- *Corresponding Author:
- Yanping Zhang
Department of Gastroenterology, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital,
Third Hospital of Shanxi Medical University, Taiyuan 030032, China
E-mail: caoping842@126.com
| Date of Received | 30 March 2022 |
| Date of Revision | 12 February 2023 |
| Date of Acceptance | 28 September 2023 |
| Indian J Pharm Sci 2023;85(5):1422-1428 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The study includes the function of tramadol on the oncogenic phenotypes of SNU-1 gastric cancer cells and its mechanism. SNU-1 cells were divided into control group, tramadol group, microRNA-negative control group, microRNA-212-5p group, 300 μg/ml tramadol+anti-microRNA-negative control group and 300 μg/ ml tramadol+anti-microRNA-212-5p group. SNU-1 cell proliferation, invasiveness and migration were quantified. Reverse transcription-quantitative polymerase chain reaction and Western blot examined the microRNA-212-5p and beta-catenin expression. The proliferation, invasiveness and migration of SNU-1 cells in tramadol (75 μg/ml, 150 μg/ml or 300 μg/ml) group were significantly reduced. Tramadol elevated microRNA-212-5p level in SNU-1 cells, and microRNA-212-5p elevation suppressed SNU-1 cell proliferation, migration and invasion. Moreover, microRNA-212-5p deficiency reversed the anticancer activity of tramadol in SNU-1 cells. Additionally, tramadol declined beta-catenin protein in SNU-1 cells, while microRNA-212- 5p deficiency abolished this effect of tramadol. Tramadol inhibited SNU-1 gastric cancer cell proliferation, invasiveness and migration by the microRNA-212-5p/Wnt/β-catenin pathway.
Keywords
Tramadol, gastric cancer, microRNA-212-5p, Wnt/β-catenin, chemotherapy
Currently, gastric cancer remains the main cause of deaths worldwide, even the improvement in treatment strategies such as surgical resection and chemotherapy[1]. Moreover, the high recurrence rates and tumor metastasis is still the main obstacles for prolonging the survival rate of gastric cancer patients[2]. Accordingly, identifying the regulatory mechanisms for gastric cancer progression is crucial for developing new treatment strategies.
Tramadol is a well-tolerated nonsteroidal anti- inflammatory drugs widely used for the treatment of various pain syndromes and postoperative pain, including cancer-related pain[3,4]. Clinical application has proved that the analgesic effect of tramadol is better than most non-opioid drugs, and the toxicity is lower than morphine and pethidine. Tramadol is the only non-narcotic analgesic drug in the guidelines of the Ministry of Health's three-step analgesic therapy for cancer patients with moderate pain. Recent studies have manifested that anesthetic analgesics such as morphine and propofol, can impair malignant behaviors of tumor cells, meanwhile, the application of them in cancer surgery is expected to reduce the risk of postoperative recurrence and metastasis[5-7]. In addition, Tramadol was proved to suppress cell mobility and survival in lung adenocarcinoma via blocking Phosphoinositide 3-Kinase (PI3K)/ Protein Kinase B (AKT) through PTEN[8]. Tramadol repressed the metastasis and enhanced cytotoxic effects in breast cancer[9]. Nevertheless, effects of tramadol on gastric cancer cell malignant behaviors are till vague. microRNA (miR)-212-5p is a tumor-regulated miRNA. It was found to miR- 212-5p overexpression reduced proliferation in gastric cancer cells, which might be related to long non-coding RNA (lncRNA) MIF-AS1/miR-212-5p axis[10]. Through the preliminary experiment, we found tramadol treatment significantly increased miR-212-5p content in gastric cancer cells. Here, we speculated that tramadol might exert its anticancer function in gastric cancer by affecting miR-212-5p level.
Here, this study analyzed the functions of tramadol and miR-212-5p on gastric cancer cell oncogenic phenotypes in vitro, aiming to lay a theoretical foundation for the application of tramadol in gastric cancer therapy.
