- Corresponding Author:
- Purnima Amin
Pharmaceutical Sciences and Technology Division, Institute of Chemical Technology, University of Mumbai, Matunga, Mumbai - 400 019, India. E-mail: dramin@vsnl.net
| Date of Submission | 19 July 2006 |
| Date of Revision | 27 June 2007 |
| Date of Acceptance | 25 July 2007 |
| Indian J Pharm Sci, 2007, 69 (4): 562-567 |
Abstract
Acyclovir, an antiviral is used in the treatment of ocular infections. Acyclovir is effective against human herpes viruses including Herpes simplex virus type 1 and 2, Varicella Zoster virus, Epstein-Barr virus and Cytomegalovirus. Acyclovir is available as a 3%w/w eye ointment to be applied 5 times a day in the eye. The present investigation was aimed at designing a twice a day ocular inserts of acyclovir by melt extrusion technique to improve patient compliance, using hydroxypropylcellulose as a thermoplastic polymer. Also, the developed formulation would overcome the greasy nature of the eye ointment. The developed inserts were stable, non-irritant and provided release of the drug over a period of 10 hours in vitro .
Keywords
Ocular insert, hot-melt extrusion, hydroxypropylcellulose, Klucel®
Topical ophthalmic application is considered the preferred way to achieve therapeutic levels of drugs used to treat ocular diseases [1]. The conventional preparations for this route are solutions, suspensions, semisolids like ointments, etc. Bioavailability, particularly for ocular solutions ranges from 1-10% of the total administered dose. This is due in part to the rapid precorneal clearance kinetics resulting from reflex tearing and blinking, where half-life times of instilled isotonic solutions or suspensions approximate only 15 s in humans. The problem associated with the use of ophthalmic ointment is poor patient acceptance [2]. To overcome these problems, various novel ophthalmic delivery systems such as inserts, in situ gels, etc have been investigated in an attempt to extend the ocular residence time of medication for topical application to the eye.
Melt extrusion is a technique in which during extrusion, a polymer melt is pumped through a shaping die and formed into a profile. This profile can be a plate, a film, a tube, or have any shape of its cross section [3]. The process often is referred to as profile or line extrusion in which the shape of the extrudate like a tube is determined by the die. The extruded profile proceeds horizontally to the cutoff equipment, which controls its length. Profiles may be further processed, for example, as in film extrusion, blow molding, or injection molding. In film extrusion, the polymer melt is extruded through a long slit die onto highly polished cooled rolls which form and wind the finished sheet. This is known as cast film [4].
Melt extrusion technology has been exploited in polymer industries since 1930′s [5]. Since then it has been extensively used in polymer [6], food [7,8], chemical [9], rubber [10] and metal industries [11]. In pharmaceutical industries this technology is also exploited in preparation of pellets [12,13], solid dispersion [14-16], topical dosage forms [17], powder coating [18], gastroretentive dosage forms [19], tablets [20] and sustained release oral dosage forms [21-23]. However this technique has not yet been exploited in preparation of sustained release ophthalmic formulations.
Acyclovir has an in vitro and in vivo inhibitory activity against human herpes viruses including Herpes Simplex virus (HSV) type 1 and 2, Varicella Zoster virus, Epstein→Barr virus and Cytomegalovirus [24]. Acyclovir is available as a 3% w/w eye ointment to be applied 5 times a day in the eye [25]. The objective of the present investigation was to prepare long acting ocular inserts of acyclovir to be placed in the eye twice a day, by melt extrusion technique.
Materials and Methods
Acyclovir was obtained as a gift sample from Cipla Limited. Methocel® and Starch 1500 were obtained as a gift sample from Colorcon Asia Pvt. Ltd. Klucel® was gifted by Signet Chemical Corporation. All other chemicals and solvents used were of analytical grade.
Formulation considerations
Extrusion of each plain polymer Methocel® A (methylcellulose), Methocel® E and Methocel® K (hydroxypropylmethylcellulose), Klucel® (hydroxypropylcellulose, HPC), Natrosol® (hydroxyethylcellulose) and Starch 1500 were carried out on Melt Flow Rate Apparatus, Model 3/80, Davenport. Extrusion dies of dimensions 1 mm, 1.2 mm and 1.5 mm were tried to afford the product suitable for instillation in the eye. The polymer used for the study was medium viscosity grade Klucel® GFF. The dose of Acyclovir was calculated so that an ocular insert for twice a day use could be fabricated using the technique of melt extrusion. Plasticizers such as propylene glycol, glycerine and polyethylene glycol 400 were tried as they are nonirritant for ocular use and their concentrations were optimised.
