- *Corresponding Author:
- Hongwei Gu
Department of Otolaryngology,
Nanjing Integrated Traditional Chinese and Western Medicine Hospital, Xuanwu,
Nanjing,
Jiangsu 210014,
China
E-mail: hongweiisu@hotmail.com
| This article was originally published in a special issue, “Recent Developments in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(4) Spl Issue “114-120” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Chronic rhinosinusitis is a chronic inflammatory condition that caused by many factors. However, the underlying mechanism of chronic rhinosinusitis remains unclear. The current study focused on the roles of triptolide on hydrogen peroxide treated nasal epithelial cells and the underling mechanism. Hydrogen peroxide treated human nasal epithelial cells were cultured and treated by different concentrations of triptolide, cell growth and cell death were determined by cell counting kit-8 and flow cytometry methods. Moreover, effects of triptolide on expression of superoxide dismutase as well as malondialdehyde in nasal epithelial cells treated by hydrogen peroxide were evaluated by enzyme-linked immunosorbent assay analysis. Furthermore, effects of triptolide on expression of phosphatase and tensin homolog deleted on chromosome 10/phosphatidylinositol 3-kinase/protein kinase B molecules in hydrogen peroxide treated nasal epithelial cells have been examined by Western blot method. We found treatment of hydrogen peroxide markedly inhibited growth of the nasal epithelial cells in dose-dependent manner and triptolide could reverse the effect induced by hydrogen peroxide. Moreover, triptolide significantly decreased the apoptosis of the nasal epithelial cells induced by hydrogen peroxide in dose-dependent manner; moreover, triptolide reduced superoxide dismutase as well as malondialdehyde levels in dose dependent manner. Finally, triptolide downregulated phosphatase and tensin homolog deleted on chromosome 10 and upregulated the phosphatidylinositol 3-kinase related signalling in hydrogen peroxide treated cells in vitro. In conclusion, triptolide may exert anti-apoptotic and anti-oxidative behaviors in chronic rhinosinusitis via affecting phosphatase and tensin homolog deleted on chromosome 10/phosphatidylinositol 3-kinase/protein kinase B signaling. The results of current work study may provide new evidence for the research as well as management of chronic rhinosinusitis.
Keywords
Chronic rhinosinusitis, chronic inflammatory, nasal epithelial cells, phosphatidylinositol 3-kinase/protein kinase B
Chronic Rhinosinusitis (CRS) is mainly due to the persistence of chronic inflammation of nasal cavity and paranasal sinus mucosa caused by many factors[1,2]. Although the control of patients with nasal polyps has been greatly improved with the improvement of treatment measures such as drugs and surgery, there is still a high recurrence rate of CRS after treatment and this recurrence is more obvious in nasal polyps[3,4]. The existence of these conditions may be related to the controversial and unclear etiology and pathogenesis of CRS. Many factors have been reported to be related to the pathogenesis of CRS, including bacterial and viral infection; allergic factors; impairment of epithelial barrier function; obstruction of ostium nasal complex; immune dysfunction and so on[5-9]. The role of epithelial cells in the pathogenesis of CRS has attracted more and more attention. As the first natural barrier to contact external stimuli and an important structure of innate immunity, epithelial cells on the surface of nasal mucosa play an important role in protecting the body from external harmful factors[10-12]. Once the epithelial cell barrier function is damaged, it is mainly manifested in epithelial cell abscission and dysfunction in CRS, which will provide conditions for the invasion of external harmful substances, such as bacteria, virus invasion and allergen exposure, so as to cause and aggravate the chronic inflammation of nasal cavity and paranasal sinus mucus membrane and lead to the accumulation of inflammatory cells and inflammatory factors[13,14]. There are many factors causing epithelial cell injury and abscission, such as inflammatory factor stimulation, but at present, Interferon (IFN) has been found-gamma (γ) it can cause epithelial cell apoptosis in CRS, but the specific mechanism is not clear.
Under normal conditions, the oxidation and antioxidant system in the human body maintain a relative balance. For example, some enzymatic and non-enzymatic antioxidants will prevent the invasion of Reactive Oxygen Species (ROS)[15-17]. When this balance is broken by excessive ROS, it is called “oxidative stress”. Oxidative stress can lead to diseases, including cancer, neurological diseases, hypertension, ischemia and reperfusion[18-20], diabetes, chronic obstructive pulmonary disease and asthma[21-23]. ROS plays an important role in the regulation of important cellular processes such as oxidative stress, apoptosis and gene expression. It is one of the most active frontier fields in life science and molecular medicine.