Materials and Methods
Cell culture and treatment:
SNU-1 cells (Procell, Wuhan, China) were maintained in Roswell Park Memorial Institute (RPMI)-1640 (BIOSUN, Shanghai, China) plus 10 % Fetal Bovine Serum (FBS) (BIOSUN) with 5 % Carbon dioxide (CO2) at 37°.
Tramadol was obtained from Huarenrizhao (Shandong, China). SNU-1 cells were treated with 75 μg/ml, 150 μg/ml, or 300 μg/ml tramadol for 48 h. 300 μg/ml tramadol was used for further analysis.
Cell transfection:
The miR-212-5p mimic, inhibitor (anti-miR-212-5p) and the negative control (miR-NC and anti- miR-NC) were designed by RiboBio (Guangzhou, China). Cell transfection was conducted followed in full compliance with the commercial instructions of Lipofectamine 3000.
Cell Counting Kit-8 (CCK-8) assay:
SNU-1 cells (1×104 cells/well) were inoculated in a 96-well plates with 10 μl CCK-8 solution, followed by recoding the absorbance at 450 nm after 2 h incubation.
Colony formation assay:
SNU-1 cells were incubated for 14 d in a 5 % CO2 incubator. Cell colonies were stained with 0.1 % crystal violet (Beyotime, Shanghai, China) and colonies (≥50 cells) were counted.
Transwell assay:
Place the 24-well plate on flat ice and gently move it into the 24-well plate by holding the edge of the transwell chamber with sterile tweezers.
Take an appropriate amount of medium in to the chamber and then suction it out to make the chamber wet and easy to spread glue. The Matrigel diluted by medium was spread on the transwell cell polycarbonate film in a volume of 35 µl for invasion assay, and uncoated polycarbonate film was used for migration. Accurately l×105 cells/ well were put into the upper chamber, and the cell suspended fluid volume is 200 µl. 24 h later, the images of migrated cells were collected and migrated cells were counted after dyeing with 0.1 % crystal violet.
Western blot:
SNU-1 cells are cleaved in Radioimmunoprecipitation Assay (RIPA) buffer to collect supernatant. After separating by Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and transferring to nitrocellulose membranes, membranes were incubated with primary antibodies at 4° all night, and following secondary antibody for 2 h at 37°. After Electrochemiluminescence (ECL) incubation, Quantity One software was adopted to measure the gray value of protein bands.
Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR):
TRIzol reagent (TaKaRa, Beijing, China) was adopted for the isolation of total Ribonucleic Acid (RNAs). The extracted RNA was reversely transcribed into complimentary Deoxyribonu- cleic Acid (cDNA), and cDNA was subsequently quantified using the SYBR-Green (TaKaRa) with following conditions; 94° 5 min, 94° 30 sec for 30 cycles, 57° 45 sec and 72° 45 sec. miR-212-5p levels were assayed by the 2-ΔΔCt method with U6 as an internal reference. The sequences of prim- ers; miR-212-5p forward 5’-GGA AAC ATC CTC GAC TG-3’ and reverse 5’-ATT GAA CGT GCC TCC GTG TTG AGG-3’.
Statistical analysis:
The data were presented by x͞ ±s. The comparison of the two groups or multiple groups was conduct- ed using t-test or Analysis of Variance (ANOVA). p<0.05 meant significant difference.
