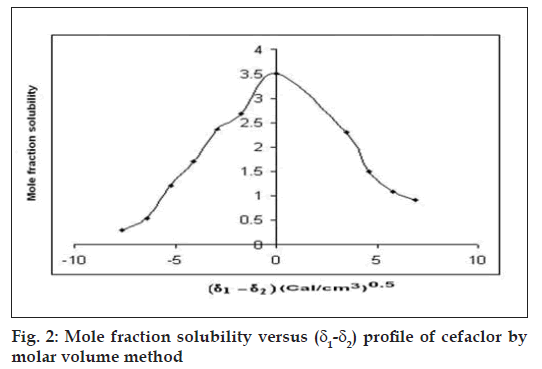
The solubility parameter of cefaclor was determined by molar volume method by calculating the mole fraction solubility (Xi2 ) of cefaclor in solvent blends containing water and propylene glycol in different ratios as shown in Table 1. The mole fraction solubility was calculated by using the following equation, Xi2 = n2/n1+n2 (1), where n1= number of moles of solvent and n2 = number of moles of solute. A plot of mole fraction solubility of cefaclor in the various ratios of the binary mixtures was made against Δδ (δ1–δ2) .The solubility parameter of the solvent blend (δ 1) in which cefaclor showed peak mole fraction solubility represented the solubility parameter of cefaclor (δ2)8.
The method of Lin and Nash is based on the use of experimental mole fraction solubility of drug in given solvent blends. Thus δ2 can be determined by use of the following equation,δ2= ΣXi 2δ1/ ΣXi2 (2), in which δ2 is the solubility parameter of cefaclor, Xi2is the mole fraction solubility of the solute in a given solvent and δ1 is the solubility parameter of the solvent [9].
Formulation of dry suspension of cefaclor for reconstitution
Dry suspensions for reconstitution were prepared using the formulae shown in Table 2. All the ingredients were mixed in geometric proportion in a glass pestlemortar and a suffi cient volume of granulating agent (starch paste, 5% w/v for F1-F5 and alcohol 95% v/v for F6 and F7) was incorporated slowly. After enough cohesiveness was obtained, the mass was sieved though mesh #16. The granules were dried in oven (Jindal, Scientifi c Inst. Pvt. Ltd., India) at 60° for 30 min and the dried mass was passed through mesh #22. The dry products were reconstituted with reconstitution medium (water: propylene glycol, 20:80) for further evaluations.
| Ingredients (mg) | F1 | F2 | F3 | F4 | F5 | F6 | F7 |
|---|---|---|---|---|---|---|---|
| Cefaclor | 125 | 125 | 125 | 125 | 125 | 125 | 125 |
| Sodium benzoate | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| Sucrose (g) | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 |
| Colloidal silica | - | - | - | - | - | - | - |
| Acacia | 250 | 250 | 250 | 250 | 250 | 250 | 250 |
| Xanthan gum | - | - | - | - | - | - | - |
| Sodium citrate | 75 | 75 | 75 | 75 | 75 | 75 | 75 |
| Citric acid | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| Corn starch (5% aqueous paste) | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. |
| Methyl cellulose | 125 | 250 | 500 | - | - | - | - |
| Microcrystalline cellulose | - | - | - | 250 | 500 | - | - |
| Sodium starch glycolate | - | - | - | - | - | 100 | - |
| Polyplasdone XL | - | - | - | - | - | - | 100 |
| Color (orange) | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. |
| Flavor (orange) | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. |
To be reconstituted to 5 ml with water: PG (20:80).
Table 2: The Different Formulations of Cefaclor and Their Composition.
Evaluation of reconstituted suspensions
The pH of the reconstituted suspensions was measured using pH meter (DB-1011, HICON, India). The sedimentation volume (Hu/Ho) calculated against time was described in terms of the ratio of equilibrium settled height (HU) to original height (Ho)10. The degree of fl occulation was determined as the ratio of sedimentation volume of the fl occulated suspension to the sedimentation volume of deflocculated suspension [11]. Ease of redispersion of suspensions was quantified by counting the number of strokes (given at an angle of 180°) required to redisperse the dispersed phase in 10 ml of the sample [12]. For single point viscosity determination of the reconstituted suspension, Brookfi eld viscometer (DV-II, Brookfi eld Eng. Lab. INC, USA), attached with spindle RV No. 4 was used. Suspension (75 ml) was taken in 100 ml beaker and the viscosity was measured at 100 rpm at room temperature. For drug content determination 1 ml of each suspension was dissolved in 100 ml of double distilled water, filtered, diluted as required and analyzed spectrophotometrically at 264 nm. Each value represents the mean content of three replicates [13].