In recent years, more and more attention has been paid to the role of oxidative stress in the pathogenesis of airway diseases. The airway is the only organ exposed to high oxides and antioxidants. Under normal conditions, oxides and antioxidants maintain a relative balance, which is very necessary to protect the normal function of the airway. When the airway is exposed to environmental factors such as animal hair, dust, mites, fungi, pollen, cigarette smoke, volatile chemicals, occupational dust, automobile exhaust and sulfur dioxide[18,19,23,24], these irritants stimulate epithelial cells to produce too many harmful oxides, break the balance between oxides and antioxidants, and finally lead to oxidative stress and airway inflammation.
Triptolide, also known as triptolide and triptolide alcohol, is the most deeply studied traditional Chinese medicineimmunosuppressant[25,26]. is a diterpene compound isolated from the roots of Tripterygium wilfordiiinCelastraceae.Triptolide is a light yellow crystal with stable physical and chemical properties[27,28]. It is insoluble in water, slightly soluble in alcohol and easily soluble in organic solvents. It has the dual effects of anti-inflammatory and immunosuppression[25,29,30]. Through clinical application and experimental research, triptolide has anti-inflammatory and immunosuppressive effects.
In the present work, we will focus on the function of triptolide for the treatment of CRS using nasal epithelial cells as cell models. Hydrogen peroxide (H2O2) has been used to induce ROS and modulate the oxidative stress. We hypothesized that triptolide may inhibit the apoptosis and ROS production in H2O2.
Materials and Methods
Cells and cell culture:
The Human Nasal Epithelial Cells (HNEpC) have been placed in Dulbecco’s modified eagle’s medium (Thermo Fisher Scientific, Waltham, MA, USA) combing with 10 % fetal bovine serum (Gibco), 1 % penicillin as well as streptomycin. Cells have been cultured in an incubator with 5 % Carbon dioxide (CO2) at 37°.
Cell growth analysis:
Cell Counting Kit-8 (CCK-8) method was used to detect cell growth in each group. First, 4×103 cells were seeded into 96-well plates. Then, a total of 4×103 cells were inoculated with 96-well plates per hole. CCK-8 reagent (Tokyo Dojindo, Japan) was added at 0, 24, 48, 72 h respectively. The plate was then incubated at 37° for 60 min using an automatic microboard reader (BioTek, USA). The measurement of Optical Density (OD) was performed with a microplate at the wavelength of 450 nm.
Cell apoptosis analysis:
After 48 h of culture, cells were washed twice with Phosphate Buffered Saline (PBS), followed by digestion and resuspension, Then cells were double- stained with FITC Annexin V/Propidium Iodide (PI) at 4°. Cell apoptosis was calculated with FACSCaliburTM flow cytometry (BD Biosciences, USA) using FlowJo software (version 7.6.5; Tree Star, USA).
Enzyme-Linked Immunosorbent Assay (ELISA) assay:
Treated HNEpC cells were cultured at 37°, 5 % CO2 environment for 12 h and treated with Radio Immunoprecipitation Assay (RIPA) lysis buffer. Then, the supernatant was obtained by centrifugation at 4°, 10 000 r/min for 5 min. Finally, the Superoxide Dismutase (SOD) and Malondialdehyde (MDA) contents of each group were measured using corresponding commercial ELISA kits.
Western blot:
Cells were harvested at 48 h and lysed by RIPA (Sigma-Aldrich, Germany). Protein concentration was calculated by a bicinchoninic acid kit (Pierce, USA).
Then the protein was separated with 12 % Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride membranes, which were blocked with 5 % non-fat milk. Next, the membranes were incubated with primary antibodies, purchased from Boster Biological Technology, China, overnight at 4°; 2nd d, the membranes were incubated with Horseradish Peroxidase (HRP)-goat-anti-rabbit antibody (Beyotime, China) and treated with BeyoECL Plus (Beyotime, China). The bands were visualized with a microscope (Bio-Rad, USA).
Statistical analysis:
All the data were analyzed by Statistical Package for the Social Sciences (SPSS) 19.0 software and expressed as mean±standard error. When the sample size is two groups, the T-test was used to compare the mean value and analysis of variance for multi-group. p<0.05 was considered a significant difference.