Results and Discussion
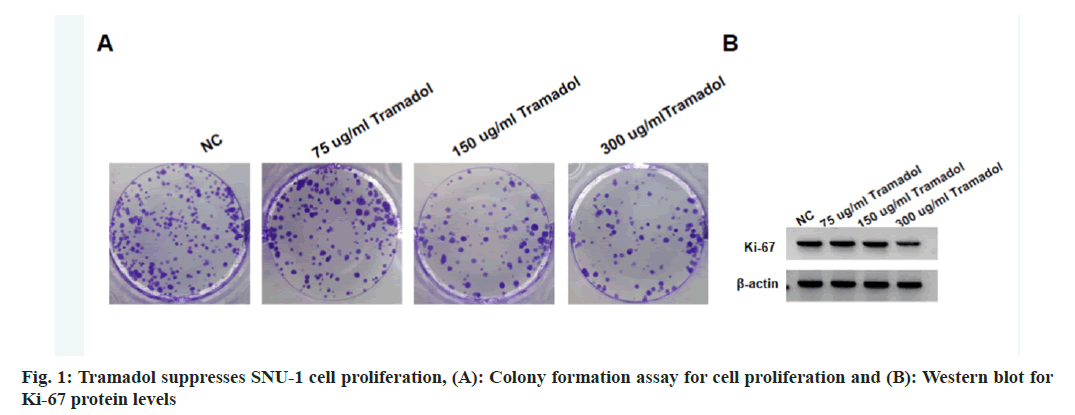
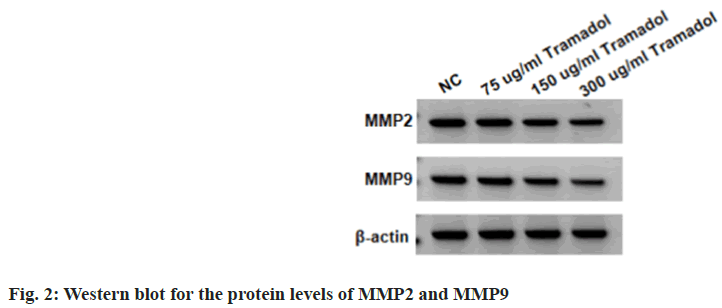
Compared with the NC group, the viability and DNA synthesis activity, as well as levels of Ki-67 were significantly reduced in SNU-1 cells with 75 μg/ml, 150 μg/ml, or 300 μg/ml tramadol treatment as shown in fig. 1A and fig. 1B, and Table 1. The migratory and invasion abilities of SNU-1 cells were notably repressed in 75 μg/ml, 150 μg/ml or 300 μg/ml tramadol treated group relative to the NC group as shown in fig. 2 and Table 2.
| Group | A values | Colonies | Ki-67 |
|---|---|---|---|
| NC | 1.135±0.10 | 124±10.36 | 0.83±0.08 |
| 75 μg/ml | 0.923±0.08* | 105±8.37* | 0.71±0.06* |
| 150 μg/ml | 0.635±0.07* | 73±5.96* | 0.57±0.05* |
| 300 μg/ml | 0.584±0.05* | 57±5.03* | 0.44±0.04* |
| F | 100.716 | 138.975 | 73.085 |
| p | 0.000 | 0.000 | 0.000 |
Note: Relative to NC group, *p<0.05
Table 1: Tramadol Suppresses SNU-1 Cell DNA Synthesis Activity (x͞±s, n=9)
| Group | Migration number | Invasion number | MMP2 | MMP9 |
|---|---|---|---|---|
| NC | 218±18.15 | 184±15.02 | 0.92±0.09 | 0.75±0.07 |
| 75 μg/ml | 182±15.03* | 154±13.28* | 0.72±0.07* | 0.62±0.05* |
| 150 μg/ml | 141±10.37* | 121±10.24* | 0.54±0.06* | 0.48±0.04* |
| 300 μg/ml | 105±8.35* | 81±7.34* | 0.45±0.04* | 0.37±0.03* |
| F | 118.348 | 125.716 | 85.5 | 99.515 |
| P | 0 | 0 | 0 | 0 |
Note: Relative to NC group, *p<0.05
Table 2: Tramadol Suppresses SNU-1 Cell Migration and Invasion (x͞±s, n=9)
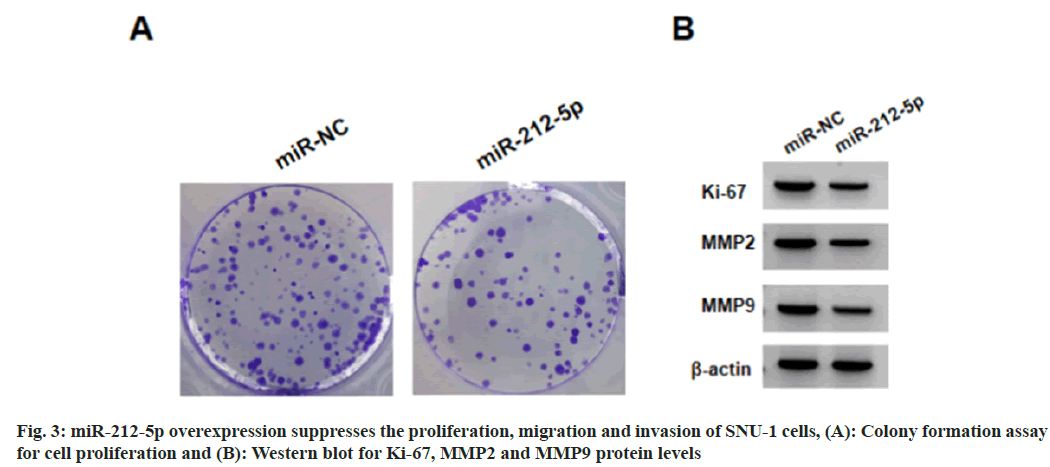
Relative to the NC group, we found miR-212-5p levels were markedly elevated in SNU-1 cells with 75 μg/ml, 150 μg/ml, or 300 μg/ml tramadol treatment (Table 3). As exhibited in fig. 3 and Table 4, miR-212-5p mimic introduction markedly elevated miR-212-5p expression, functionally, forced expression of miR-212-5p suppressed cell viability, DNA synthesis activity, migration and invasion, as well as reduced the levels of Ki-67, Matrix Metalloproteinase (MMP)-2 and MMP9 in SNU-1 cells.
| Group | miR-212-5p |
|---|---|
| NC | 1.00±0.10 |
| 75 μg/ml | 1.76±0.15* |
| 150 μg/ml | 2.38±0.21* |
| 300 μg/ml | 2.72±0.25* |
| F | 147.994 |
| p | 0.000 |
Note: Relative to NC group, *p<0.05
Table 3: Tramadol Elevates miR-212-5p Expression in SNU-1 Cells (x͞±s, n=9)
