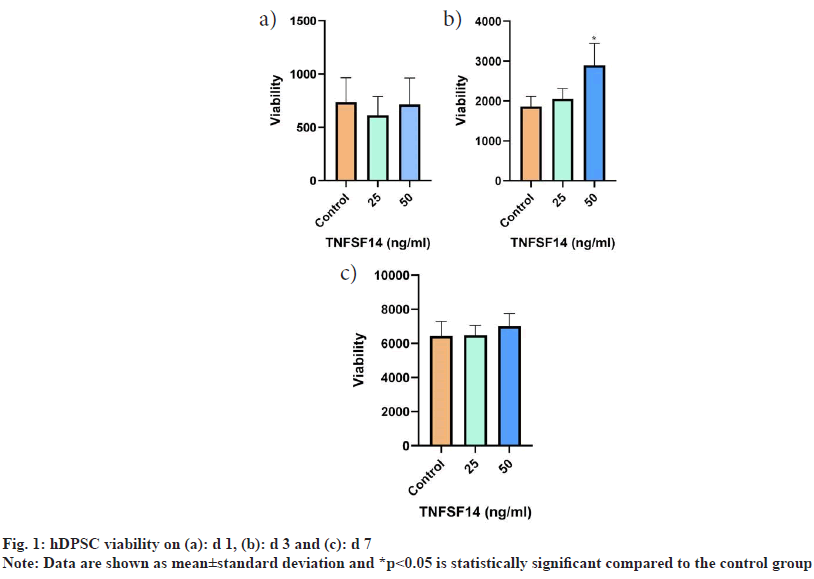
- *Corresponding Author:
- Solaiman Al-Hadlaq
Department of Restorative Dental Sciences, College of Dentistry, King Saud University, Riyadh 11545, Saudi Arabia
E-mail: salhadlaq@ksu.edu.sa
| This article was originally published in a special issue,“Integrative Approaches in Biomedical Sciences for Drug Discovery and Development” |
| Indian J Pharm Sci 2024:86(6) Spl Issue “85-91” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study aimed to evaluate the effect of tumor necrosis factor superfamily 14 on the viability and differentiation of human dental pulp stem cells. Human dental pulp stem cells were treated with 25 ng/ ml and 50 ng/ml tumor necrosis factor superfamily 14. Cell viability, alkaline phosphatase activity and messenger ribonucleic acid of odontogenic markers were assessed via quantitative reverse transcription polymerase chain reaction. Mineral deposit formation was determined using alizarin red staining. Statistical analysis was conducted using a one-way analysis of variance parametric test. Results were considered statistically significant if the p value was <0.05. Tumor necrosis factor superfamily 14 at 50 ng/ml significantly enhanced human dental pulp stem cells viability on d 3 and alkaline phosphatase activity on d 7 and d 14 compared with the control group. Tumor necrosis factor superfamily 14 (50 ng/ml) significantly upregulated the messenger ribonucleic acid expression of odontogenic markers including alkaline phosphatase, osteocalcin, dentin sialophosphoprotein and dentin matrix protein-1. Runt-related transcription factor 2 expression was significantly increased by treatment with 25 ng/ml tumor necrosis factor superfamily 14. The treatment groups showed higher expression of osteopontin messenger ribonucleic acid, but the difference was not statistically significant. Furthermore, an increased formation of mineral deposits was observed in the tumor necrosis factor superfamily 14-treated groups after alizarin red staining. Tumor necrosis factor superfamily 14 promotes human dental pulp stem cells expression of odontogenic differentiation markers and formation of calcific deposits.
Keywords
Calcific deposits, differentiation, human dental pulp stem cells, tumor necrosis factor superfamily 14
Dental pulp is located within a solid chamber consisting of dentin, enamel and cementum. This chamber offers firm mechanical support and protects the dental pulp from a microorganism-filled oral environment[1]. Pulp and dentin function physiologically as a unit called the pulp-dentin complex. This complex is a dynamic tissue that responds to mechanical, bacterial or chemical irritation to decrease its effects [2].