Results and Discussion
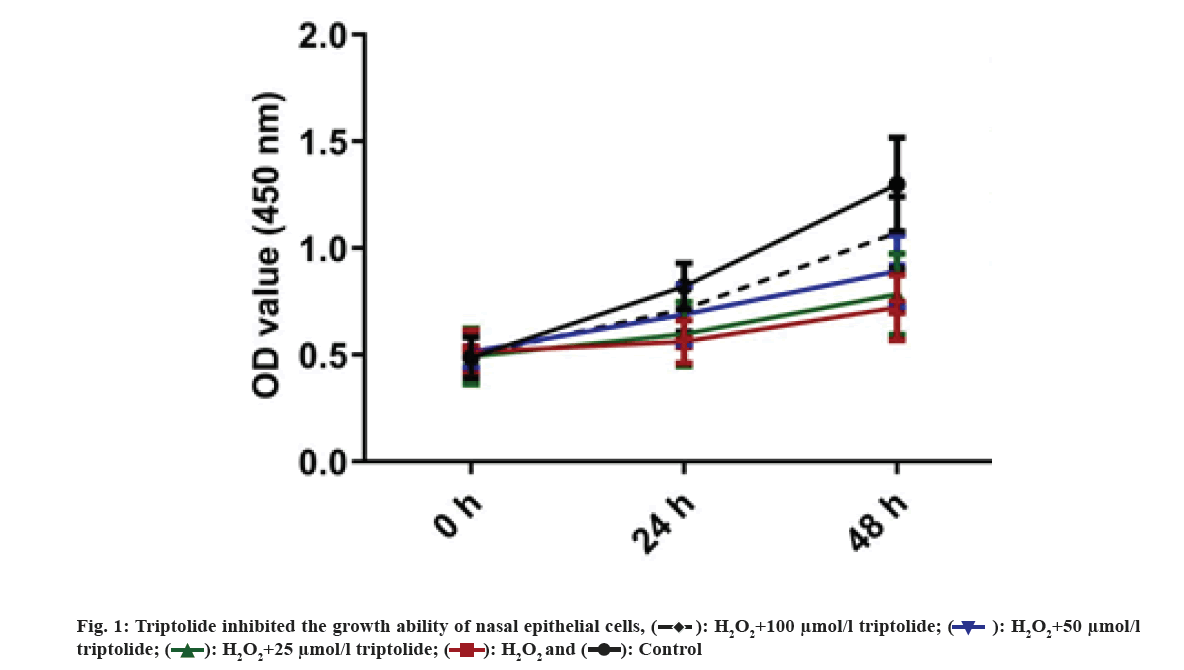
First, H2O2 treated nasal epithelial cells have been randomly divided to five groups. Cells have been cultured using triptolide of different concentrations. The growth of the cells has been examined by CCK-8 assay. We found that H2O2 inhibited nasal epithelial cell growth and treatment of triptolide markedly increased growth of the nasal epithelial cells in dose-dependent manner as shown in fig. 1. Nasal epithelial cells were stimulated with H2O2 and treated with different concentrations of triptolide. CCK-8 analysis was applied for determining cell viability. **p<0.01, H2O2 vs. control group.
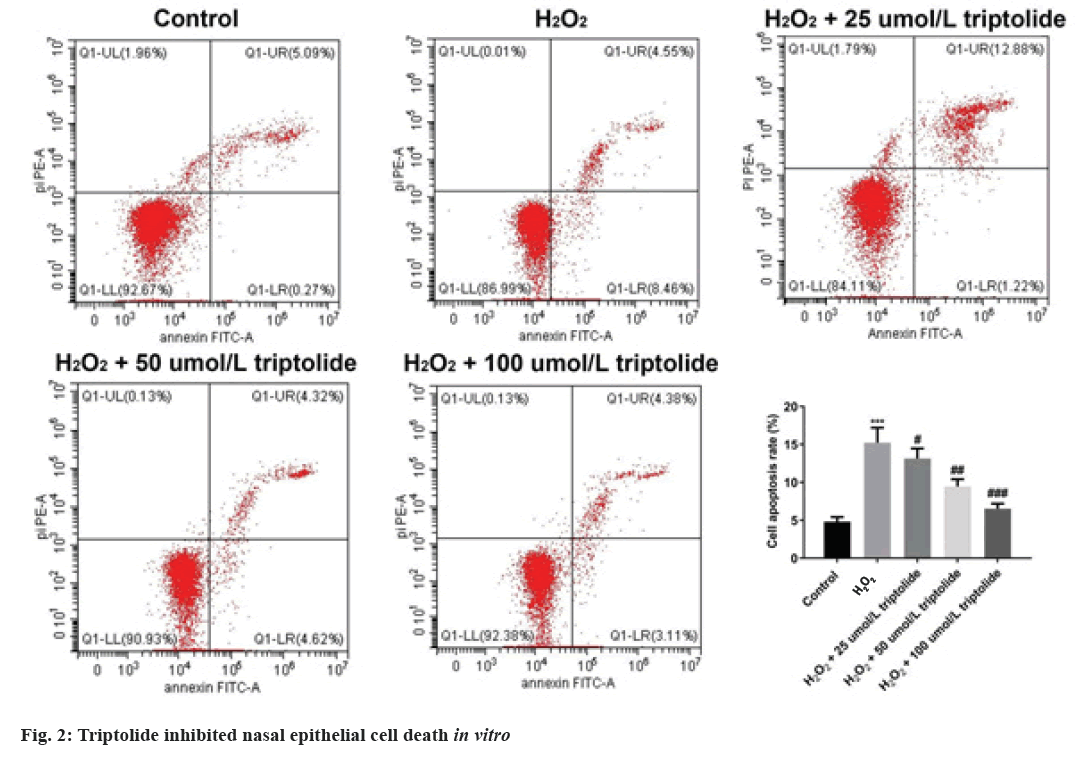
Furthermore, H2O2 treated nasal epithelial cells have been randomly divided to five groups and the cell death have been determined by flow cytometry assay. We found that after exposing to H2O2, cell apoptosis was novelty increased; however, treatment