| Group | miR-212-5p | A values | Colonies | Ki-67 | MMP2 | MMP9 | Migration number | Invasion number |
|---|---|---|---|---|---|---|---|---|
| miR-NC | 1.00±0.11 | 1.134±0.11 | 128±9.67 | 0.84±0.07 | 0.93±0.09 | 0.73±0.07 | 214±17.63 | 181±14.33 |
| miR-212-5p | 2.34±0.19* | 0.594±0.05* | 61±5.34* | 0.41±0.04* | 0.46±0.02* | 0.39±0.03* | 97±8.31* | 76±6.51* |
| t | 18.311 | 13.407 | 18.196 | 16 | 15.294 | 13.393 | 18.009 | 20.013 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Relative to the miR-NC group, *p<0.05
Table 4: miR-212-5p Overexpression Suppresses the Proliferation, Migration and Invasion of SNU-1 Cells
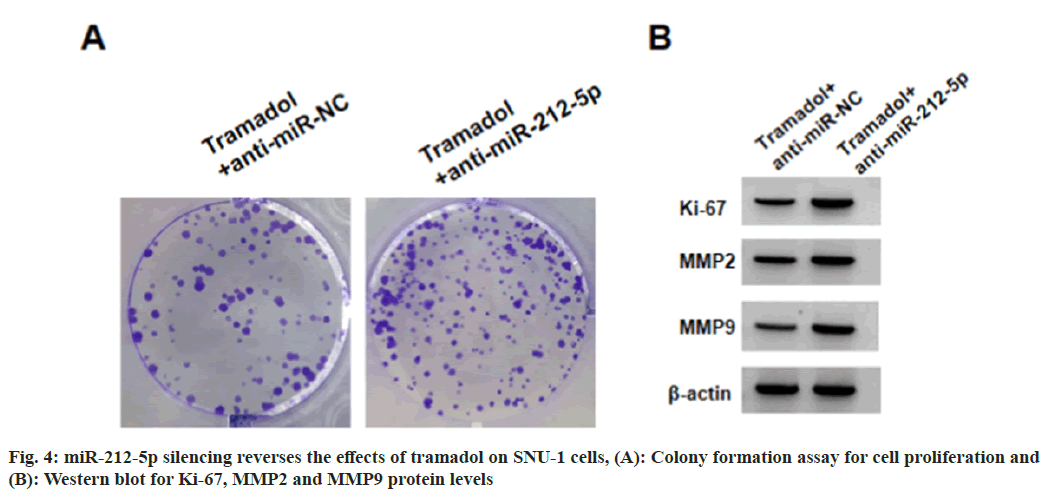
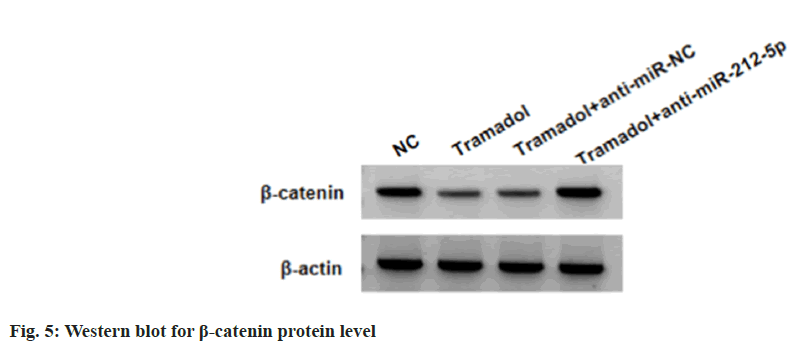
SNU-1 cells were transduced with anti-miR-212-5p or anti-miR-NC, followed by treating with 300 μg/ml tramadol. As expected, anti-miR-212-5p introduction reduced tramadol-evoked elevation of miR-212-5p levels in SNU-1 cells (Table 5). Functionally, the suppression of tramadol on SNU-1 cell viability, DNA synthesis activity, migration and invasion, as well as the reduction of tramadol on Ki-67, MMP2 and MMP9 protein levels in SNU-1 cells were abolished by anti-miR- 212-5p introduction (Table 5, fig. 4A and fig. 4B). Tramadol treatment reduced the protein levels of β-catenin, which was rescued by miR-212-5p silencing (fig. 5 and Table 6).
| Group | miR-212-5p | A values | Colonies | Ki-67 | MMP2 | MMP9 | Migration number | Invasion number |
|---|---|---|---|---|---|---|---|---|
| Tramadol+anti-miR-NC | 1.00±0.10 | 0.581±0.04 | 59±5.14 | 0.43±0.04 | 0.47±0.04 | 0.36±0.03 | 103±9.17 | 83±7.12 |
| Tramadol+anti-miR-212-5p | 0.41±0.04* | 1.096±0.09* | 127±10.66* | 0.86±0.08* | 0.94±0.09* | 0.78±0.07* | 228±20.06* | 191±15.37* |
| t | 16.434 | 15.687 | 17.238 | 14.423 | 14.316 | 16.545 | 17.002 | 19.127 |
| P | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Relative to the tramadol+anti-miR-NC group, *p<0.05
Table 5: miR-212-5p Silencing Reverses the Effects of Tramadol on SNU-1 Cells
| Group | β-catenin |
|---|---|
| NC | 0.71±0.07 |
| Tramadol | 0.32±0.04* |
| Tramadol+anti-miR-NC | 0.34±0.03 |
| Tramadol+anti-miR-212-5p | 0.75±0.06# |
| F | 175.636 |
| p | 0.000 |
