- *Corresponding Author:
- A. J. Rajamma
Department of Pharmacy, KLEUs College of Pharmacy, Bengaluru-560 010, India
E-mail: abburjayaramu6@gmail.com
| Date of Submission | 09 May 2017 |
| Date of Revision | 24 October 2017 |
| Date of Acceptance | 29 May 2018 |
| Indian J Pharm Sci 2018;80(4):647-656 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The objective of this study is to demonstrate an effective technique with potential for commercialization for microsphere production. Ultrasound-assisted ionic gelation of sodium alginate was used to encapsulate simvastatin. Chitosan was used as a mucoadhesive polymer for gastro-retention of microsphere. Sodium alginate solution was sonicated using probe sonicator at a frequency of 20±3 KHz and ultrasonic power of 130 W with an input voltage range of 170-270 AC, 50 Hz, at a temperature of 50°. The influence of ultrasound waves on drug entrapment efficiency, yield and particle size dispersion was studied in comparison to mechanical stirring method. Mucoadhesiveness, release pattern and kinetics of drug release were also characterized. Ultrasound treatment caused small fissures and depressions on the surface of alginate as evidenced in SEM. Ultrasound of 20±3 KHz for 12 min duration was the optimum frequency and time in the experimental set up to obtain microspheres with uniform and smaller microparticles. The encapsulation efficiency of simvastatin was directly proportional to the sonication effect, concentration of alginate and extent of its cross linkage with calcium ions. DSC studies revealed that ultrasound treatment did not alter the structural integrity of the drug component. Formulation was found to be dissolution efficient and drug release pattern was concentration dependent, which followed non-Fickian diffusion mechanism with an ‘n’ value of 0.8. Therefore, it could be concluded that ultrasound can be used as an efficient technology to develop drug-entrapped microsphere for controlled delivery.
Keywords
Sonication, acoustic cavitation, microencapsulation, simvastatin, ionic gelation, drug release kinetics
In the present era, there is an immense need for the pharmaceutical industries to step forward, contrive novel effectual drug formulations and techniques to ameliorate the existing products. On the other hand, lack of scale up techniques and commercial procedures were the reason for the failure of the novel drug delivery system (DDS) to enter the market. Therefore, the pharmaceutical industries are in search of effective, reproducible and simple methods for producing DDS. A continuous search is on to find out better techniques for generating DDS, and to make a positive impact on the conventional techniques. Recently, ultrasound cavitation has gained importance due to its widespread use in a variety of processes i.e. physical, chemical and biological [1]. Research in ultrasound-activated novel delivery has emerged widely in the last two decades with the origination of gas bubbles [2]. High energy ultrasonic vibrations are tried for designing and formulating the various novel DDS [3]. Recently, in novel aspects, advantages of ultrasound technology in terms of intensification and low energy requests for microencapsulation are emphasized [4]. Generation of microspheres using cavitation approach is highly energy efficient and also flexible to control particle size over other conventional mechanical and high-pressure techniques [5]. The impact of process parameters such as the flow rate and liquid properties on the size distribution and effect of other equipment parameters like the operating frequency, power dissipation have also been evaluated over conventional methods [6].
Review of literature emphasized that ultrasound assisted microencapsulation, which is the scope of this research work appeared to be more competitive and attractive or even superior in terms of simplicity, reproducibility and energy efficiency compared to other conventional formulation approach [7,8]. In view of this, ultrasound-assisted microencapsulation has been proposed for the preparation of simvastatin microspheres in the current work.
Simvastatin is one of the widely used drugs in the treatment of hyperlipidemic condition and for the prophylactic treatment of obesity. It is highly effective in lowering the levels of low-density lipoprotein and triglycerides in the blood and for improving the lipid profile in hypercholesterolemic diabetic patients [9]. However, simvastatin has a few limitations for its conventional delivery. Simvastatin has a short halflife (<2 h) and therefore requires to be administered multiple times a day [10]. The bio-absorption of this drug is comparatively high in the upper portion of the GIT; therefore delivery of this drug by conventional methods result in variable and non-uniform absorption [11]. Lower duration of residence in the stomach and varying gastric emptying time may have a significant impact on the bioavailability of this drug [12]. Hence, a special delivery technique should be designed, which extends the gastric emptying time and deliver higher amount of drug in the stomach. Also, a formulation that would deliver the drug for a sustained span of time would be ideal. Drug-entrapped mucoadhesive microspheres have been designed for gastroretentive drug delivery [13]. These forms reduce the chances of dumping of dose and also inter-subject variability in absorption. Therefore the objective of this study is to investigate ultrasound waves for the production of simvastatin-entrapped microspheres.
Materials and Methods
Simvastatin was obtained as a gift sample from Biocon, Bangalore, India. Sodium alginate and chitosan were purchased from Sigma-Aldrich, St. Louis, Missouri, United States. All other polymers, chemicals and reagents used were of analytical reagent grade.