of triptolide markedly inhibited the apoptosis of the nasal epithelial cells in a dose-dependent manner as shown in fig. 2. Nasal epithelial cells were triggered with H2O2 and treated with different concentrations of triptolide. Flow cytometry analysis was conducted to evaluate cell apoptosis. ***p<0.001, H2O2 vs. control; #p<0.05, ##p<0.01 and ###p<0.001 vs. H2O2.
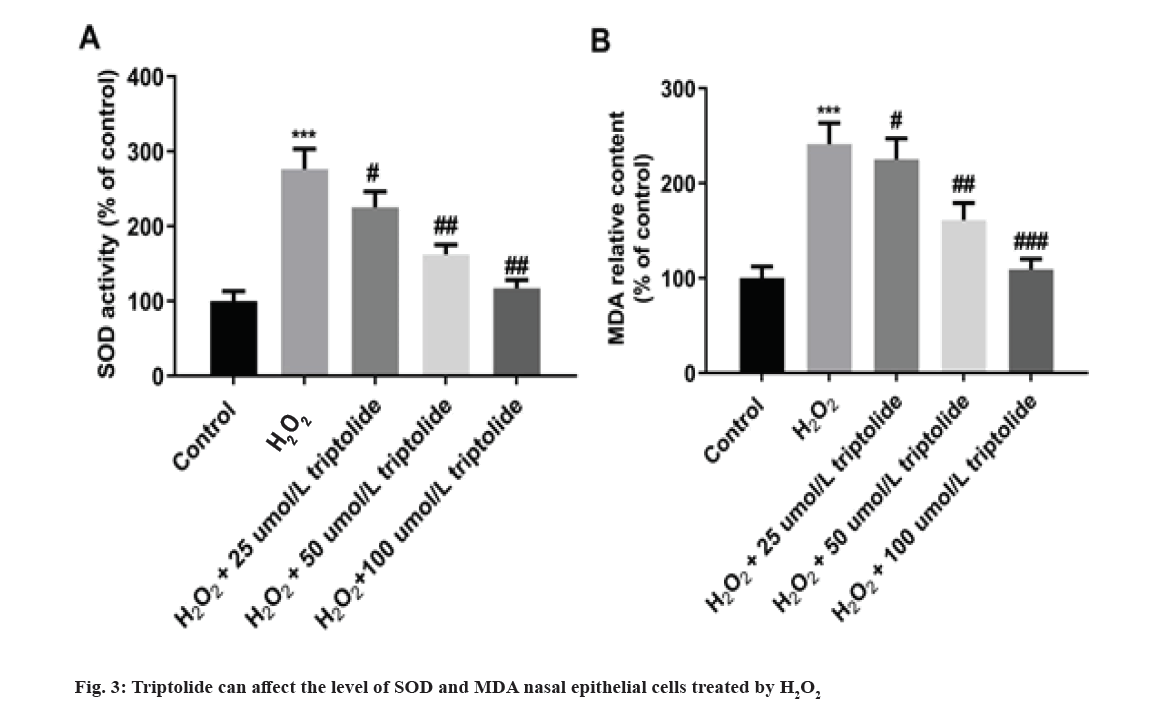
Furthermore, effects of triptolide on activity of SOD as well as MDA in nasal epithelial cells treated by H2O2 were evaluated by ELISA analysis. In fig. 3, H2O2 markedly increased the levels of SOD and MDA. And 25, 50 and 100 µmol/l of triptolide decreased SOD as well as MDA levels induced by H2O2 in dose dependent manner as shown in fig. 3. Nasal epithelial cells were triggered with H2O2 and treated with different concentrations of triptolide. ***p<0.001, H2O2 vs. control; #p<0.05, ##p<0.01, ###p<0.001 vs. H2O2.