In cases where the dental pulp is exposed due to dental caries, root canal treatment has been considered as the treatment of choice; however, there has been increased interest in procedures that attempt to preserve pulp vitality using Vital Pulp Therapy (VPT) techniques. These procedures included direct pulp capping, partial pulpotomy and complete pulpotomy[3]. VPT has shown increased success in recent years[4-6]. VPT depends on recruiting undifferentiated stem cells for differentiation into cells involved in regenerating lost dentin pulp complex tissues[7].
Hard tissue formation on the site of pulp injury is considered a sign of successful management in vivo; therefore, in vitro studies use various markers to evaluate successful stem cells differentiation to odontoblast-like cells when exposed to different materials[8]. Several markers and transcription factors are involved in stem cell differentiation into odontoblasts and odontoblast-like cells. These markers include Dentin Matrix Protein 1 (DMP-1), Dentin Sialophosphoprotein (DSPP), Osteopontin (OP), Osteocalcin (OC), Alkaline Phosphatase (ALP) and Runt-related transcription factor 2 (RUNX 2) [9-11]. Interestingly, most markers of odontoblastic differentiation such as ALP, OC and RUNX2 are shared with osteoblastic differentiation[12].
Multiple biomaterials have been used in VPT, ranging from calcium hydroxide to the more recent different forms of calcium silicates, to promote undifferentiated stem cell differentiation into odontoblast-like cells[6,13]. Although considerable success has been achieved, the quality of newly formed tissue differs from that of the original physiological structures. In fact, the newly formed dentin bridge in VPT is made of osteodentin with irregular tubular dentin and some tunnel defects[14].
Despite the availability of multiple biomaterials, the endodontic field still lacks a material that promotes high-quality regeneration of the pulp-dentin complex with reduction or complete resolution of the inflammatory process. Therefore, finding a material that promotes odontoblast differentiation while reducing the inflammatory response at the site of tissue regeneration could potentially improve the observed outcome.
Tumor Necrosis Factor Superfamily 14 (TNFSF14) also known as Lymphotoxin-like Inducible proteins that competes with Glycoprotein D for binding to Herpes virus entry mediator on T cells (LIGHT), is an immunomodulatory cytokine that is a member of the tumor necrosis factor superfamily[15]. TNFSF14 modulates innate and adaptive immune responses by promoting the homeostasis of lymphoid organs, liver and bone[16]. When used on human Bone Marrow Mesenchymal Stem Cells (BM-MSCs) it enhances survival, proliferation, differentiation into osteocytes and osteogenesis[17]. Interestingly, when TNFSF14 was added to BM-MSCs, markers associated with both osteogenesis and odontogenesis were upregulated[12,17].
To date, no study has investigated the effect of TNFSF14 on the odontogenic differentiation of human Dental Pulp Stem Cells (hDPSCs). Therefore, this study aimed to evaluate the effect of TNFSF14 on the viability and odontogenic differentiation of hDPSCs. The null hypothesis that guided this work was TNFSF14 had no effect on odontoblastic differentiation of hDPSCs.
Materials and Methods
Cell culture:
hDPSCs purchased from Lonza (Basel, Switzerland; Catalog No.: PT-5025) were expanded and cultured in alpha-modified Minimum Essential Medium (α-MEM) (Thermo Fisher Scientific, Waltham, United States of America) supplemented with 10 % Fetal Bovine Serum (FBS) (Thermo Fisher Scientific), 1 % penicillin/ streptomycin and 1 % MEM Non-Essential Amino Acids (NEAA) solution, subsequently referred to as supplemented α-MEM. All cells were cultured at 37° at 5 % CO2 and 95 % humidity. Cells were transferred to 96, 24 and 12-well plates with the supplemented α-MEM once they reached 90 % confluency in advance of the subsequent experiments. Passages 4-6 of hDPSCs were used in the experiments.