Construction of standard curve of simvastatin
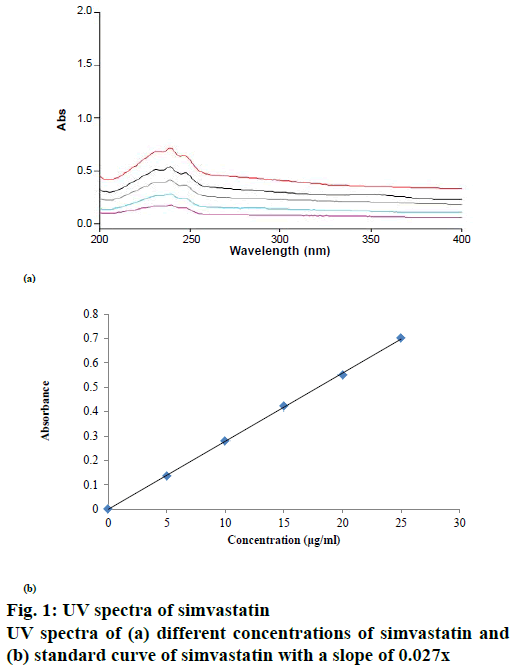
Simvastatin solution bearing a concentration of 1000 μg/ml was prepared in methanol [14]. Distilled water was used to further dilute the initial stock solution in methanol to obtain solutions with concentration ranging from 5-30 μg/ml. Simvastatin solutions of different concentration were scanned to detect the absorption maxima (λmax), which was found to be 239 nm (Figure 1). Then, the standard curve of simvastatin was constructed at 239 nm using a spectrophotometer (UV-1800, Shimadzu, Kyoto-Japan). Standard curve was found to be linear at this wavelength and the correlation coefficient (r2) value obtained was 0.999.
Fourier-transform infrared spectroscopy (FTIR) study
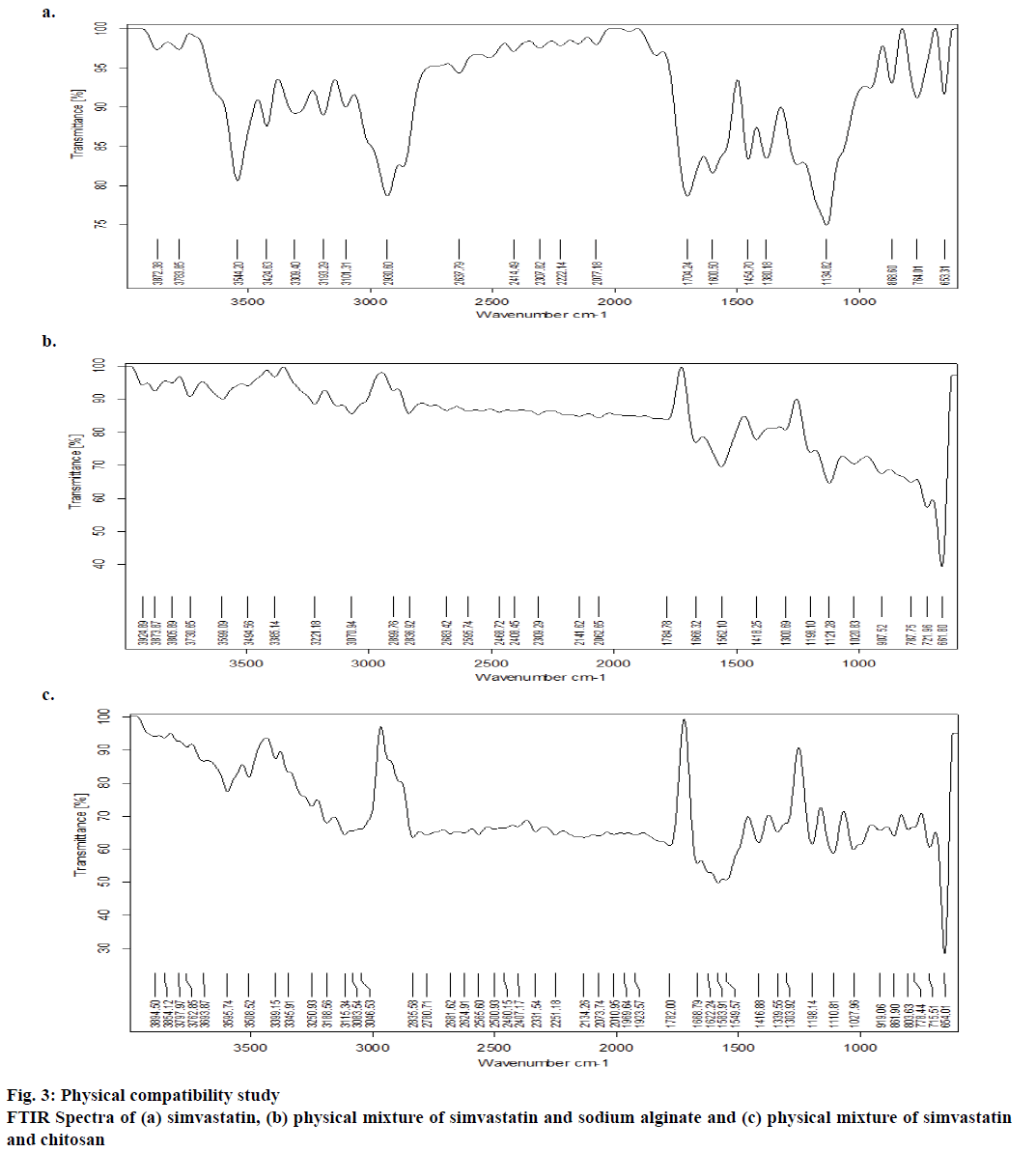
FTIR study was employed to characterize and quantify the physicochemical compatibility between simvastatin and the polymers incorporated in the formulation. FTIR spectra were recorded for the mixture of drug and polymer at 1:1 ratio and for the drug separately. The spectra of the samples were obtained using an FTIR (Bruker Optics, model Tensor 27; Opus software) instrument. The sample was analysed in the ambit of 400-4000 cm-1; wave number versus percent transmittance spectra was plotted.