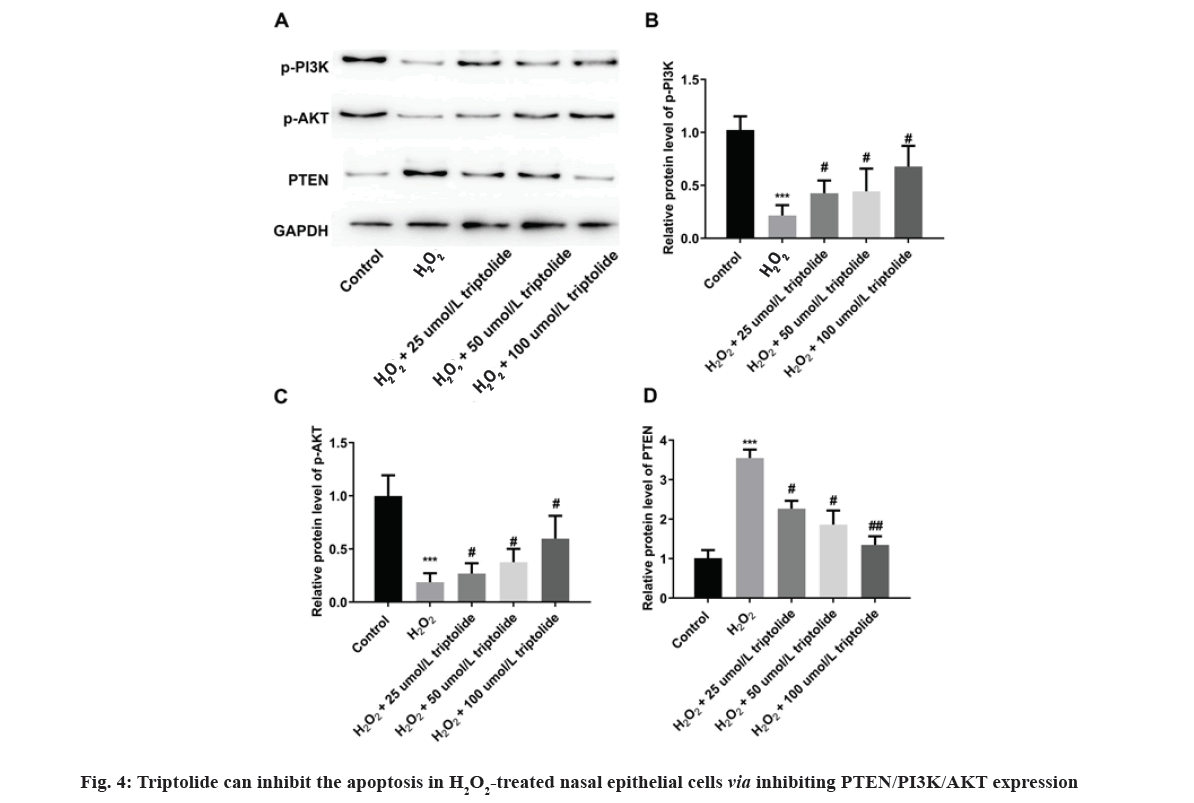
Finally, effects of triptolide on expression of Phosphatase and Tensin homolog deleted on chromosome 10/Phosphatidylinositol 3-Kinase/Protein Kinase B (PTEN/PI3K/AKT) molecules in H2O2treated nasal epithelial cells have been examined. We found that H2O2 significantly promoted PTEN expression and inhibited phosphorylated-PI3K (p-PI3K) as well as p-AKT expression, while on the other hand, triptolide inhibited PTEN and the PI3K related signaling in H2O2 treated cells in vitro as shown in fig. 4. Nasal epithelial cells were triggered with H2O2 and treated with different concentrations of triptolide. Western blot assay was utilized to determine PTEN/PI3K/ATK activation under H2O2 conditions. ***p<0.001, H2O2 vs. control; #p<0.05 and ##p<0.01 vs. H2O2.