Reagents:
Recombinant human TNFSF14 (R&D Systems, Minneapolis, United State of America; Catalog No: 664-LI-025), was dissolved in 0.1 % Bovine Serum Albumin (BSA)-Phosphate-Buffered Saline (PBS) and stored at -20° until use according to the manufacturer’s instructions.
Osteogenic medium preparation:
The Osteogenic differentiation Medium (OM) consisted of α-MEM supplemented with 10 % FBS, 1 % penicillin-streptomycin, 50 μg/ml L-ascorbic acid (Wako Chemicals, Neuss, Germany), 10 nmol/l calcitriol 1α,25-dihydroxy vitamin D3 (Sigma-Aldrich, Burlington, United State of America), 10 mmol/l β-glycerophosphate (Sigma-Aldrich) and 10 nmol/l dexamethasone (Sigma-Aldrich).
Cellular viability:
Alamar blue assay: The effect of TNFSF14 on the viability of hDPSCs was evaluated at 25 ng/ml and 50 ng/ml on d 1, d 3 and d 7 using an Alamar blue assay according to standard protocols[18]. Briefly, the cells were seeded in 96-well plates at a density of 5×103 cells/well. At the end of each time point, 10 % AlamarBlueTM reagent (Bio-Rad Inc. Hercules, United States of America) was added to each well, after 1 h of incubation, fluorescence was measured at an excitation and emission wavelength of 530 nm and 590 nm, respectively using the SpectraMax® M5/ M5e Multimode Plate Reader (Molecular Devices, San Jose, United States of America). Data were collected using SoftMax® pro 6 microplate data acquisition and analysis software (Molecular Devices). Experiments were performed in duplicate.
Evaluation of odontogenesis:
ALP assay: hDPSCs were seeded into a 24-well plate in α-MEM with a density of 5×104 cells/well for ALP activity. After cells reached a confluency of 90 %, TNFSF14 was added to each group with α-MEM for 24 h. The following day, the cells were cultured in OM supplemented with 25 ng/ml or 50 ng/ml TNFSF14. ALP activity was evaluated on d 7 and d 14[19]. ALP Diethanolamine Activity Kit (Thermo Fisher Scientific) was used to quantify ALP activity by absorbance level measured at 405 nm by the SpectraMax® M5/M5e Multimode Plate Reader (Molecular Devices). The ALP assay was conducted in two independent experiments with three samples per group.
Calcified deposits evaluation:
Alizarin red staining: Alizarin red staining was conducted on d 14 and d 21 according to standard protocols[17]. Briefly, hDPSCs were exposed to 25 ng/ml and 50 ng/ml TNFSF14 in 24-well plates at a density of 1×105 cells/well. Wells were rinsed and then fixed with 4 % paraformaldehyde followed via incubation with 2 % Alizarin red stain (ScienCell Research Laboratories, Carlsbad, United States; Catalog No.: 0223) for 30 min at room temperature and then washed with distilled water three times for 5 min each time. Light microscopy images were recorded and assessed by two blinded examiners in each group. Alizarin red staining was performed in duplicate.
Quantitative reverse transcription polymerase chain reaction:
The hDPSCs were seeded in 12-well plates in OM supplemented with 25 ng/ml and 50 ng/ml TNFSF14 at a density of 2×105 cells/well. On d 7, the cells were collected and Ribonucleic Acid (RNA) lysis buffer was added to the cell pellet. RNA was isolated using the RNeasy Mini Kit (RNeasy; Qiagen, Hilden, Germany) and quantified using a Nanodrop spectrophotometer (Nanodrop 2000, Thermo Fisher Scientific)[20]. The extracted RNA was reverse-transcribed using a High-Capacity complementary Deoxyribonucleic Acid (cDNA) Reverse Transcription kit (Thermo Fisher Scientific) and cDNA was synthesized using a Multigene thermocycler (Labnet International, Inc., Edison, United States of America). Messenger RNA (mRNA) expression was analyzed using fast SYBRTM Green polymerase chain reaction master mix (Thermo Fisher Scientific) under the following thermal conditions according to the manufacturer; 95° for 12 min followed by 40 cycles of 95° for 15 s, 65° for 30 s, and 72° for 30 s. The primer sequences used for ALP, RUNX-2, OC, OP, DSPP and DMP-1 (OligoTM, Seoul, South Korea) are listed in Table 1. Experiments were conducted in duplicate. Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) was used as an endogenous control. The data was normalized using the comparative delta-delta Cycle threshold (Ct) method (2-ΔΔ CT). This normalization is represented by the relative fold change in percentage compared to the values obtained from the control group[21].