Differential scanning calorimetry (DSC) analysis
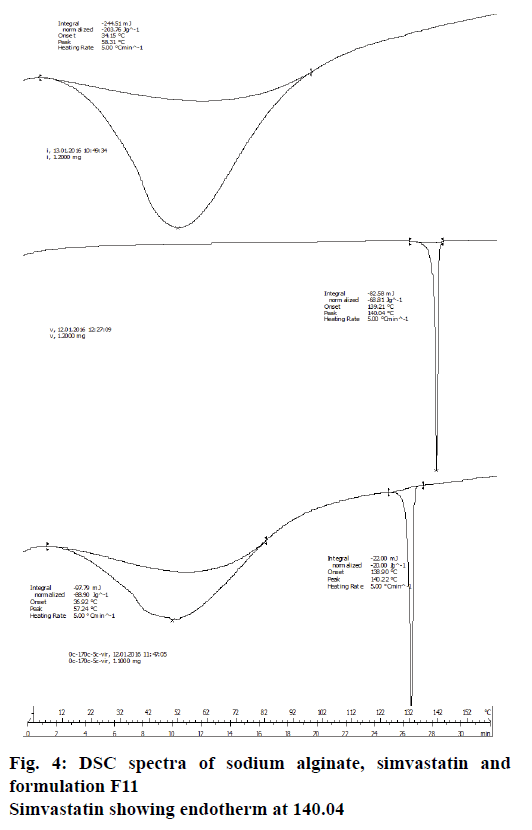
DSC analysis was performed on a differential thermal analyser 2100 (Dupont Co., Parkersburg, WV, USA) in an open pan system under stable atmospheric considerations. Initial DSC was obtained by an analyser, which was provided with data developing system. Alumina was used as the standard, which is inert in nature. The samples for analysis were packed in an aluminium pan and the thermal readings were observed at a room temperature of 160° at the rate of 10° min−1. They were then cooled to room temperature. All samples were kept under nitrogen while cooling curves were recorded.
Scanning electron microscope (SEM) studies
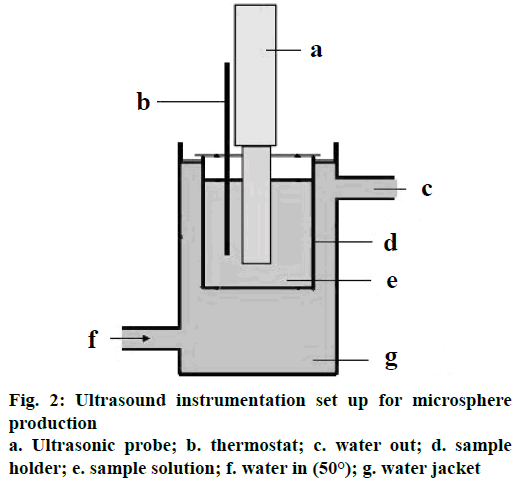
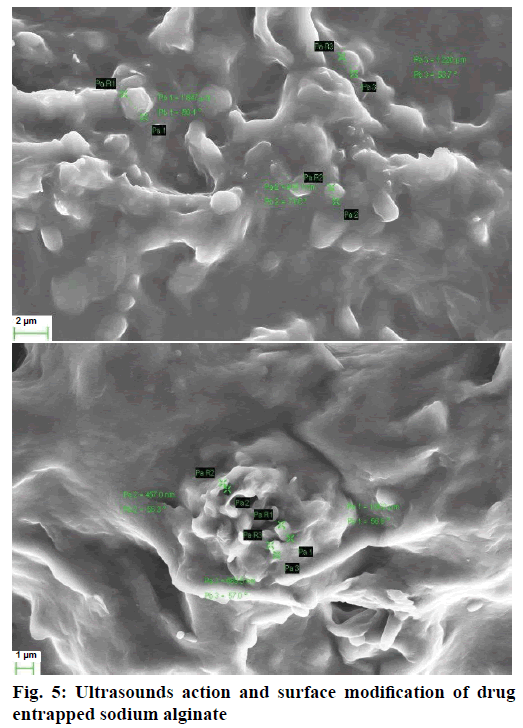
Sodium alginate solution was treated with ultrasound for 10 min at 50° using Sonoplus HD 2200 homogenizer (Bandelin Electronic, Berlin, Germany). Cavitation produced at a frequency of 20±3 KHz and ultrasonic power of 130 W with an input voltage range of 170- 270 AC, 50 Hz were observed under SEM (Zeiss, IISc, Bangalore) at specified magnification (x5 and x10) in room temperature.
Formulation of gastroretentive microspheres
Mucoadhesive gastroretentive microspheres entrapped with simvastatin were produced by ionic gelation method combined with ultrasonication [15]. Formulation composition and design of experiment has been shown in Table 1. The polymers tested in the formulation were chitosan and sodium alginate. The effect of ultrasound waves on drug entrapment efficiency, percent yield and particle size dispersion were studied in comparison to mechanical stirring method. Mucoadhesiveness, release pattern and kinetics of drug release were also characterized.