In current work, we investigated the effects of triptolide on growth, apoptosis and ROS production of nasal epithelial cells in vitro. The results suggested that triptolide may regulate growth as well as apoptosis and the ROS production of nasal epithelial cells through PTEN/PI3K/AKT signaling. Our results found that triptolide may function as alternative medicine for CRS management.
With the in-depth research on the roles of traditional medicine in different diseases, the roles of traditional medicine in many types of diseases have been elucidated. For example, it has been reported that ginsenoside-Rg5 may decrease the proliferation and promote the cell death of tumor cells through decreasing BCL2 expression[31]; on the other hand, wogonin could decrease the formation of nasal polyp via increasing the apoptosis of eosinophil via regulating the expression of Hypoxia-Inducible Factor (HIF)-1 alpha as well as surviving[32]. The function of triptolide for the treatment of CRS remains unclear. In current work, nasal epithelial cells have been treated by triptolide, the growth of the cell, cell death and ROS production has been determined. Our data suggested that triptolide may regulate the proliferation as well as death of nasal epithelial cells. Furthermore, triptolide decreased ROS production in nasal epithelial cells. The present data suggested that triptolide may decrease the apoptosis as well as ROS production of nasal epithelial cells. Our results proved that triptolide may exert anti-apoptotic and anti-oxidative effects in CRS.
The PI3K/AKT signaling was known as the key cellular signaling pathway that involved in many different biological activities, including the carcinogenesis process[33-35]. The roles of PI3K/AKT signaling in CRS have also been discussed previously[36,37]. Therefore, we further examined whether the management of triptolide may regulate the expression of PI3K/AKT signaling. As expected, PTEN was markedly decreased, while p-PI3K and p-AKT were significantly increased in triptolide treated nasal epithelial cells. Our results proved that triptolide may regulate nasal epithelial cells proliferation as well as ROS production via PI3K/AKT signaling pathway.
In conclusion, our data suggested triptolide may exert anti-apoptotic and anti-oxidative behaviors in CRS via affecting PTEN/PI3K/AKT signaling. The results of current work study may provide new evidence for the research as well as management of CRS.
Acknowledgement:
This work was supported by 2020 Jiangsu Province TCM science and technology development plan project (No. YB2020032). Xiaoyan Zhu, Qinghua Guo and Xiaoyang Zhang were as considered co-second authors.
Conflict of interests:
The authors declared no conflict of interests.
References
- Sedaghat AR.
- Lai Y, Hu L, Yang L, Hu X, Song X, Yang J, et al. Interaction between serum/glucocorticoid-regulated kinase 1 and interleukin-6 in chronic rhinosinusitis. Allergy Asthma Immunol Res 2021;13(5):776-90.
[Crossref] [Google Scholar] [Pub Med]
- Yuan X, Xie S, Jing Q, She Y, Jiang W, Zhang H, et al. The role of serum macrophage migration inhibitory factor in preoperative prediction of chronic rhinosinusitis with nasal polyps endotypes. Int Immunopharmacol 2021;100:108084.
[Crossref] [Google Scholar] [Pub Med]
- Wu Q, Yuan L, Qiu H, Wang X, Huang X, Zheng R, et al. Efficacy and safety of omalizumab in chronic rhinosinusitis with nasal polyps: A systematic review and meta-analysis of randomised controlled trials. BMJ open 2021;11(9):e047344.
[Crossref] [Google Scholar] [Pub Med]
- Patel GB, Kudlaty EA, Guo A, Yeh C, Kim MS, Price CP, et al. Impact of type 2 targeting biologics on acute exacerbations of chronic rhinosinusitis. Allergy Asthma Proc 2021;42(5):417-24.
[Crossref] [Google Scholar] [Pub Med]
- Kato A, Peters AT, Stevens WW, Schleimer RP, Tan BK, Kern RC. Endotypes of chronic rhinosinusitis: relationships to disease phenotypes, pathogenesis, clinical findings and treatment approaches. Allergy 2022;77(3):812-26.
[Crossref] [Google Scholar] [Pub Med]
- Ramakrishnan VR, Arbet J, Mace JC, Suresh K, Shintani Smith S, Soler ZM, et al. Predicting olfactory loss in chronic rhinosinusitis using machine learning. Chem Senses 2021;46.