| Gene | Sequence (5´-3´) |
|---|---|
| GAPDH | Sense (Forward primer) |
| 5`-CTGGTAAAGTGGATATTGTTGCCAT-3` | |
| Antisense (Reverse primer) | |
| 5`-TGGAATCATATTGGAACATGTAAACC-3` | |
| ALP | Sense (Forward primer) |
| 5`-GACGGACCCTCGCCAGTGCT-3` | |
| Antisense (Reverse primer) | |
| 5`-AATCGACGTGGGTGGGAGGGG-3` | |
| RUNX-2 | Sense (Forward primer) |
| 5`-ACGIGGCTAAGAATGTCATC-3` | |
| Antisense (Reverse primer) | |
| 5`-CTGGTAGGCGATGTCCTTA-3` | |
| OC | Sense (Forward primer) |
| 5`-GGCAGCGAGGTAGTGAAGAG-3` | |
| Antisense (Reverse primer) | |
| 5`-CTCACACACCTCCCTCCTG-3` | |
| OP | Sense (Forward primer) |
| 5'-CAGTTCAGAAGAGGAGG-3' | |
| Antisense (Reverse primer) | |
| 5'-TCAGCCTCAGAGTCTTCATC- 3' | |
| DMP-1 | Sense (Forward primer) |
| 5`-CAGGAGCACAGGAAAAGGAG-3` | |
| Antisense (Reverse primer) | |
| 5`-CTGGTGGTATCTTGGGCACT-3` | |
| DSPP | Sense (Forward primer) |
| 5`-AATGGGACTAAGGAAGCTG-3` | |
| Antisense (Reverse primer) | |
| 5`-AAGAAGCATCTCCTCGGC-3` |
Table 1: Primer Sequences for ALP, RUNX-2, OC, OP, DMP-1 and DSPP
Statistical analysis:
The normality of the distribution was assessed using the Shapiro-Wilk test. A parametric test (one-way Analysis of Variance (ANOVA)) was performed when data followed a normal distribution. The Levene test was performed to confirm the equality of variances and Welch ANOVA with Games-Howell post-hoc tests were applied if the assumption was not observed. Results were considered statistically significant if the p value was <0.05. The analysis was conducted using the International Business Machines (IBM) statistical package for the social sciences statistics software version 29. GraphPad Prism 10 (GraphPad Software, San Diego, United States of America) was used to design the graphs.
Results and Discussion
On d 1, no statistically significant differences were observed between the control and treatment groups. On d 3, the group treated with 50 ng/ml showed a statistically significant increase in viability compared to the groups treated with 25 ng/ml and the control group (p<0.001). The 50 ng/ml group showed higher viability on d 7, but the difference between the groups was not statistically significant (fig. 1).
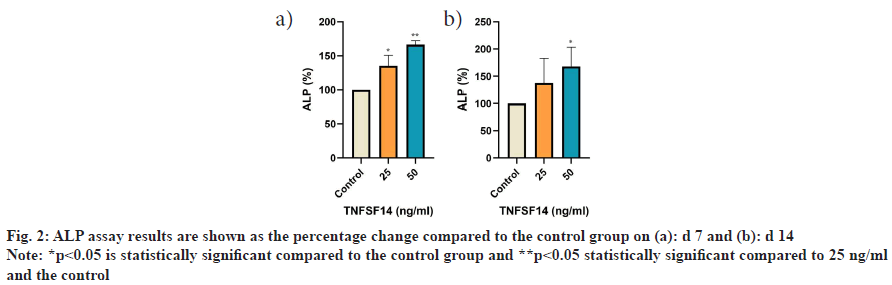
On d 7, the activity of ALP on hDPSCs significantly increased in the treatment groups compared with the control group 25 ng/ml (p=0.006), 50 ng/ml (p≤0.001). Furthermore, significantly higher ALP activity was observed at 50 ng/ml compared to 25 ng/ml (p=0.008). On d 14, the 50 ng/ml group showed a significant increase in activity compared to the control group (p=0.013). Similarly, the 50 ng/ml groups showed an increase in ALP activity compared to the 25 ng/ml group; however, the difference was not statistically significant (fig. 2).