In vitro drug release study
Reconstituted suspension (5 ml) was taken in the donor compartment of lab fabricated glass diffusion cell (d= 2.4 cm) and 50 ml of double distilled water in the receptor compartment was stirred at 100 rpm and maintained at 37±0.5°. The HIMEDIA dialysis membrane-50 soaked in double distilled water for 24 h was used as a barrier membrane. Samples were withdrawn at different time intervals of 15, 30, 60, 90, 120, 150, 180, 240 and 360 min diluted with double distilled water and analyzed spectrophotometrically at 264 nm with reference to suitably constructed calibration curve. Release study was performed in triplicate. Marketed formulation (Kefl or®, Ranbaxy, India) was also evaluated for in vitro drug release and compared with the best formulation.
Results and Discussion
Solubility of cefaclor was evaluated in solvent blends containing water:PG for the determination of δ 2 as the varying blends of these provided a range of 14.80 - 23.40 (Cal/cm3)0.5 of δ 1. The peak solubility (X2) of 29.93 mg/ml for cefaclor was observed in a solvent blend of water: PG (20:80) with δ1 of 16.52 (Cal/cm3)0.5. Thus the solubility parameter for cefaclor can be defi ned as 16.52 (Cal/cm3)0.5 as according to the solubility measurement method,δ 2 is that value of δ 1 at which the drug exhibits maximum solubility. Table 1 lists the solvent blends, the Hildebrand solubility parameter (δ1) of the solvent blends and the experimentally determined solubilities (mg/ml) of cefaclor.
The molar volume method was used to determine the peak mole fraction solubility of cefaclor in various solvent blends and the mole fraction solubilities Xi2 of cefaclor and Δδ are tabulated in Table 1. Peak mole fraction solubility was determined to be 3.51×10-3 in solvent blend (water:PG, 20:80) with δ1 value 16.52 (Cal/cm3)0.5, which is in agreement with solubility measurement method. A plot of δ1 and Xi2 (fig. 1) showed a bell shaped curve suggesting that both at lower and higher values δ1 = 16.52 (Cal/cm3)0.5 the solubility of cefaclor decreased. When Δδ was plotted against Xi2 (fi g. 2), the solubility parameter of cefaclor was confi rmed at 16.52 (Cal/cm3)0.5 as it is that value of δ1 at which cefaclor exhibited peak mole fraction solubility and Δδ = 0. δ2 determined by the method of Lin and Nash were found to be 16.35 (cal/cm3)0.5, which is comparable to the value obtained by solubility measurement method and molar volume method.
Granular formulations of dry suspension for reconstitution were designed based on of two types of disintegrants, gel forming (methyl cellulose and sodium starch glycolate) and non-gel forming (microcrystalline cellulose and polypasdone XL) in order to assess their role on drug release (Table 2). Table 3 summarizes the physical and rheological characteristics of the reconstituted suspensions. The pH was found to be the range of 3.74–3.82, which is desirable for the stability of cefaclor14 and also accenuates palatability of the oral dosage form. The formulation F7, that showed maximum value (0.89 closest to 1 as compared to other formulations) of sedimentation volume and degree of fl occulation is suggested to be homogenous in appearance and may not exhibit caking on long term storage. The viscosity of all the formulations ranged between 42–56 cps.