| Formulation code | Sodium alginate and chitosan solution | Calcium chloride solution | Sodium alginate, chitosan and calcium chloride solution (volume ratio) | Sonication time period (min) | |
|---|---|---|---|---|---|
| Conc. (% w/v) |
Conc. ratio | Conc. (% w/v) |
|||
| F1 F2 F3 F4 F5 F6 F7 F8 F9 F10 F11 |
0.2:0.4 0.3:0.4 0.4:0.4 0.5:0.4 0.2:0.6 0.3:0.6 0.4:0.6 0.5:0.6 0.2:0.8 0.3:0.8 0.5:0.8 |
1:2 1.5:2 2:2 2.5:2 1:3 1.5:3 2:3 2.5:3 1:4 1.5:4 2.5:4 |
0.5 0.5 0.5 0.5 0.5 0.5 0.5 0.5 0.5 0.5 0.5 |
10:4:2 10:4:2 10:4:2 10:4:2 10:4:2 10:4:2 10:4:2 10:4:2 10:4:2 10:4:2 10:4:2 |
04 08 12 16 04 08 12 16 04 08 12 |
| Encapsulation without sonication | |||||
| F12 F13 F14 |
0.4: 0.4 0.5: 0.6 0.5: 0.8 |
2:2 2.5:3 2.5:4 |
0.5 0.5 0.5 |
10:4:2 10:4:2 10:4:2 |
10 10 10 |
Table 1: Formulation of Simvastatin Microspheres
Preparation of polymer solution
Simvastatin equivalent to 20 mg was dissolved in sufficient volume of ethanol on a magnetic stirrer by stirring at 300 rpm at room temperature. Simvastatin solution was incorporated in calcium chloride solution with uninterrupted stirring on a magnetic stirrer at a speed of 300 rpm to obtain homogenous solution. Sodium alginate solution was prepared in dilute HCl (pH 5.5) with continuous stirring for 8 h at a slow speed (100 to 300 rpm) on a magnetic stirrer to get a clear solution. Chitosan solution in 1 % acetic acid (pH 5.4) was prepared on a magnetic stirrer at a speed of 300 rpm [16].
Sodium alginate solution, 150 ml per batch was sonicated in a glass beaker of 250 ml capacity and a diameter of 7 cm. Probe sonicator at a frequency of 20±3 KHz and ultrasonic power of 130 W with an input voltage range of 170-270 AC, 50 Hz was used (Figure 2). Probe of diameter 2 cm and immersed in the suspension to a depth of 3 cm in a beaker 2 cm away from the bottom. Sonication was in continuous mode with temperature control to 50°. During the sonication, simvastatin dispersed in calcium chloride solution was added drop-wise to sodium alginate solution.
Another set of microencapsulation was also carried out for selected formulation composition (Table 1) using similar procedure mentioned above but without sonication. At the time of addition of simvastatindispersed calcium chloride solution, sodium alginate solution was stirred at a speed of 900 to 1100 rpm on a magnetic stirrer.
Chitosan coating
Immediately after cross linking of alginate dispersion, it was placed over magnetic stirrer and chitosan solution was added drop-wise at a stirring speed of 300 rpm. Preparation was kept overnight, filtered and dried at 50° to collect free flowing microspheres [17].
Entrapment efficiency
Entrapment efficiency was conducted to quantitatively estimate the amount of drug present in microspheres. Microspheres were crushed in a clean dry mortar. A sufficient volume of methanol was added to it in order to dissolve the drug. The solutions were sonicated to obtain homogenous solution. The resultant solution was strained through a membrane filter (0.45 μ). Clear filtrate of the drug extract was diluted appropriately with methanol and subsequently with distilled water. Drug concentration was measured spectrophotometrically on a UV/Vis instrument at 239 nm [18]. The product yield was determined for all the formulations using the following Eqn., % yield = (practical yield/theoretical yield)×100. The percent encapsulation efficiency was calculated from the following Eqn., % encapsulation efficiency = (amount of encapsulated drug/amount of added drug)×100.
Particle size distribution study
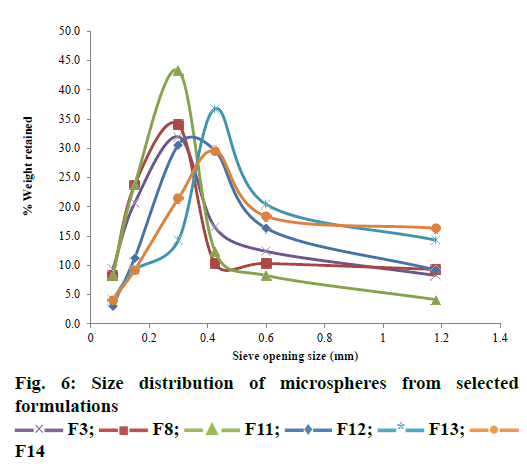
Standard USP procedure (method I) for size analysis was followed to understand the size dispersion of microspheres. A set of seven standard sieves (0.15- 1.18 mm) were used in the study, sieves were arranged such that course sieve to the top and finest sieve to the bottom. Entire sieves array was mounted on the sieve shaker. Dried sample of microspheres weighing accurately 100 g was placed on the top (coarse) sieve. The sieve shaker was operated for 10 min. The samples retained on each sieve was then collected and processed for analysis. Homogeneity of the formulation was determined by plotting % retained weight vs. sieve size [19]. Assessment of size distribution was made with the help of a grading curve, i.e. log sieve size versus % fines. D30, D60 and D90 values were ascertained from the grading curve, which corresponded to 30, 60 and 90 % fines [20]. To check the spread of the range of the particle sizes the coefficient of uniformity (CU) and coefficient of curvature (CC) was also calculated using the Eqn., CU = D60/D10; CC = [D30]2/D10D60.