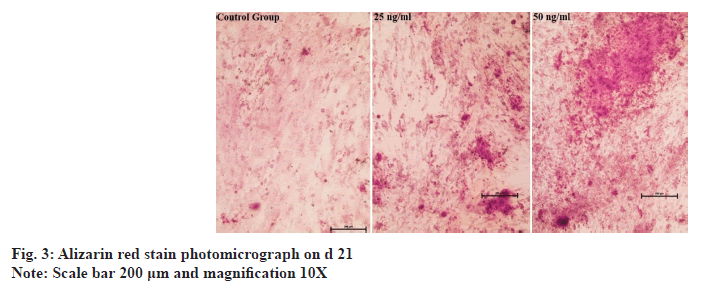
hDPSCs stained with alizarin red on d 14 showed that none of the groups exhibited calcified nodules. On d 21, the quantity, color and size of the calcified nodules in the TNFSF14-treated groups were noticeably higher than those in the control group. The 50 ng/ml concentration group had more calcified nodules than the 25 ng/ml group (fig. 3).
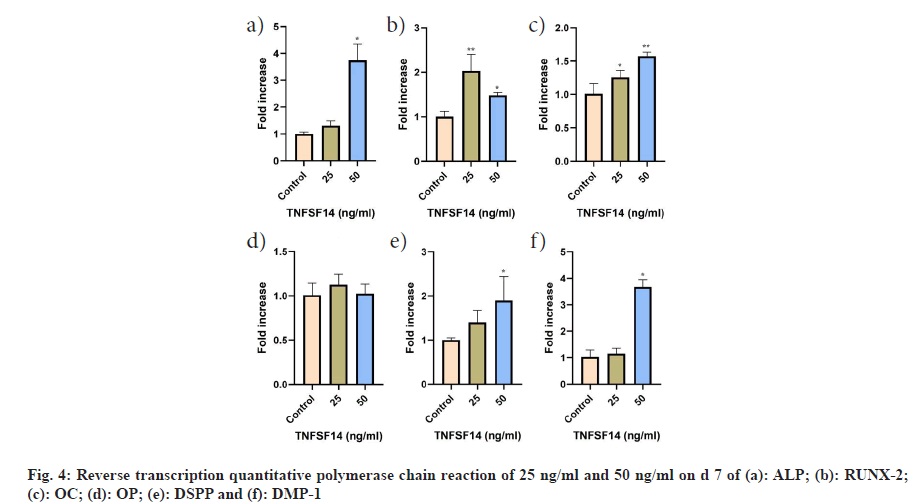
In comparison to the control group, the TNFSF14 concentration of 50 ng/ml exhibited the highest mean value for the messenger RNA (mRNA) of ALP (p<0.001), OC (p<0.001), DSPP (p=0.023), and DMP-1 (p<0.001). Additionally, OC levels were significantly higher in the 25 ng/ml group than in the control group (p=0.028). ALP, DSPP and DMP-1 levels were higher in the 25 ng/ml group than in the control group, however these differences were not statistically significant. RUNX-2 demonstrated a significant increase at a concentration of 25 ng/ml compared to the control and 50 ng/ml groups. Conversely, OP gene expression was higher in the experimental groups than that in the control group, however the difference was not statistically significant (fig. 4).
hDPSCs have the capacity to differentiate into multipotent mesenchymal stem cells. These cells can then further differentiate into osteoblast-like or odontoblast-like cells, which are responsible for the process of mineralization[22]. In this article, it has been demonstrated that adding TNFSF14 to hDPSC has the ability to induce upregulation of specific odontogenic marker mRNAs, such as ALP, RUNX-2, OC, DMP- 1 and DSPP. In addition, it promotes increased ALP activity. Furthermore, an increased calcific deposit formation was observed in response to TNFSF14 application. Moreover, hDPSC viability increased when cells were grown in the presence of TNFSF14, similar to the effect of TNFSF14 on BM-MSCs[23].