| Parameters | pH | Sedimentation volume | Degree of flocculation |
Redispersibility (strokes) | Viscosity (cps) | Drug content (mg /ml) | |
|---|---|---|---|---|---|---|---|
| F1 | 0day | 3.76 | 0.48 | 1.01 | 2 | 50 | 25.20 |
| At 7thday | 3.95 | 0.51 | 1.03 | 2 | 48 | 24.30 | |
| F2 | 0day | 3.77 | 0.60 | 1.08 | 2 | 54 | 25.17 |
| At 7thday | 3.90 | 0.62 | 1.10 | 2 | 50 | 24.69 | |
| F3 | 0day | 3.84 | 0.62 | 1.02 | 2 | 42 | 25.02 |
| At 7thday | 3.89 | 0.64 | 1.02 | 2 | 42 | 24.94 | |
| F4 | 0day | 3.81 | 0.66 | 1.05 | 2 | 48 | 24.06 |
| At 7thday | 3.92 | 0.67 | 1.07 | 2 | 45 | 24.69 | |
| F5 | 0day | 3.74 | 0.72 | 0.95 | 2 | 52 | 25.60 |
| At 7thday | 3.95 | 0.74 | 0.96 | 2 | 49 | 24.24 | |
| F6 | 0day | 3.78 | 0.77 | 1.07 | 2 | 58 | 25.09 |
| At 7thday | 3.87 | 0.79 | 1.03 | 2 | 55 | 23.02 | |
| F7 | 0day | 3.82 | 0.89 | 1.25 | 2 | 56 | 25.35 |
| At 7thday | 3.90 | 0.88 | 1.24 | 2 | 56 | 24.06 | |
Table 3: Comparative Physical and Rehological Parameters of Formulations F1 - F7.
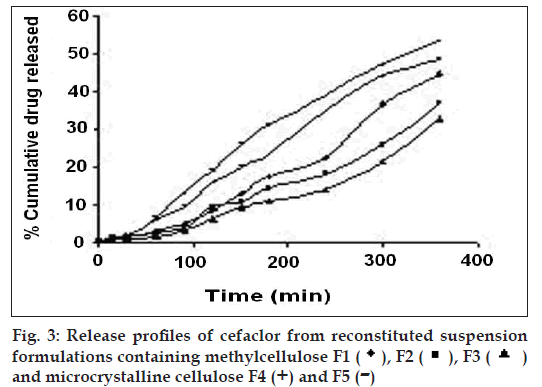
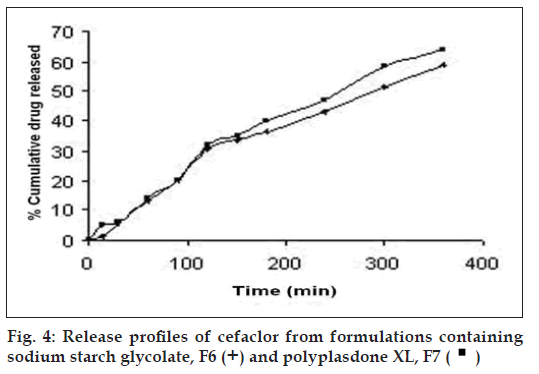
The release profiles of the freshly reconstituted suspensions are reported in fi gs. 3 and 4. The order of percentage cumulative drug release (%CDR) at 360 min is F7>F6>F5>F4>F1>F2>F3 with values of 64.14, 58.74, 53.48, 48.33, 44.50, 37.08, 32.51, respectively. In formulations F1, F2 and F3 a decrease in %CDR was observed as the concentration of gel forming disintegrants increased irrespective of the amount of the suspending agent used whereas in formulations F4 and F5, an increase in %CDR drug release was observed with increasing amounts of non-gel forming disintegrants. In an attempt to enhance the %CDR, granular formulations containing superdisintegrant were made by non-aqueous granulation. Formulation F6 containing sodium starch glycolate, the gel forming superdisintegrant showed lesser drug release when compared to F7 containing polyplasdone XL, both used in similar concentrations. The swelling of superdisintegrant by gel formation can delay dissolution, as the drug must diffuse through the gel layer before being released [15]. Highest drug release obtained with formulation F7 containing polyplasdone XL may be attributed to high cross-link density, swelling without gelling and thus not hindering the dissolution of drug.
Stability studies were accomplished at two levels, one for the reconstituted suspension for one week (Table 3) and other for the dried product and reconstituting medium for a period of three months (Table 4). No signifi cant changes were observed after 3 months in physical and rheological characteristics of the suspension reconstituted with water: propylene glycol (20:80) as well as for the dried product including chemical stability (defi ned as maintenance of more than 80% of initial concentration) and drug release.
| Sample | Evaluated | 0 | 1 | 2 | 3 |
|---|---|---|---|---|---|
| Dry | pH | 3.85 | 3.79 | 3.77 | 3.77 |
| Suspension | Drug content | 23.35 | 23.41 | 23.23 | 23.32 |
| (mg/ml) | |||||
| % Cumulative | 64.14 | 64.55 | 63.40 | 63.78 | |
| drug release | |||||
| Recon- | Color change | - | - | - | - |
| stituting | pH | 4.95 | 4.90 | 4.93 | 4.98 |
| Medium |
Table 4: Stability data of dry suspension and Reconstituting medium.