[Crossref] [Google Scholar] [Pub Med]
- Wee JH, Min C, Jung HJ, Park MW, Choi HG. Association between air pollution and chronic rhinosinusitis: a nested case-control study using meteorological data and national health screening cohort data. Rhinology 2021
[Crossref] [Google Scholar] [Pub Med]
- Napolitano M, Maffei M, Patruno C, Leone CA, Di Guida A, Potestio L, et al. Dupilumab effectiveness for the treatment of patients with concomitant atopic dermatitis and chronic rhinosinusitis with nasal polyposis. Dermatol Ther 2021;34(6):e15120.
[Crossref] [Google Scholar] [Pub Med]
- Caliaperoumal VB, Dharanya GS, Velayutham P, Krishnaswami B, Krishnan KK, Savery N. Correlation of clinical symptoms with nasal endoscopy and radiological findings in the diagnosis of chronic rhinosinusitis: A prospective observational study. Cureus 2021;13(7).
- Xie Y, Li M, Chen K, Zhu H, Tang M, Zhou C, et al. Necroptosis underlies neutrophilic inflammation associated with the chronic rhinosinusitis with nasal polyps (CRSwNP). J Inflamm Res 2021;14:3969.
[Crossref] [Google Scholar] [Pub Med]
- da Silva GS, dos Santos Isoppo K. Therapeutic ultrasound as a treatment for chronic rhinosinusitis: A systematic review. Clin Respir J 2021;15(12):1275-85.
[Crossref] [Google Scholar] [Pub Med]
- Li J, Kang H, Hong S, Shen Y. Effect of postoperative specific immunotherapy combined with nasal irrigation on chronic rhinosinusitis with allergic rhinitis. Iran J Allergy Asthma Immunol 2021;20(4):432-40.
- Akhlaghi A, Darabi A, Mahmoodi M, Movahed A, Kaboodkhani R, Mohammadi Z, et al. The frequency and clinical assessment of COVID-19 in patients with chronic rhinosinusitis. Ear Nose Throat J 2021:01455613211038070.
[Crossref] [Google Scholar] [Pub Med]
- Zhao T, Wu W, Sui L, Huang Q, Nan Y, Liu J, et al. Reactive oxygen species-based nanomaterials for the treatment of myocardial ischemia reperfusion injuries. Bioact Mater 2022;7:47-72.
[Crossref] [Google Scholar] [Pub Med]
- Al Humam NA. Bacterial phagocytosis and reactive oxygen species production by camel neutrophils and monocytes are influenced by the type of anticoagulation agent. Vet World 2021;14(7):1888-93.
[Crossref] [Google Scholar] [Pub Med]
- Ortega GA, Del Sol-Fernandez S, Portilla Y, Cedeno E, Reguera E, Srinivasan S, et al. Rodlike particles of polydopamine-CdTe quantum dots: An actuator as a photothermal agent and reactive oxygen species-generating nanoplatform for cancer therapy. ACS Appl Mater Interfaces 2021;13(36):42357-69.
[Crossref] [Google Scholar] [Pub Med]
- Liu X, Zhang Z. A double‐edged sword: Reactive oxygen species (ROS) during the rice blast fungus and host interaction. FEBS J 2021.
- Veselova S, Nuzhnaya T, Burkhanova G, Rumyantsev S, Maksimov I. Reactive oxygen species in host plant are required for an early defense response against attack of Stagonospora nodorum Berk. necrotrophic effectors SnTox. Plants 2021;10(8):1586.
[Crossref] [Google Scholar] [Pub Med]
- Romolini G, Gambucci M, Ricciarelli D, Tarpani L, Zampini G, Latterini L. Photocatalytic activity of silica and silica-silver nanocolloids based on photo-induced formation of reactive oxygen species. Photochem Photobiol Sci 2021;20(9):1161-72.
[Crossref] [Google Scholar] [Pub Med]
- Park WH. Propyl gallate decreases the proliferation of Calu‐6 and A549 lung cancer cells via affecting reactive oxygen species and glutathione levels. J Appl Toxicol 2022;42(3):436-49.
- Ivanova JS, Pugovkina NA, Neganova IE, Kozhukharova IV, Nikolsky NN, Lyublinskaya OG. Cell cycle-coupled changes in the level of reactive oxygen species support the proliferation of human pluripotent stem cells. Stem Cells 2021;39(12):1671-87.
[Crossref] [Google Scholar] [Pub Med]
- Jan H, Shah M, Andleeb A, Faisal S, Khattak A, Rizwan M, et al. Plant-based synthesis of zinc oxide nanoparticles (ZnO-NPs) using aqueous leaf extract of Aquilegia pubiflora: Their antiproliferative activity against HepG2 cells inducing reactive oxygen species and other in vitro properties. Oxid Med Cell Longev 2021;2021.