Note: Relative to the NC group, *p<0.05 and relative to the tramadol+anti-miR-NC group, #p<0.05
Table 6: Tramadol Inactivates WNT/β-Catenin Pathway by miR-212-5p
In traditional drugs, painkillers are mostly narcotic analgesics and some psychotropic drugs, which are easy to produce a certain degree of dependence and tolerance, resulting in medical personnel having certain concerns when using pain-relieving drugs, and they are reluctant to use them easily, resulting in many pain symptoms not being controlled in time. Tramadol is a type of artificially synthesized central analgesic drug that acts on μ-opioid receptors, as well as norepinephrine and serotonin systems, are used to alleviate moderate to severe pain[11]. The main problem with the use of opioid painkillers is their side effects, especially in outpatient patients and patients who need to take long-term medication, which can cause nausea, vomiting, dizziness, drowsiness, and cardiovascular and respiratory depression, as well as drug dependence. Tramadol has fewer side effects than the former, and has no inhibitory effect on the respiratory and cardiovascular systems, and rarely produces body dependence and tolerance. At present, the effects of anesthetic analgesics on tumor growth and metastasis are currently one of the hotspots in oncology research. Research shows that tramadol repressed the mobility and survival of breast cancer cells via blocking α2-adrenoceptor signaling[12]. At the same time, tramadol could induce apoptosis and reduce Reactive Oxygen Species (ROS) production in endometrial cancer cells, and enhanced the cytotoxic effects of cisplatin or doxorubicin in endometrial cancer[13]. In addition, tramadol had cytotoxic effects on colon cancer stem cells and triggered cell apoptosis[14]. Thereafter, this study displayed that tramadol treatment significantly impaired the proliferative, migratory and invasive abilities of gastric cancer cells. Ki-67 protein is a widely used marker for proliferation in cancer cells, and has function in mitotic and interphase cells[15]. MMP-2 and MMP- 9 participate in the degradation of key components of the basement membrane and extracellular matrix, leading to tumor metastasis[16]. We observed tramadol treatment reduced Ki-67, MMP2 and MMP9 levels, further implying the anticancer activity of tramadol in gastric cancer.
