- *Corresponding Author:
- Lili Deng
Department of Cardiovascular Medicine, Ankang People Hospital, Ankang, Shaanxi Province 725000, China
E-mail: denglili0414@163.com
| Date of Received | 30 October 2022 |
| Date of Revision | 25 June 2023 |
| Date of Acceptance | 05 April 2024 |
| Indian J Pharm Sci 2024;86(2):494-501 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the protective mechanism of total flavonoids from Eucommia ulmoides Oliver leaves (thin-film electroluminescent) on lipopolysaccharide-induced cardiomyocyte H9C2 injury. Cardiomyocytes were divided into control, lipopolysaccharides, lipopolysaccharides+thin-film electroluminescent-low group, lipopolysaccharides+thin-film electroluminescent-middle, lipopolysaccharides+thin-film electroluminescent-high, lipopolysaccharides+microRNA-negative control, lipopolysaccharides+microRNA-494, lipopolysaccharides+thin-film electroluminescent+anti-microRNA-negative control, and lipopolysaccharides+thin-film electroluminescent+anti-microRNA-494 groups. The commercial kits were used to evaluate superoxide dismutase and glutathione peroxidase activities, as well as malondialdehyde levels. Flow cytometry was utilized for the estimation of cell apoptosis. Western blotting was employed to detect cleaved caspase-3 and cleaved caspase-9 protein levels. Reverse transcription-quantitative polymerase chain reaction was selected to detect microRNA-494 expression. Lipopolysaccharides significantly decreased malondialdehyde levels, increased glutathione peroxidase and superoxide dismutase activities, induced cell apoptosis, and upregulated cleaved caspase-3 and cleaved caspase-9 protein levels in cardiomyocytes, suggesting that lipopolysaccharides facilitated cardiomyocyte oxidative stress and apoptosis. Thin-film electroluminescent overtly weakened lipopolysaccharides induced cardiomyocyte oxidative stress and apoptosis in a dose-dependent mode. Thin-film electroluminescent partly overturned lipopolysaccharides induced downregulation of microRNA-494 expression in cardiomyocytes. Furthermore, elevation of microRNA-494 impaired lipopolysaccharides-induced cardiomyocyte oxidative stress and apoptosis. In addition, microRNA-494 knockdown weakened thin-film electroluminescent mediated repressive effects on lipopolysaccharides induced cardiomyocyte oxidative stress and apoptosis. Thin-film electroluminescent could alleviate lipopolysaccharides induced cardiomyocyte oxidative stress and apoptosis, which was achieved by upregulating microRNA-494 expression.
Keywords
Eucommia flavonoids, lipopolysaccharide, cardiomyocytes, oxidative stress, cardiomyopathy
Sepsis is a syndrome involving multi-organ dysfunction and systemic inflammatory response that is attributed to infection. Cardiomyopathy injury is the most important feature of sepsis and it can significantly increase mortality in septic patients[1]. A clinical cohort study showed that 63 % of sepsis patients experienced left heart dysfunction[2]. Sepsis-Induced Myocardial Injury (SIMI) can cause structural remodeling and electrical activity dysfunction of the heart, leading to cardiac pumping failure[3]. Currently, certain drugs have been identified to improve myocardial contractile function in sepsis, such as dobutamine, mitochondrial protectors, and inflammatory factor inhibitors[4], but the therapeutic effect is poor. Therefore, searching for a therapeutic approach to alleviate myocardial dysfunction during sepsis is vital.
In the context of sepsis, mitochondria generate overload of Reactive Oxygen Species (ROS), contributing to a cascade of events including oxidative damage and apoptosis in cardiomyocytes, ultimately bringing about cardiomyocyte death[5]. Accordingly, drugs with antioxidant and anti-apoptotic properties may have a protective effect against SIMI[6,7]. Eucommia ulmoides Oliver (EUO) is a traditional Chinese medicine in China, with various active ingredients such as flavonoids and lignans[8]. Total flavonoids from EUO leaves (Thin-Film Electroluminescent (TFEL)) are potential natural antioxidants that can directly scavenge free radicals and hydroxyl radicals, as well as inhibit ROS production[9,10]. In addition, TFEL has a protective effect against hydrogen peroxide-induced injury in intestinal epithelial cells[11]. However, its effect on myocardial injury in sepsis is not well understood.
MicroRNAs (miRNAs) are single-stranded Ribonuclic Acid (RNA) (approximately 22 nt), which are widely involved in cardiomyocyte essential activities by regulating their downstream target messenger RNA (mRNA) expression[12]. It was pointed out that miR-494 was downregulated in septic shock patients and had a negative correlation with myocardial injury, and miR-494 overexpression was shown to restrain SIMI and protect cardiomyocyte function[13]. Lipopolysaccharide (LPS) is a major initiator of organ dysfunction in sepsis[14]. Our study used LPS-treated cardiomyocytes H9C2 to simulate cardiomyocyte injury models for investigating the protection of TFEL against LPS-induced cardiomyocyte injury. Furthermore, we also explored the potential mechanisms involved in its protective action by surrounding miR-494.