[Crossref] [Google Scholar] [Pub Med]
- Huang X, He D, Pan Z, Luo G, Deng J. Reactive-oxygen-species-scavenging nanomaterials for resolving inflammation. Mater Today Bio 2021;11:100124.
[Crossref] [Google Scholar] [Pub Med]
- Li Y, Guo L, Hou Z, Gong H, Yan M, Zhang B. Role of MicroRNA-155 in triptolide-induced hepatotoxicity via the Nrf2-dependent pathway. J Ethnopharmacol 2021;281:114489.
[Crossref] [Google Scholar] [Pub Med]
- Piao X, Zhou J, Xue L. Triptolide decreases rheumatoid arthritis fibroblast-like synoviocyte proliferation, invasion, inflammation and presents a therapeutic effect in collagen-induced arthritis rats via inactivating lncRNA RP11-83J16. 1 mediated URI1 and β-catenin signaling. Int Immunopharmacol 2021;99:108010.
[Crossref] [Google Scholar] [Pub Med]
- Qiu H, Zhang X, Yu H, Gao R, Shi J, Shen T. Identification of potential targets of triptolide in regulating the tumor microenvironment of stomach adenocarcinoma patients using bioinformatics. Bioengineered 2021;12(1):4304-19.
[Crossref] [Google Scholar] [Pub Med]
- Guan YM, Shen Q, He LF, Chen LM, Zang Z, Liu L, et al. Pharmacokinetics and tissue distribution of combined triptolide and paeoniflorin regimen for percutaneous administration in rats assessed by liquid chromatography-tandem mass spectrometry. Evid Based Complement Alternat Med 2021;2021.
- Wang S, Jiang H, Wang J, Wu H, Wu T, Ni M, et al. Superior in vitro anticancer effect of biomimetic paclitaxel and triptolide co-delivery system in gastric cancer. J Biomed Res 2021;35(4):327.
[Crossref] [Google Scholar] [Pub Med]
- Yu L, Wang Z, Mo Z, Zou B, Yang Y, Sun R, et al. Synergetic delivery of triptolide and Ce6 with light-activatable liposomes for efficient hepatocellular carcinoma therapy. Acta Pharm Sin B 2021;11(7):2004-15.
- Cui Y, Su Y, Deng L, Wang W. Ginsenoside-Rg5 inhibits retinoblastoma proliferation and induces apoptosis through suppressing BCL2 expression. Chemotherapy 2018;63(5):293-300.
[Crossref] [Google Scholar] [Pub Med]
- Khalmuratova R, Lee M, Mo JH, Jung Y, Park JW, Shin HW. Wogonin attenuates nasal polyp formation by inducing eosinophil apoptosis through HIF-1α and survivin suppression. Sci Rep 2018;8(1):1-2.
- Yu S, Jia B, Liu N, Yu D, Zhang S, Wu A. Fumonisin B1 triggers carcinogenesis via HDAC/PI3K/Akt signalling pathway in human esophageal epithelial cells. Sci Total Environ 2021;787:147405.
[Crossref] [Google Scholar] [Pub Med]
- Liu Y, Cao J, Zhu YN, Ma Y, Murtaza G, Li Y, et al. C1222C deletion in exon 8 of ABL1 is involved in carcinogenesis and cell cycle control of colorectal cancer through IRS1/PI3K/akt pathway. Front Oncol 2020;10:1385.
[Crossref] [Google Scholar] [Pub Med]
- Streleckiene G, Inciuraite R, Juzenas S, Salteniene V, Steponaitiene R, Gyvyte U, et al. MiR-20b and miR-451a are involved in gastric carcinogenesis through the PI3K/AKT/mTOR signaling pathway: Data from gastric cancer patients, cell lines and ins-gas mouse model. Int J Mol Sci 2020;21(3):877.
[Crossref] [Google Scholar] [Pub Med]
- Li A, Zhao F, Zhao Y, Liu H, Wang Z. ATF4-mediated GDF15 suppresses LPS-induced inflammation and MUC5AC in human nasal epithelial cells through the PI3K/Akt pathway. Life Sci 2021;275:119356.
[Crossref] [Google Scholar] [Pub Med]
- Jia M, Chen X, Liu J, Chen J. PTEN promotes apoptosis of H2O2‑injured rat nasal epithelial cells through PI3K/Akt and other pathways. Mol Med Rep 2018;17(1):571-9.
[Crossref] [Google Scholar] [Pub Med]