Method of preparation of ocular insert
Acyclovir and the polymer were sieved through 60#, weighed and blended geometrically. The plasticizer was added and blended. The blend was then charged to the barrel of Melt Flow Rate apparatus and extruded. The extrudate was cut into appropriate size of 4.5 mm × 1 mm and packed in polyethylene lined aluminium foil (thickness 100 µ), heat sealed and sterilized by gamma radiation (2.5 Mrad for 4 h).
Evaluation of insert
The developed inserts were evaluated for several parameters viz. appearance, uniformity of weight, dimensions, drug content, uniformity of content, Differential Scanning Calorimetric (DSC) analysis, eye irritation test and in vitro release studies. The inserts were observed for appearance/elegance, colour, surface irregularities, air bubbles, tackiness and suitability for ocular use. Twenty inserts were weighed and the average weight was determined. Deviation of individual insert′s weight with respect to average weight was determined. Three inserts from a batch were powdered and dissolved in 50 ml of purified water by stirring on a magnetic stirrer for 2 h. The absorbance of each of these solution was then measured on a Jasco V530 UV/Vis Spectrophotometer at 251 nm. The concentration was extrapolated from the standard curve. Six inserts from a batch were individually crushed and dissolved in 50 ml of purified water. The absorbance of this solution was then measured spectrophotometrically at 251 nm. The concentration was extrapolated from the standard curve.
Differential scanning calorimetric (DSC) analysis
DSC of the selected samples was carried out to study the thermal behaviour under specified conditions. Each sample was heated over the temperature range from ambient to 425° at a heating rate of 10°/min under nitrogen environment (20 ml/min). The instrument used was Perkin Elmer Differential Scanning Calorimeter. Thermograms were integrated using Pyris 6 software.
Ocular irritation test
Ocular irritation studies were performed according to the Draize technique. Assessment of ocular irritation potential of ophthalmic formulations is an extremely important step in the development of ophthalmic formulations. The test has been standardized at the international level, e.g. using the OECD guideline No.40526. Acute eye irritation/corrosion and is the most widely used test for classification and labelling of chemicals according to their ocular safety. Six female rabbits each weighing 2-3 kg were used for the study of the formulations. The sterile formulations were placed twice a day for a period of 21 d and the rabbits were observed periodically for redness, swelling and watering of the eyes.
in vitro release studies
The in vitro release studies were performed in a modified dissolution apparatus as per USP specification27. The dissolution conditions were: temperature was kept at 37±1°, horizontal amplitude was 3.8 cm and frequency was set at 32 cycles/min. Each insert was tied in muslin cloth and was placed in the test tube containing 10 ml dissolution medium with the help of the hanger, in triplicates. Aliquots were withdrawn at 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 h. The aliquots were suitably diluted and analysed by spectrophotometrically at 252 nm. The % cumulative release of the drug was computed and graph of % cumulative release vs. time was plotted.
Sterilisation studies
The inserts were packed in polyethylene lined aluminium foil (thickness 100 µ), heat sealed and sterilized by gamma radiation (2.5 Mrad for 4 h). Radio sterilised inserts were evaluated for appearance, uniformity of weight, dimensions, content and uniformity of content, in vitro release profile, DSC characterisation and sterility testing
Accelerated stability studies
The optimized formulation in its final pack was stored at ambient conditions, 30±2°/65±5% RH and 40±2°/75±5% RH. Sampling was done at 0, 1, 2 and 3 mo and the formulations were evaluated for physical parameters, in vitro release, sterility and drug content.
Results and Discussion
Melt extrusion offers the advantages of being a single step, simple, continuous process with relatively high throughput rates. It provides the facility of mixing inside the extruder body thus bypassing problem of segregation during premixing. It obviates the need for organic solvents in processing and circumvents associated hazards. Also, it obviates the need for water and hence can work for water sensitive drugs. Also, there is no time consuming drying step involved. The bioavailability of the drug substance could be improved when it is dispersed at the molecular level in hot-melt extruded dosage forms.