The %CDR of cefaclor from the selected reconstituted formulation F7 was compared with that of the marketed formulation FM and a higher release was obtained with F7 (64.14%) when compared to the marketed formulation FM (45.66%) (fig. 5). The f1 (dissimilarity factor) and f2 (similarity factor) factors were calculated to assess similarity of dissolution profiles of formulations. The f1 and f2 factors were equal to 43.62 and 17.65, respectively. A higher f1 and f2 value below 50 indicates difference between the two dissolution profi les16. Conclusively the solubility studies based on solubility parameter are useful indicator for selection of appropriate solvent blend for the formulation of a stable and effi cacious liquid dosage form.
References
- Ivama MV, Rodrigues NC, Guaratin CI, Zanoni BM. Spectrophotometric determination of cefaclor in pharmaceutical preparations. Quim Nova 1999;22:201-4.
- Swarbrick J, Boylan JC. Encyclopedia of pharmaceutical technology.2nd ed. Marcel Dekker New York: 2002. p. 658-69.
- Karanth H, Shenoy VS. Solubility parameter: Influence on In vitro release and antibacterial activity of sparfloxacin. Indian Drugs 2005;42:222-5.
- Subrahmanyam CV, Reddy MS, Rao JV, Rao PG. Irregular solution behaviour of paracetamol in binary solvents. Int J Pharm 1992;78:17-24.
- Pena MA, Reillo A, Escalera B, Bustamante P. Solubility parameter of drugs for predicting the solubility profi le type within a wide polarity range in solvent mixtures. Int J Pharm 2006;321:155-61.
- Barra J, Bustamante P, Pena MA. Partial solubility parameters of naproxen and sodium diclofenac. J Pharm Phamacol 1998;50:975-82.
- Hancock BC, York P, Rowe RC. The use of solubility parameters in pharmaceutical dosage form design. Int J Pharm 1997;148:1-21.
- Josyula VR, Karanth H. Studies on solubility of amoxycillintrihydrate: Influence on In vitro release and antibacterial activity. J Pharm Sci 2005;67:342-5.
- Bustamante P, Romero S, Escalera B. Solubility behaviour of polymorphs I and II of mefenamic acid in solvent mixtures. Int J Pharm 1999;178:193-202.
- Martin A, Swarbrick J, Cammarata A. Physical pharmacy. 3rd ed. KM Varghese Company Bombay; 1991. p. 314-46.
- Nappinnai M, Kishore SV, Kumar VR. Formulation and evaluation of hydrocalcite suspensions. Indian Pharmacist 2006;5:89-92.
- Prabakaran L, Bajpai M, SainiTR. Formulation development and optimization studies of satranidazole oral suspension. J Pharm Res 2005;4:19-23.
- British Pharmacopeia, Vol II. The department of health, social services and public safety.Her Majesty?s Stationary OfficeLondon 2004. p. 367-70
- Florey K. Analytical profi les of drug substances. Vol 9. Marcel Dekker New York 2005. p. 107-22.
- Marshall VP, Pope DG, Carstensen JT. Methods for the assessment of the stability of tablet disintegrants. J Pharm Sci 1991;80:899-903.
- Costa P, Lobo JMS. Modeling and comparison of dissolution profi les.Eur J Pharm Sci 2001;13:123-33.
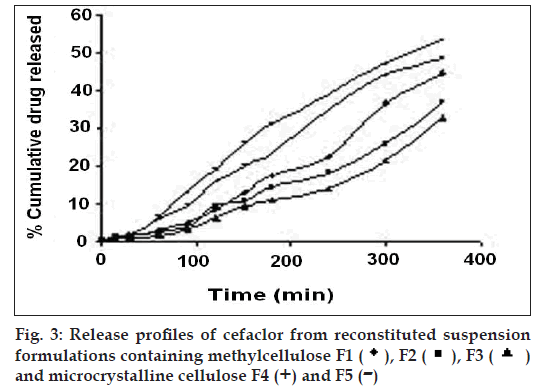
 ), F3 (
), F3 (  )
and microcrystalline cellulose F4 (+) and F5 (-)
)
and microcrystalline cellulose F4 (+) and F5 (-)