Research displayed that miR-212-5p overexpression was able to impede breast cancer cell Epithelial-to-Mesenchymal Transition (EMT)[17]. The clear renal cell carcinoma showed decreased miR-212-5p level, and its elevation impaired cell growth and migration[18]. Glioma cells also displayed low miR-212-5p expression, which elevation could hinder glioma tumor growth in nude mice[19]. miR-212-5p performed tumor suppressing activity in breast cancer by impairing cell glycolysis, growth and metastasis, which might be related to circWHSC1/miR-212-5p/ AKT3 axis[20]. Propofol suppressed ovarian cancer cell glycolysis and growth by elevating miR-212- 5p through decreasing circ-ZFR expression[21]. In our work, gastric cancer cell showed increased miR-212-5p after tramadol treatment, moreover, forced expression of it restrained gastric cancer cell mobility and proliferation, importantly, and its silencing reversed the anticancer action of tramadol on tumor cells.
The Wnt/β-catenin pathway is implicated in diverse physiological processes, and the abnormal activation of this pathway is closely linked with the occurrence and metastasis of tumors, and targeting inhibition of Wntn/β-catenin pathway has been recognized as a potential cancer treatment strategy[22]. In gastric cancer, research reports that noncoding RNAs, such as LINC01133[23] and miR-6838-5p[24], inactivated Wnt/β-catenin pathway to impair multiple malignant processes of gastric cancer. The aberrant Wnt/β-catenin activation could enhance the arrest of ferroptotic death in gastric cancer cells via promoting the transcription of GPX4[25]. SERPINH1 evoked Wnt/β-catenin activation to boost the metastasis of gastric cancer[26]. In addition, the apoptosis induction and proliferation arrest activity of miR-212-5p in acute myeloid leukemia were achieved by the blockage of Wntn/β-catenin pathway[27]. Besides that, LINC00115 activated Wnt/β-catenin axis in prostate cancer cell by increasing FZD5 through absorbing miR-212-5p, thereby promoting the invasiveness and survival of prostate cancer cells[28]. Also, this study confirmed tramadol treatment mediated the blockage of Wnt/β-catenin pathway via miR-6838-5p.