In vitro mucoadhesion test
This study was performed in the simulated gastric fluid. The scraped gastric mucosal layers were obtained freshly from goat and mounted on the wooden piece (6×1.5 cm). Microspheres (100 mg) were placed on the mounted tissue and incubated for duration of 5 min and placed in the cylindrical tubes of a disintegration test apparatus containing simulated gastric fluid (900 ml). Then the disintegration apparatus was operated. The tissue specimen was given a slow, up and down movements in the test fluid at 37°, at a speed of 31 dips/min. After every 1 h time interval the fluid was filtered and number of micro particles that falls out of the tissue was counted and the procedure was continued for 12 h [21,22].
In vitro drug release study
This study of release pattern for the selected formulations (F3, F8, F11) were carried out using an amber-coloured USP XXIV dissolution apparatus (TDT-08T, Electrolab) type-II (paddle) method for 12 h. Drug-entrapped microspheres (100 mg) were placed in 0.1 N HCl (900 ml, pH 1.2) solution maintained at 37±0.5° and stirred at 75 rpm. A known volume of dissolution sample was withdrawn each time and was replenished with the same volume of pre-warmed fresh dissolution media. The amount of simvastatin released was analysed in a UV/Vis spectrophotometer at 239 nm. The experiments were performed in triplicate.
Drug release kinetics were analysed with various mathematical models such as Higuchi, Korsemeyer- Peppas and zero order kinetics. The correlation coefficient values (r2) were used to determine how well it fits in a model and also evaluation of the drug release pattern [23,24].
Dissolution efficiency (DE)
DE of a pharmaceutical dosage form is defined as the area under the dissolution curve between defined time points [25]. DE was used to compare the dissolution profiles of three formulations F3, F8 and F11, considering the amount of drug dissolved in 12 h as the maximum. The following equation was used to characterize the % DE. % DE = (∫toy.dt/y100.t)×100.
Results and Discussion
The rationale of this work was to improve the therapeutic efficacy of simvastatin through preparing gastroretentive mucoadhesive microspheres. A gastroretentive mucoadhesive microspheres entrapped with simvastatin was developed by ultra-sonication combined with ionic gelation of sodium alginate. Ultrasound frequency of 20±3 KHz (130 W) with an input voltage range of 170-270 AC, 50 Hz, was applied to fabricate simvastatin-entrapped microsphere [26]. Biocompatible polymer, chitosan was employed to impart mucoadhesive character to the microspheres, thereby these could be retained in the stomach by adhesion to the mucosal wall. Drug release from these microspheres then would be continuous to mucosal tissues and eventually to get absorbed in to the systemic circulation. Simvastatin microspheres cross-linked with calcium ions help in the delivery of the drug for a prolonged period of time [4].
Many variables control the characteristics of drugentrapped microspheres. The impact of ultrasound waves on drug encapsulation efficiency, yield and particle size of microspheres was investigated in comparison to mechanical stirring method. The mucoadhesiveness and drug release kinetics from the microspheres were also characterized to understand efficiency of DDS [27].
Drug compatibility was explored using the FTIR method, and simvastatin was found to be stable in the presence of formulation ingredients (Figure 3). Frequencies of the functional groups were within the standard range (Table 2). Further, treatment with ultrasound waves did not modify the integrity of the drug substance; as was clearly evident from the DSC studies (Figure 4).
| Functional Group | IR Standard Range (cm-1) | Simvastatin (cm-1) | Simvastatin with sodium alginate (cm-1) | Simvastatin with chitosan (cm-1) |
|---|---|---|---|---|
| C=O stretch O-H stretch(H-bonded) C-H stretch C=C stretch C-O bend |
1760-1665 3500-3300 3000-2850 3100-3010 1150-1050 |
1704 3424 2893 3101 1134 |
1666 3397 2833 3083 1114 |
1666 3385 2899 3070 1121 |
Table 2: FTIR Spectra of Simvastatin and Physical Mixtures with Formulation Ingredients
Initially, in order to standardize the ultrasound frequency required to produce cavities in the polymeric structure, many trials were run employing ultrasound at the frequencies ranging from 20 to 33 KHz and at a fixed concentration of alginate solution. Ultrasound at the frequencies of 20±3 KHz produced small fissures and depressions on the surface of the sodium alginate solution (Figure 5). This effect enabled the polymeric structure to develop cavities [28]. Intense shock waves caused cavitation bubble to collapse violently inwards in the liquid leading to the formation of liquid jets of high speed. This resulted in the formation of numerous cracks and scratches on the polymer which in turn helped in the formation of mean droplet sizes in the range of micrometres [29]. The power required to disperse a liquid phase in to small droplets was rendered by the high, intensive ultrasound. Formation of cavities inside the polymeric structure enabled the microspheres to be uniform (Figure 6) and observed with highest drug entrapment. Therefore, the frequency of 20±3 KHz was chosen for further investigation.