The formation of calcific bridges is an important indicator of successful pulp recovery and regeneration[8]. In this study, the upregulation of odontogenic marker genes, including ALP, OC, DMP-1 and DSPP in response to TNFSF14 treatment, combined with increased ALP activity, suggests that TNFSF14 stimulates odontogenic differentiation pathways in hDPSCs, which is an essential foundation for the formation of mineralized tissues[12].
TNFSF14 has been previously shown to promote the osteogenic differentiation of BM-MSCs by upregulating osteogenic/odontogenic markers such as ALP, RUNX-2 and OC, which is in agreement with the current findings[17]. OC is a protein that is found in both bones and teeth. It has a role in the later stages of mineralization of the extracellular matrix[24]. The up-regulation of OC, along with the activation of ALP activity, significantly promotes the differentiation of hDPSCs into odontoblast-like cells[11].
OP is a multifunctional protein involved in various cellular processes including mineralization[25]. It is possible that the timing of our analysis did not capture peak OP expression. It is plausible that OP expression may have been more pronounced at later time points beyond the duration of our study[26]. Thus, assessing OP expression at additional time points may provide further insight into its role in odontogenic differentiation.
RUNX-2 together with ALP is key marker of early odontogenic differentiation[27,28]. The findings of the current study suggest that a concentration of 50 ng/ml TNFSF14 may have exceeded the necessary threshold to achieve RUNX-2 peak expression. Consequently, the observed effect on the expression of RUNX-2 may have reached a plateau or decreased in comparison with the lower concentration of 25 ng/ml. This saturation effect may explain why lower concentrations have a more significant impact on RUNX-2 expression[29]. Conversely, the mRNA levels were higher at 50 ng/ ml TNFSF14 compared to 25 ng/ml in BM-MSCs, possibly due to the difference in cell types used in the two studies[17].
The differentiation markers examined, DSPP and DMP- 1, have been identified as being primarily expressed in odontoblasts and are the primary indicators of odontoblast differentiation. The continuous presence of DMP-1 and DSPP during development indicates that these proteins play an important role in maintaining the balance of the dentin matrix[28].
Alizarin red staining is an effective method for examining the development of calcified nodules[30]. TNFSF14 induced calcific nodule production serves as an indicator of the odontogenic differentiation of hDPSC. These findings are consistent with the previous report on BM-MSCs[17].
Although in vitro studies, like this offer valuable insights into the cellular responses to stimuli, they fail to accurately recreate the complex microenvironment and physiological conditions of the living body. Therefore, further studies using in vivo models and clinical trials are required to verify the findings of the present study.
Our study provides novel insights into the ability of TNFSF14 to promote the odontogenic differentiation of hDPSCs. TNFSF14 has the potential to be a therapeutic agent for the management of pulp conditions requiring calcific bridge formation.
Ethical approval:
The research was approved by the Institutional Review Board (IRB) at King Saud University Medical City (KSUMC) and the College of Dentistry Research Centre (CDRC) at King Saud University (KSU) with IRB Project No. E-23-7902 and CDRC No PR 0160.
Acknowledgements:
The authors would like to thank College of Dentistry Research Center and Deanship of Scientific Research at King Saud University, Saudi Arabia for funding this research project.
Conflict of interests:
The authors declared no conflict of interests.
References
- Yu C, Abbott PV. An overview of the dental pulp: Its functions and responses to injury. Aust Dent J 2007;52:S4-6.