In summary, tramadol impaired the proliferative, migratory and invasive abilities of gastric cancer cells via modulating miR-212-5p/Wntn/β-catenin axis. These data provide important evidences for tramadol application in surgical treatment of gastric cancer.
Conflict of interests:
The authors declared no conflict of interests.
References
- Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol 2020;18(3):534-42.
[Crossref] [Google Scholar] [PubMed]
- Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: Epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol 2023;20(5):338-49.
[Crossref] [Google Scholar] [PubMed]
- Barakji J, Korang SK, Feinberg JB, Maagaard M, Mathiesen O, Gluud C, et al. Tramadol for chronic pain in adults: Protocol for a systematic review with meta-analysis and trial sequential analysis of randomized clinical trials. Syst Rev 2023;12(1):145.
[Crossref] [Google Scholar] [PubMed]
- Kuramochi T, Sano M, Kajiwara I, Oshima Y, Itaya T, Kim J, et al. Effects of tramadol via a µ-opioid receptor on pancreatic ductal adenocarcinoma in vitro and in vivo. Reg Anesth Pain Med 2023.
[Crossref] [Google Scholar] [PubMed]
- Zhang B, Hou Q, Zhang X, Ma Y, Yuan J, Li S, et al. Anesthetic propofol inhibits ferroptosis and aggravates distant cancer metastasis via Nrf2 upregulation. Free Radic Biol Med 2023;195:298-308.
[Crossref] [Google Scholar] [PubMed]
- Cao Y, Fan L, Li L, Zhou J. Propofol suppresses cell proliferation in gastric cancer cells through NRF2-mediated polyol pathway. Clin Exp Pharmacol Physiol 2022;49(2):264-74.
[Crossref] [Google Scholar] [PubMed]
- Vecera L, Prasil P, Srovnal J, Berta E, Vidlarova M, Gabrhelik T, et al. Morphine analgesia, cannabinoid receptor 2 and opioid growth factor receptor cancer tissue expression improve survival after pancreatic cancer surgery. Cancers 2023;15(16):4038.
[Crossref] [Google Scholar] [PubMed]
- Xia M, Tong JH, Ji NN, Duan ML, Tan YH, Xu JG. Tramadol regulates proliferation, migration and invasion via PTEN/PI3K/AKT signaling in lung adenocarcinoma cells. Eur Rev Med Pharmacol Sci 2016;20(12):2573-80.
[Google Scholar] [PubMed]
- Huang YH, Sue SH, Wu ZS, Huang SM, Lee SY, Wu ZF. Antitumorigenic effect of tramadol and synergistic effect with doxorubicin in human breast cancer cells. Front Oncol 2022;12:811716.
[Crossref] [Google Scholar] [PubMed]
- Li L, Li Y, Huang Y, Ouyang Y, Zhu Y, Wang Y, et al. Long non-coding RNA MIF-AS 1 promotes gastric cancer cell proliferation and reduces apoptosis to upregulate NDUFA 4. Cancer Sci 2018;109(12):3714-25.
[Crossref] [Google Scholar] [PubMed]
- Barakat A. Revisiting tramadol: A multi-modal agent for pain management. CNS Drugs 2019;33(5):481-501.
[Crossref] [Google Scholar] [PubMed]
- Xia M, Tong JH, Zhou ZQ, Duan ML, Xu JG, Zeng HJ, et al. Tramadol inhibits proliferation, migration and invasion via α2-adrenoceptor signaling in breast cancer cells. Eur Rev Med Pharmacol Sci 2016;20(1):157-65.
[Google Scholar] [PubMed]
- Liu LC, Wu ZS, Chen JL, Wu ZF, Lai HC, Huang YH. Mitochondrial dysfunction involved in the cytotoxicity of tramadol in human endometrial carcinoma cells. Int J Mol Sci 2022;24(1):99.