Of the various polymers evaluated for melt extrusion, only all grades of Klucel® could be melt extruded. Methocel® A, Methocel® E, Methocel® K, Natrosol® and Starch 1500 could not be melt extruded. Polymers were extruded using different die of diameters 1, 1.2 and 1.5 mm at 122-128°. The compression force required to extrude the polymer was found to be 15 kg for 1.5 mm die diameter and was 21.5 kg for 1 mm and 1.2 mm die diameter, respectively. However, smallest diameter (1 mm) die was chosen for further studies after considering the size of the marketed formulation i.e. Lacrisert® (dimension: 5 mm×1.16 mm). The same die was used for further studies. Acyclovir is available as a 3%w/w ointment to be placed in the eye five times a day as a 1cm ribbon each25. The weight of such 5 ribbons approximates 66 mg of the ointment that contains around 2 mg of acyclovir daily. Hence, it was decided to formulate ocular inserts containing 1 mg of acyclovir for twicedaily use.
Plasticizers are normally used with polymers in melt extrusion to ensure smooth, uniform melt flow and flexible, homogeneous end products. The advantages of plasticizers in melt extrusion are lower processing temperatures and ease of manufacturing [3]. The effect of plasticizers at a concentration of 5% w/w viz. propylene glycol, polyethylene glycol and glycerine on processing conditions using Klucel® GFF was as shown in Table 1. The incorporation of the plasticizer was found to reduce the processing temperature as well as the compression force for melt extrusion. Propylene glycol was chosen as a plasticizer for further studies since it afforded a lowest processing temperature and therefore its concentration was optimised using Klucel® GFF as a melt extrudable polymer. The results are as depicted in Table 2. Propylene glycol was optimised as a plasticizer at a concentration of 5% w/w.
| Plasticizer | Concentration % w/w | Polymer | Extrusion temperature (°) | Compression force (kg) | Appearance |
|---|---|---|---|---|---|
| Propylene glycol | 5 | Klucel® GFF | 104 | 5 | Good |
| Glycerine | 5 | Klucel® GFF | 116 | 5 | Good |
| Polyethylene glycol 400 | 5 | Klucel® GFF | 120.8 | 5 | Good |
Table 1: Selection of plasticizer for extrusion
| Plasticizer concentration | Polymer | Extrusion temperature (°) | Compression force (kg) | Appearance |
|---|---|---|---|---|
| 2.5% w/w | Klucel® GFF | 105.3 | 5 | Brittle |
| 5% w/w | Klucel® GFF | 104 | 5 | Good |
| 7.5% w/w | Klucel® GFF | 102.5 | 5 | Tacky |
Table 2: Optimization of concentration of propylene glycol as a plasticizer
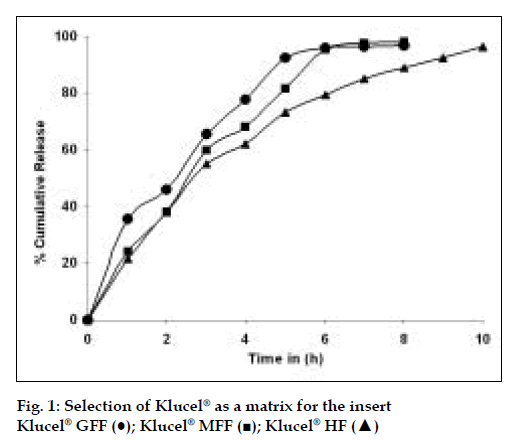
Klucel® GFF, Klucel® MFF and Klucel® HF (Table 3) were tried as a matrix for the insert along with propylene glycol (5%w/w) as the plasticizer and the polymer that afforded a desired release profile of more than 90% at the end of 10 h, in an in vitro dissolution study was selected as the optimum formulation. Klucel® HF gave the desired release profile. The results are as depicted in fig. 1.