[Crossref] [Google Scholar] [PubMed]
- Chogle SM, Goodis HE, Kinaia BM. Pulpal and periradicular response to caries: Current management and regenerative options. Dent Clin North Am 2012;56(3):521-36.
[Crossref] [Google Scholar] [PubMed]
- Lin LM, Ricucci D, Saoud TM, Sigurdsson A, Kahler B. Vital pulp therapy of mature permanent teeth with irreversible pulpitis from the perspective of pulp biology. Aust Endod J 2020;46(1):154-66.
[Crossref] [Google Scholar] [PubMed]
- Cushley S, Duncan HF, Lappin MJ, Chua P, Elamin AD, Clarke M, et al. Efficacy of direct pulp capping for management of cariously exposed pulps in permanent teeth: A systematic review and meta‐analysis. Int Endod J 2021;54(4):556-71.
[Crossref] [Google Scholar] [PubMed]
- Elmsmari F, Ruiz XF, Miró Q, Feijoo-Pato N, Durán-Sindreu F, Olivieri JG. Outcome of partial pulpotomy in cariously exposed posterior permanent teeth: A systematic review and meta-analysis. J Endod 2019;45(11):1296-306.
[Crossref] [Google Scholar] [PubMed]
- Taha NA, Abdulkhader SZ. Full pulpotomy with biodentine in symptomatic young permanent teeth with carious exposure. J Endod 2018;44(6):932-7.
[Crossref] [Google Scholar] [PubMed]
- Cooper PR, Holder MJ, Smith AJ. Inflammation and regeneration in the dentin-pulp complex: A double-edged sword. J Endod 2014;40(4):S46-51.
[Crossref] [Google Scholar] [PubMed]
- Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review—part III: Clinical applications, drawbacks, and mechanism of action. J Endod 2010;36(3):400-13.
[Crossref] [Google Scholar] [PubMed]
- Almushayt A, Narayanan K, Zaki AE, George A. Dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene Ther 2006;13(7):611-20.
[Crossref] [Google Scholar] [PubMed]
- Teti G, Salvatore V, Ruggeri A, Manzoli L, Gesi M, Orsini G, Falconi M. In vitro reparative dentin: A biochemical and morphological study. Eur J Histochem 2013;57(3):e23.
[Crossref] [Google Scholar] [PubMed]
- Baldión PA, Velandia-Romero ML, Castellanos JE. Odontoblast‐like cells differentiated from dental pulp stem cells retain their phenotype after subcultivation. Int J Cell Biol 2018;2018(1):6853189.
[Crossref] [Google Scholar] [PubMed]
- Sabbagh J, Ghassibe-Sabbagh M, Fayyad-Kazan M, Al-Nemer F, Fahed JC, Berberi A, et al. Differences in osteogenic and odontogenic differentiation potential of DPSCs and SHED. J Dent 2020;101:103413.
[Crossref] [Google Scholar] [PubMed]
- Barthel CR, Rosenkranz B, Leuenberg A, Roulet JF. Pulp capping of carious exposures: treatment outcome after 5 and 10 years: A retrospective study. J Endod 2000;26(9):525-8.
[Crossref] [Google Scholar] [PubMed]
- Mehrvarzfar P, Abbott PV, Mashhadiabbas F, Vatanpour M, Tour Savadkouhi S. Clinical and histological responses of human dental pulp to MTA and combined MTA/treated dentin matrix in partial pulpotomy. Aust Endod J 2018;44(1):46-53.
[Crossref] [Google Scholar] [PubMed]
- Sedy J, Bekiaris V, Ware CF. Tumor necrosis factor superfamily in innate immunity and inflammation. Cold Spring Harb Perspect Biol 2015;7(4):a016279.
[Crossref] [Google Scholar] [PubMed]
- Brunetti G, Belisario DC, Bortolotti S, Storlino G, Colaianni G, Faienza MF, et al. LIGHT/TNFSF14 promotes osteolytic bone metastases in non‐small cell lung cancer patients. J Bone Miner Res 2020;35(4):671-80.