Another investigation of this study was to understand the effect of duration of ultrasound waves on the properties of microspheres (Table 3). The microspheres were produced with varying the sonication time from 4 to 16 min and also varying the concentration of alginate. The production yield was largest (78.61± 2.07 %) when formulation was sonicated for 10 min. The lowest yield (55.23±1.25) was observed when sonicated for 4 min. The yield of the formulations F3, F8 and F11 was 70 % when sonicated for a period of 12 min. Because, ultrasound provided the local molecular vibration to disentangle polymer chains and slight reduction of molecular weight, both of which improved drug dispersion. Increasing the ultrasound power level increased the local power density in the polymer matrix and resulted in a more mechanochemical degradation that provided a greater reduction in tensile strength [30]. As a result the yield and encapsulation efficiency was significantly higher. Whereas, mechanical stirring failed to disentangle the polymeric chains and did not induce fissures and depressions on the surface of the polymer. It did not cause any local molecular vibrations and therefore failed to disentangle polymer chains. As a result, the yield and entrapment efficiency was found to be less in the case of F12, F13 and F14.
| Formulation Code | % Yield | Encapsulation efficiency | % Particles retained on tissue after 12 h | AUC | DE (%) |
|---|---|---|---|---|---|
| F1 F2 F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 F13 F14 |
55.23 ± 1.25 62.47 ± 0.15 71.56 ± 2.16 74.56 ± 1.85 58.45 ± 2.05 65.24 ± 3.08 67.13 ± 2.54 77.03 ± 1.58 70.31 ± 1.27 74.05 ± 2.65 78.61 ± 2.07 63.26 ± 0.15 65.12 ± 0.05 66.05 ± 1.41 |
48.08 ± 2.05 54.26 ± 2.12 61.26 ± 1.78 62.21 ± 3.01 51.24 ± 2.85 56.24 ± 2.45 64.51 ± 2.22 65.52 ± 1.09 52.18 ± 3.12 62.51 ± 3.54 71.58 ± 0.12 46.22 ± 1.25 49.25 ± 1.55 53.36 ± 1.32 |
56.25 ± 1.25 55.27 ± 2.02 69.24 ± 0.15 71.43 ± 0.35 61.34 ± 1.01 59.24 ± 1.23 79.32 ± 0.15 72.45 ± 1.15 76.54 ± 0.34 69.47 ± 1.23 76.23 ± 1.07 73.42 ± 2.07 76.34 ± 1.13 75.72 ± 1.12 |
450.21 ± 3.01 600.14 ± 2.11 702.00 ± 3.04 779.00 ± 6.54 526.31 ± 6.17 565.21 ± 3.73 711.31 ± 3.06 709.58 ± 4.11 760.21 ± 6.04 821.04 ± 3.21 920.41 ± 3.31 483.05 ± 3.28 503.12 ± 4.07 523.31 ± 6.08 |
66.59 ± 1.14 76.73 ± 1.16 83.66 ± 0.88 89.49 ± 2.13 71.60 ± 1.1 74.12 ± 1.12 77.70 ± 2.11 73.52 ± 2.12 75.85 ± 2.20 92.17 ± 2.14 96.88 ± 2.22 69.86 ± 2.09 69.25 ± 2.10 69.80 ± 1.12 |
Table 3: Formulation Properties of Simvastatin Microspheres
The drug encapsulation efficiency was found to be highest at an alginate concentration of 0.5 % w/v. Higher viscosity of the polymer solution owing to the increased polymer concentration decreased the drug on the external surface resulting in higher encapsulation efficiency [5] (Table 3). The encapsulation efficiency of simvastatin was found to be directly proportional to the concentration of the alginate, extent of its cross linkage with calcium ions and ultrasound time. Lower alginate concentrations reduced the viscosity of the mixture which lead to lower encapsulation. There was no appreciable increase in encapsulation efficiency upon increase in chitosan concentration as chitosan did not undergo ionic gelation.
The mucoadhesiveness of the microspheres also increased as the concentration of chitosan increased. The mucoadhesiveness of all the formulations was found to be above 60 % and lasted for more than 12 h. Mucoadhesiveness of the formulations with highest concentration of chitosan (F9, F10, and F11) was above 75 %. Therefore, the concentration of chitosan appeared to be of great significance in retaining microspheres in the upper gastric environment (Table 3).
Bulk flow, size distribution, surface properties were reported to directly affect the dissolution and drug release properties [31,32]. Therefore, to understand the effect of sonication and mechanical stirring on the micromeritic properties, formulations F3, F8, F11, F12, F13 and F14 were examined by deriving D10, D30, D60, D90 values and coefficients such as CU and CC (Table 4). The ultrasound has an appreciable effect on the size of the microspheres. Increased sonication frequency and time duration resulted in smooth and smaller sized particles. Lower alginate concentration in combination with increased sonication time (16 min) produced smaller and smoother microspheres. The combination of lower sonication time (4 min) and higher alginate concentration resulted in largesized, irregular and rough-surfaced microspheres. The percent fines obtained was significantly increased with increasing the sonication time. The size and surface characteristics of F3, F8 and F11 formulations were found to be smaller and smoother in comparison to F12, F13 and F14 as shown by the size distribution pattern in Figure 6.
| Formulation code | Parameter* | |||||
|---|---|---|---|---|---|---|
| D10 | D30 | D60 | D90 | CU | CC | |
| F3 F8 F11 F12 F13 F14 |
2.10 ± 0.04 2.05 ± 0.11 2.15 ± 0.13 2.01 ± 0.35 2.09 ± 0.06 2.0 ± 0.01 |
2.40 ± 0.12 2.35 ± 0.12 2.40 ± 0.26 2.24 ± 0.06 2.3 ± 0.12 2.27 ± 0.14 |
2.6 ± 0.01 2.55 ± 0.24 2.50 ± 0.24 2.68 ± 0.05 2.54 ± 0.32 2.59 ± 0.17 |
2.95 ± 0.28 3.0 ± 0.17 2.80 ± 0.16 2.81 ± 0.26 2.79 ± 0.28 2.88 ± 0.22 |
1.23 ± 0.08 1.24 ± 0.14 1.16 ± 0.14 1.33 ± 0.22 1.21 ± 0.26 1.29 ± 0.16 |
1.05 ± 0.24 1.06 ± 0.26 1.07 ± 0.04 0.92 ± 0.08 1.0 ± 0.16 0.99 ± 0.14 |
Table 4: Size Distribution of Microsphere of Selected Formulations
D10 referred to 10 % of the particles are finer and 90 % of the particles are coarser than that particular particle size D10. Similarly, D60 means diameter of the microparticles for which 60 % of the particles are finer and 40 % of the particles are coarser than D60. Hence in the present investigation, D10, D30, D60 and D90 values were used as measures of gradation. The D10, D30, D60, and D90 values obtained were within satisfactory range indicating that the % fines obtained were not to a greater extent. Therefore, the ultrasound of 20±3 KHz for 12 min duration was the optimum frequency and time to obtain microspheres with uniform and smaller sized microparticles.