The drug release from the Klucel® matrices was found to exhibit first order kinetics. This can be exhibited by the value of R and the values for T25, T50, T75 and T90% for the drug release from the matrix as shown in Table 3
| Ingredients Formulation | Quantity per insert | ||||
|---|---|---|---|---|---|
| GFF | MFF | HF | |||
| Acyclovir | 1.00 mg, | 1.00 mg | 1.00 mg | ||
| Propylene glycol | 0.30 mg (5% w/w) | 0.30 mg (5% w/w) | 0.30 mg (5% w/w) | ||
| Klucee GFF q.s. | 6.00 mg | - | - | ||
| Klucell' MFF q.s. | - | 6.00 mg, | - | ||
| Klucel'& HF q.s. | - | - | 6.00 mg | ||
| Extrusion Temperature (°C) | 104.0 | 114.0 | 115.8 | ||
| Compression Force (Kg). | 5 | 5 | 5 | ||
| Observations | Good | Good | Good | ||
| Release kinetics | |||||
| R-value First | -0.978 | -0.9748 | -0.98978 | ||
| Zero | 0.9753 | 0.9732 | 0.9752 | ||
| Higuchi | 0.9744 | 0.974 | 0.9744 | ||
| Time in hours | |||||
| T25 | 0.9 | 1.377 | 1.383 | ||
| T50 | 1.72 | 2.12 | 2.69 | ||
| T75 | 3.12 | 3.38 | 4.95 | ||
| T90 | 4.97 | 5.04 | 7.9 | ||
Table 3: Optimization of grade of klucel®
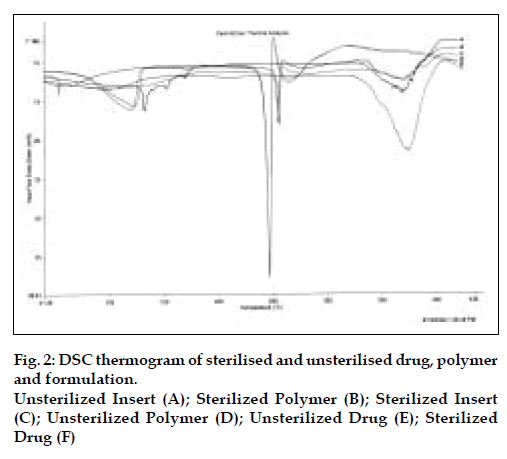
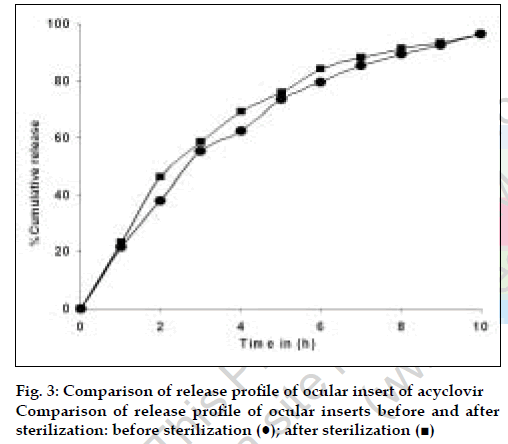
The results of the evaluation of the ocular inserts of acyclovir are as depicted in Table 4. There were no changes in the quality control parameters of the insert before and after sterilization. Similarly, no change was observed in the release kinetics before and after sterilization as depicted in Table 4. The DSC studies confirm that there is no degradation of the drug as evident by the absence of any additional peaks in the DSC Thermograms. As illustrated in fig. 2 the reduction in the peak area of DSC thermogram of insert confirms the partial solubilisation of the drug in the polymer.
| Parameter | Results | |
|---|---|---|
| Before Sterilization | After Sterilization | |
| Appearance | White and smooth devoid of air bubbles | White and smooth devoid of air bubbles |
| Uniformity of weight (mg) ±SD | 5.97±0.17 | 5.892±0.21 |
| Diameter (mm) ±SD | 1.23 ± 0.0295 | 1.22±0.0286 |
| Length (mm) ±SD | 4.56±0.22 | 4.52±0.235 |
| Content % | 99.56 | 99. |
| Content uniformity ±SD | 99.38±1.4 | 99.83±1.94 |
| Ocular irritation test | Non irritant | |
| Differential Scanning Calorimetric (DSC) analysis | As shown in the fig. 3 | As shown in the fig. 3 |
| In vitro release | More than 90% release at the end of 10 hours (fig. 4) | More than 90% release at the end of 10 hours (fig. 4) |
| Sterility testing | Sterile | |
| Release kinetics | ||
| R First | -0.9898 | -0.9989 |
| Zero | 0.9752 | 0.9792 |
| Higuchi | 0.9744 | 0.9700 |
| Time in hours | ||
| T25 | 1.383 | 1.03 |
| T50 | 2.69 | 2.35 |
| T75 | 4.95 | 4.62 |
| T90 | 7.9 | 7.61 |
Table 4: Evaluation of the inserts
No irritation was observed in the rabbit eye during ocular irritation test. The overall irritation was found to be 4 out of 110 on the scale of scores for reading the severity of ocular lesions given by OECD guidelines no. 40526. It was also observed that after 12 h, the inserts completely dissolved in eye indicating biodegradable nature of the inserts.
Stability studies were carried out at ambient conditions, 30°±2°/65% RH±5%, 40°±2°/75% RH±5% for a period of 3 mo. The formulation was found to be stable, sterile and the drug content was found to be within limits.