[Crossref] [Google Scholar] [PubMed]
- Heo SK, Choi Y, Jeong YK, Ju LJ, Yu HM, Kim DK, et al. LIGHT (TNFSF14) enhances osteogenesis of human bone marrow-derived mesenchymal stem cells. Plos One 2021;16(2):e0247368.
[Crossref] [Google Scholar] [PubMed]
- Dou L, Yan Q, Yang D. Effect of five dental pulp capping agents on cell proliferation, viability, apoptosis and mineralization of human dental pulp cells. Exp Ther Med 2020;19(3):2377-83.
[Crossref] [Google Scholar] [PubMed]
- Peng W, Liu W, Zhai W, Jiang L, Li L, Chang J, et al. Effect of tricalcium silicate on the proliferation and odontogenic differentiation of human dental pulp cells. J Endod 2011;37(9):1240-6.
[Crossref] [Google Scholar] [PubMed]
- Guo L, Li J, Qiao X, Yu M, Tang W, Wang H, et al. Comparison of odontogenic differentiation of human dental follicle cells and human dental papilla cells. PLoS One 2013;8(4):e62332.
[Crossref] [Google Scholar] [PubMed]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 2008;3(6):1101-8.
[Crossref] [Google Scholar] [PubMed]
- Mortada I, Mortada R. Dental pulp stem cells and osteogenesis: An update. Cytotechnology 2018;70(5):1479-86.
[Crossref] [Google Scholar] [PubMed]
- Heo SK, Noh EK, Gwon GD, Kim JY, Jo JC, Choi Y, et al. LIGHT (TNFSF14) increases the survival and proliferation of human bone marrow-derived mesenchymal stem cells. PloS One 2016;11(11):e0166589.
[Crossref] [Google Scholar] [PubMed]
- Papagerakis P, Berdal A, Mesbah M, Peuchmaur M, Malaval L, Nydegger J, et al. Investigation of osteocalcin, osteonectin, and dentin sialophosphoprotein in developing human teeth. Bone 2002;30(2):377-85.
[Crossref] [Google Scholar] [PubMed]
- Christensen B, Kazanecki CC, Petersen TE, Rittling SR, Denhardt DT, Sørensen ES. Cell type-specific post-translational modifications of mouse osteopontin are associated with different adhesive properties. J Biol Chem 2007;282(27):19463-72.
[Crossref] [Google Scholar] [PubMed]
- Khanna-Jain R, Mannerström B, Vuorinen A, Sándor GK, Suuronen R, Miettinen S. Osteogenic differentiation of human dental pulp stem cells on β-tricalcium phosphate/poly (l-lactic acid/caprolactone) three-dimensional scaffolds. J Tissue Eng 2012;3(1):2041731412467998.
[Crossref] [Google Scholar] [PubMed]
- López-García S, Aznar-Cervantes SD, Pagán A, Llena C, Forner L, Sanz JL, et al. 3D Graphene/silk fibroin scaffolds enhance dental pulp stem cell osteo/odontogenic differentiation. Dent Mater 2024;40(3):431-40.
[Crossref] [Google Scholar] [PubMed]
- Spagnuolo G, de Luca I, Iaculli F, Barbato E, Valletta A, Calarco A, et al. Regeneration of dentin-pulp complex: Effect of calcium-based materials on hDPSCs differentiation and gene expression. Dent Mater 2023;39(5):485-91.
[Crossref] [Google Scholar] [PubMed]
- Salahudeen MS, Nishtala PS. An overview of pharmacodynamic modelling, ligand-binding approach and its application in clinical practice. Saudi Pharm J 2017;25(2):165-75.
[Crossref] [Google Scholar] [PubMed]
- Sanz JL, Soler-Doria A, López-García S, García-Bernal D, Rodríguez-Lozano FJ, Lozano A, et al. Comparative biological properties and mineralization potential of 3 endodontic materials for vital pulp therapy: Theracal PT, Theracal LC, and Biodentine on human dental pulp stem cells. J Endod 2021;47(12):1896-906.
[Crossref] [Google Scholar] [PubMed]