Further, to ascertain the size distribution, the CU and CC values were verified. When the CU was less than 4, the microspheres were considered to be uniform in size or monodisperse. If the value of CU was greater than 4, then the microspheres were said to be graded or polydisperse. Another coefficient to measure size distribution is coefficient of gradation or coefficient of curvature (CC) [33]. For the particles to be monodisperse, the value of CU has to be smaller than 4 and CC should be around 1. CU and CC values were less than 4 and around 1, respectively, for the formulations F3, F8 and F11 indicating that they were monodisperse in nature.
Release studies were conducted for formulations F3, F8 and F11 in simulated gastric media for 12 h. Drug release from all of these formulations was observed to be sustained as shown in Figure 7. The pattern of drug release for formulation F11 was linear and satisfactory in comparison to F3 and F8. Interpretation of dissolution data through mathematical models showed that the drug release followed first order kinetics, which indicated that the release was concentration-dependent. Further interpretation of results using Higuchi model indicated non-Fickian diffusion to be the predominant mechanism of drug release with ‘n’ value at around 0.8 (Table 5). % DE was also found to be highest for formulation F11.
| Formulation code | Zero order | First order | Higuchi model | Peppa’s model | ||||
|---|---|---|---|---|---|---|---|---|
| Slope | r2 | Slope | r2 | Slope | r2 | n | r2 | |
| F3 F8 F11 F12 F13 F14 |
4.632 4.526 5.147 5.483 5.746 6.024 |
0.73 0.755 0.736 0.553 0.478 0.522 |
–0.033 –0.031 –0.044 –0.022 –0.022 –0.025 |
0.909 0.926 0.939 0.951 0.935 0.955 |
0.799 0.819 0.892 16.65 17.53 18.33 |
0.976 0.991 0.975 0.964 0.944 0.958 |
0.699 0.619 0.792 0.416 0.401 0.401 |
0.875 0.885 0.821 0.980 0.968 0.982 |
Table 5: Results of Kinetic Modeling of Drug Release Studies
Therefore, from the results obtained it could be concluded that the production of microspheres using ultrasound waves was found to be efficient in terms of simplicity, reproducibility and process time. Ultrasound exerted a great impact on the properties of the prepared microspheres. Scale up procedure and commercialization would be possible if ultrasound technique is employed for the production of microspheres. Simvastatin microspheres formulated with a simultaneous ultrasound effect and ionic gelation of the polymer showed a desirable drug content, good micromeritic properties, and adequate release characteristics.
Acknowledgment
Authors are thankful to the Management and the Principal, Acharya & BM Reddy College of Pharmacy, Bengaluru for providing the research facilities.
Conflicts of interest
The authors have no conflict of interest.
Financial support and sponsorship
Nil.
References
- Chemat F, Zill-e-Huma, Khan MK. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason Sonochem 2011;18(4):813-35.
- Sarvazyan AP, Rudenko OV, Nyborg WL. Biomedical application of radiation force of ultrasound: Historical roots and physical basis. Ultrasound Med Bio 2010;36(9):1379-94.
- Povey MJW, McClements DJ. Ultrasonics in food engineering. Part I: Introduction and experimental methods. J Food Eng 1988;8(4):217-45.
- Gong C, Hart DP. Ultrasound induced cavitation and sono chemical yields. J Acoust Soc Am 1998;104:1-16.
- Alzorqi I, Ketabchi MR, Sudheer S, Manickam S. Optimization of ultrasound induced emulsification on the formulation of palm-oleic based nano-emulsions for the incorporation of anti-oxidant β-D-glucose polysaccharides. Ultrason Sonochem 2016;31(1):71-84.
- O'Donnell CP, Tiwari BK, Bourke P, Cullen PJ. Effect of ultrasonic processing on food enzymes of industrial importance. Trends Food Sci Technol 2010;21:358-67.
- Sander JR, Zeiger BW, Suslick KS. Sonocrystallization and sonofragmentation. Ultrason Sonochem 2014;21(6):1908-15.
- Ruecroft G, Hipkiss D, Ly T, Maxted N, Cains PW. Sonocrystallization: the use of ultrasound for improved industrial crystallization. Org Process Res Dev 2005;9:923-93.
- Sørensen HT, Horvath-Puho E, Søgaard KK, Christensen S, Johnsen SP, Thomsen RW, et al. Arterial cardiovascular events, statins, low dose aspirin and subsequent risk of venous thromboembolism: a population-based case control study. J Thromb Haemost 2009;7(1):521-8.
- Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high dosage statin therapy in hyperlipidemic patients- The PRIMO study. Cardiovasc Drug Ther 2005;19(6):403-14.
- Louis D. Formulation and evaluation of nanocrystals of lipid lowering agent. Iran J Pharm Res 2016;15(1):71-82.