[Crossref] [Google Scholar] [PubMed]
- Ozgurbuz U, Gencur S, Kurt FO, Ozkalkanlı M, Vatansever HS. The effects of tramadol on cancer stem cells and metabolic changes in colon carcinoma cells lines. Gene 2019;718:144030.
[Crossref] [Google Scholar] [PubMed]
- Sun X, Kaufman PD. Ki-67: More than a proliferation marker. Chromosoma 2018;127:175-86.
[Crossref] [Google Scholar] [PubMed]
- Jiang H, Li H. Prognostic values of tumoral MMP2 and MMP9 overexpression in breast cancer: A systematic review and meta-analysis. BMC Cancer 2021;21(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- Lv ZD, Yang DX, Liu XP, Jin LY, Wang XG, Yang ZC, et al. miR-212-5p suppresses the epithelial-mesenchymal transition in triple-negative breast cancer by targeting Prrx2. Cell Physiol Biochem 2018;44(5):1785-95.
[Crossref] [Google Scholar] [PubMed]
- Deng JH, Zheng GY, Li HZ, Ji ZG. miR-212-5p inhibits the malignant behavior of clear cell renal cell carcinoma cells by targeting TBX15. Eur Rev Med Pharmacol Sci 2019;23(24):10699-707.
[Crossref] [Google Scholar] [PubMed]
- Chong Y, Zhu C, Hu W, Jiang C, Liang W, Zhu Z. miR-212-5p suppresses glioma development via targeting SUMO2. Biochem Genet 2023;61(1):35-47.
[Crossref] [Google Scholar] [PubMed]
- Ding L, Xie Z. CircWHSC1 regulates malignancy and glycolysis by the miR-212-5p/AKT3 pathway in triple-negative breast cancer. Exp Mol Pathol 2021;123:104704.
[Crossref] [Google Scholar] [PubMed]
- Qu D, Zou X, Liu Z. Propofol modulates glycolysis reprogramming of ovarian tumor via restraining circular RNA-zinc finger RNA-binding protein/microRNA-212-5p/superoxide dismutase 2 axis. Bioengineered 2022;13(5):11881-92.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol 2020;13(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Yang XZ, Cheng TT, He QJ, Lei ZY, Chi J, Tang Z, et al. LINC01133 as ceRNA inhibits gastric cancer progression by sponging miR-106a-3p to regulate APC expression and the Wnt/β-catenin pathway. Mol Cancer 2018;17(1):126.
[Crossref] [Google Scholar] [PubMed]
- Zhou W, Ding X, Jin P, Li P. miR-6838-5p affects cell growth, migration and invasion by targeting GPRIN3 via the Wnt/β-catenin signaling pathway in gastric cancer. Pathobiology 2020;87(6):327-37.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Zheng L, Shang W, Yang Z, Li T, Liu F, et al. Wnt/beta-catenin signaling confers ferroptosis resistance by targeting GPX4 in gastric cancer. Cell Death Differ 2022;29(11):2190-202.
[Crossref] [Google Scholar] [PubMed]
- Tian S, Peng P, Li J, Deng H, Zhan N, Zeng Z, et al. SERPINH1 regulates EMT and gastric cancer metastasis via the Wnt/β-catenin signaling pathway. Aging (Albany NY) 2020;12(4):3574-93.
[Crossref] [Google Scholar] [PubMed]
- Lin JF, Zeng H, Zhao JQ. miR-212-5p regulates the proliferation and apoptosis of AML cells through targeting FZD5. Eur Rev Med Pharmacol Sci 2018;22(23):8415-22.
[Crossref] [Google Scholar] [PubMed]
- Peng N, Zhang Z, Wang Y, Yang M, Fan J, Wang Q, et al. Down-regulated LINC00115 inhibits prostate cancer cell proliferation and invasion via targeting miR-212-5p/FZD5/Wnt/β-catenin axis. J Cell Mol Med 2021;25(22):10627-37.
[Crossref] [Google Scholar] [PubMed]