The technique of melt extrusion was applied to the fabrication of acyclovir ocular inserts as solid polymeric rods to be placed in the cul-de-sac of the eyes. These inserts were retained in the eye for required period of time and sustained the release of the drug for 10 h. The polymer slowly released the drug via swelling and dissolved slowly in the tear fluid, thus avoiding the need to remove insert after drug administration. Further, the polymer is also non-greasy, thus potentially increasing patient acceptability.
Acknowledgements
Authors are grateful to UGC for providing junior research fellowship.
References
- Desai, S.D. and Blanchard, J., In: Swarbrick, J. and Boylan J.C., Eds., Ocular drug delivery systems. Vol. 11, Marcel Dekker Inc., New York, 1995, 43.
- Olejnik, O. and Mitra, A.K., Eds., In; Ophthalmic drug delivery systems. Marcel Dekker Inc., New York, 1993, 1.
- Mcginity, J.W., Koleng, J.J., Repka, M.A. and Zhang, F., In: Swarbrick, J. and Boylan, J.C., Eds., Encylopedia of Pharmaceutical Technology. Vol. 19, Marcel Dekker Inc., New York, 2000, 203.
- Breitenbach, J., Euro. J. Pharm. Biopharm., 2002, 54, 107.
- Chokshi, R. and Hossein, Z., Iranian J. Pharm. Res., 2004, 3, 3.
- Dangtungee, R., Desai, S.S., Tantayanon, S. and Supaphol, P., Polymer Testing, 2006, 10, 888.
- Bengoechea, C., Arrachid, A., Guerrero, A., Hill, S.E. and Mitchell J.R.,J. Cereal Sci., 2007, 5, 275.
- Babin, P., Valle, G.D., Dendievel, R., Lourdin, D. and Salvo, L.,Carbohyd. Polym., 2007, 68, 329.
- Chen, L., Pang, X.J. and Yu, Z.L., Mater. Sci. Eng. A, 2007, 457, 287.
- Griffon, J.M., Constr. Building Mater., 1988, 2, 73.
- Tang, Y., Tan, D., Li, W., Pan, Z., Liu, L. and Hu, W., J. Alloys Compounds, 2007, 439, 103.
- Mehuys, E., Vervaet, C. and Remon, J.P., J. Control. Release, 2004, 94, 273.
- Siepmann, F., Muschert, S., Flament, M.P., Leterme, P., Gayot, A. and Siepmann, J., Int. J. Pharm., 2006, 317, 136.
- Ghebremeskel, A.N., Vemavarapu, C. and Lodaya, M., Int. J. Pharm., 2007, 328, 119.
- Patterson, J.E., James, M.B., Forster, A.H., Lancaster, R.W., Butler, J.M. and Rades, T., Int. J. Pharm., 2007, 336, 22.
- Verreck, G., Decorte, A., Heymans, K., Adriaensen, J., Liu, D. and Tomasko, D., Int. J. Pharm., 2006, 327, 45.
- Mididoddi, P.K. and Repka, M.A., Euro. J. Pharm. Biopharm., 2007, 66, 95.
- Sauer, D., Zheng, W., Coots, L.B. and McGinity J.W., Euro. J. Pharm. Biopharm., 2007 (Article in press)
- Fukuda, M., Peppas, N.A. and McGinity, J.W., J. Control. Release, 2006, 115, 121.
- Fukuda, M., Peppas, N.A. and McGinity, J.W., Int. J. Pharm., 2006, 310, 90.
- Lyons, J.G., Devine, D.M., Kennedy, J.E., Geever, L.M., O.Sullivan, P. and Higginbotham, C.L., Euro. J. Pharm. Biopharm., 2006, 64, 75.
- Verhoeven, E., Vervaet, C. and Remon, J.P., Euro. J. Pharm. Biopharm., 2006, 63, 320.
- Lyons, J.G., Hallinan, M., Kennedy, J.E., Devine, D.M., Geever, L.M., Blackie, P. and Higginbotham, C.L., Int. J. Pharm., 2007, 329, 62.
- Mandell, G.L., Alfred G.G., editor. In: The Pharmacological basis of Therapeutics, 9th ed., 1996, 1193.
- Product Information Leaflet of Cipla given in the Acivir® eye ointment pack.
- OECD (1981, 1987, 1994) Guidelines for testing the chemicals: Guideline No. 405, .Acute eye irritation/corrosion. OECD Publication Office, Paris, 1981, 1987, 1994.
- Dissolution, In: The United States Pharmacopoeia XXIII, Asian Edition, The United States Pharmacopoeial Convention, Inc., Rockville, Maryland, U.S.A., 1995, 1227.