- Nath SD, Son S, Sadiasa A, Min YK, Lee BT. Preparation and characterization of PLGA microspheres by the electrospraying method for delivering simvastatin for bone regeneration. Int J Pharm 2013;443:87-94.
- Masaeli R, S Jafarzadeh Kashi T, Dinarvand R, Tahriri M, Rakhshan V, Esfandyari-Manesh M. Preparation, characterization and evaluation of drug release properties of simvastatin-loaded PLGA microspheres. Iran J Pharm Res 2016;15:205-11.
- Ali H, Nazzal S. Development and validation of a reversed-phase HPLC method for the simultaneous analysis of simvastatin and tocotrienols in combined dosage forms. J Pharm Biomed Anal 2009;49(1):950-6.
- Shu XZ, Zhu KJ. Chitosan/gelatin microspheres prepared by modified emulsification and ionotropic gelation. J Microencapsul 2001;18(2):237-45.
- Lemoine D, Wauters F, Bouchend’homme S, Preat V. Preparation and characterization of alginate microspheres containing a model antigen. Int J Pharm 1998;176(1):9-19.
- Chan L, Lee H, Heng P. Production of alginate microspheres by internal gelation using an emulsification method. Int J Pharm 2002;242(1-2):259-62.
- Nath B, Nath LK, Mazumder B, Kumar P, Sharma N, Sahu BP. Preparation and characterization of salbutamol sulphate loaded ethyl cellulose microspheres using water-in-oil-oil emulsion technique. Iran J Pharm Res 2010;9(2):97-105.
- Brittain H. Particle-Size Distribution, Part III-Determination by Analytical Sieving. Pharm Tech 2002;12(1):56-64.
- Yoshimatsu K, Reimhult K, Krozer A, Mosbach K, Sode K, Ye L. Uniform molecularly imprinted microspheres and nanoparticles prepared by precipitation polymerization: The control of particle size suitable for different analytical applications. Anal Chim Acta 2007;584(1):112-21.
- Wei Y, Wang Y, Zhang H, Zhou W, Ma G. A Novel Strategy for the Preparation of Porous Microspheres and Its Application in Peptide Drug Loading. J Colloid Interface Sci 2016;478:46-53.
- Dandagi PM, Mastiholimath VS, Gadad AP, Iliger SR. Mucoadhesive microspheres of propranolol hydrochloride for nasal delivery. Indian J Pharm Sci 2007;1(1):402-7.
- Enriquez GG, Rizvi SAA, D’Souza MJ, Do DP. Formulation and evaluation of drug-loaded targeted magnetic microspheres for cancer therapy. Int J Nanomedicine 2013;8:1393-402.
- Sezer AD, Akbuğa J. Comparison on in vitro Characterization of Fucospheres and Chitosan Microspheres Encapsulated Plasmid DNA (pGM-CSF): Formulation Design and Release Characteristics. AAPS PharmSciTech 2009;10(4):1193-9.
- Nagpal M, Maheshwari D, Rakha P, Dureja H, Goyal S, Dhingra G. Formulation Development and Evaluation of Alginate Microspheres of Ibuprofen. J Young Pharm 2012;4(1):13-6.
- Costa P, Lobo JMS. Modelling and comparison of dissolution profiles. Eur J Pharm Sci 2001;13:123-33.
- Gulseren I, Guzey D, Bruce BD, Weiss J. Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason Sonochem 2007;14:173-83.
- Dandagi P, Kerur S, Mastiholimath V, Gadad A, Kulkarni A. Polymeric ocular nanosuspension for controlled release of acyclovir: in vitro release and ocular distribution. Iran J Pharm Res 2009;8:79-86.
- Mortazavi SA, Mokarram AR. Preparation and evaluation of diphtheria toxoid-containing microspheres. Iran J Pharm Res 2004;3:133-43.
- Cavegn M, Douglas R, Akkermans G, Kuentz M. Study of an ultrasound based process analytical tool for homogenization of nanoparticulate pharmaceutical vehicles. J Pharm Sci 2011;100:3374-85.
- Dalmoroab A, Barbaa AA, Lambertib G, d’Amore M. Intensifying the microencapsulation process: Ultrasonic atomization as an innovative approach. Eur J Pharm Biopharm 2012;80:471-7.
- Kim H, Lee J, Won YY. A simple derivation of the critical condition for the ultrasonic atomization of polymer solutions. Ultrasonic 2015;61:20-4.
- Darji M, Rahul L, Pradhan A. Applications of Sonochemistry in drug delivery and formulations: A review. Int J Appl Biol Pharm Tech 2015;6:142-8.
 F3;
F3;  F8;
F8;  F11;
F11;  F12;
F12; F13;
F13;  F14
F14 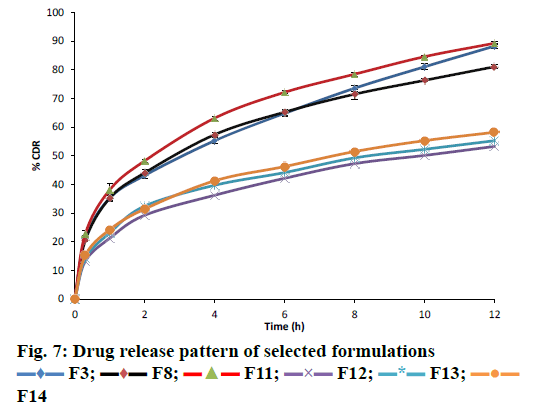
 F3;
F3; F8;
F8; F11;
F11; 