Materials and Methods
Experimental materials:
Rat cardiomyocytes H9C2 were procured from the Cell Bank of the Typical Culture Preservation Committee of the Chinese Academy of Sciences; EUO leaves were offered by the Chinese Pharmacy of our hospital; the Dulbecco's Modified Eagle Medium (DMEM) was bought from Gibco (United States of America (USA)); Fetal Bovine Serum (FBS) from Hangzhou Sijiqing Biological Company (Hangzhou, China); LPS (with purity of 97 %, No: YY11156) from Shanghai Yuanye Biological Company (Shanghai, China). The miRNA mimics, miRNA inhibitors (anti-miRNA), anti-miR-NC, miR-NC, and miRNA fluorescence quantitative Polymerase Chain Reaction (qPCR) kit from Shanghai Sangon Biological Company; Malondialdehyde (MDA) content assay kit, as well as Glutathione Peroxidase (GSH-Px) and Superoxide Dismutase (SOD) activity assay kits from Nanjing Jiancheng Biological Research Institute; Annexin V-Fluorescein Isothiocyanate (FITC) apoptosis detection kit, Radioimmunoprecipitation Assay (RIPA) lysate and Trizol reagent from Shanghai Beyotime; goat anti-rabbit Immunoglobulin G (IgG) (ab205718) and anti-Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) antibody (ab9485) from Abcam (Shanghai); anti-cleaved caspase-3 antibody (9661) from Shanghai Saixintong Biological Reagent Company; miRNA reverse transcription kit was acquired from Beijing BioLab Technology Company.
Methods:
Preparation of TFEL: For the extraction of TFEL, an experimental method mentioned by Yanni Lei was used[8]. Briefly, EUO leaves were crushed and extracted by ultrasonic extraction according to the material-liquid ratio (g/ml) of 1:15 for 2 h. The extraction was repeated three times at a power of 0.45 W/cm2, followed by purification by chromatographic column method. Using rutin as control, the content of TFEL was measured to be 81.5 %.
Culturing and transfection of cardiomyocytes H9C2: Cardiomyocytes H9C2 were cultured in DMEM medium containing 10 % FBS at 37° with 5 % Carbon dioxide (CO2). Cardiomyocytes were trypsin-digested and passaged (1:4) at 80 % fusion, and cardiomyocytes cultured to a logarithmic phase were used for experiments. After 24 h of incubation with a complete medium containing 0 (control), 25 (low), 50 (middle), or 100 μg/ml (high) of TFEL[11], cardiomyocytes were cultured for 6 h with a medium containing 10 mg/l of LPS, which were recorded as the LPS, LPS+TFEL-low, LPS+TFEL-middle, and LPS+TFEL-high groups sequentially.
Approximately 1×104 cardiomyocytes were inoculated into 24-well plates, and transfection of anti-miR-494, anti-miR-Negative Control (NC), miR-NC, and miR-494 mimics into cardiomyocytes with 40 % fusion was performed by referring to the instructions of Lipofectamine 2000, respectively. Transfection was performed for 48 h. Cardiomyocytes were collected and miR-494 expression was detected to verify the transfection efficiency.
Detection of intracellular MDA levels, as well as SOD and GSH-Px activities: Cardiomyocytes that were transfected and/or treated with LPS and/or TFEL were collected, followed by the addition of 500 μl of Phosphate Buffer Solution (PBS). Cells were broken by sonication and then centrifuged (1200 r/min, 4°, for 10 min). Supernatants were collected, and intracellular GSH-Px and SOD activities, as well as MDA production, were determined using the appropriate kits in line with the instructions.
Apoptosis detection by flow cytometry:
Specifically transfected and/or treated cardiomyocytes were collected and resuspended with 500 μl of 1×binding buffer (1×106 cells/ml). Cardiomyocytes were incubated with 5 μl of Annexin V-FITC and propidium iodide for 15 min away from light. Apoptotic rate of cardiomyocytes was estimated by flow cytometry.
Western blotting:
Lysis of cardiomyocytes with RIPA lysate was performed to extract total protein, and the protein concentration was established relying on the Bicinchoninic Acid (BCA) assay kit. Protein samples (50 μg/l) were resolved by Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and transferred to a Polyvinylidene Difluoride (PVDF) membrane. Membranes were enclosed with a solution containing 5 % skimmed milk for 90 min, followed by incubation with specific primary antibodies at 4° overnight. Thereafter, the membranes were incubated with a secondary antibody at room temperature for 1 h. Chemiluminescence was used to visualize the proteins. The optical density was determined using ImageJ software.
Reverse transcription-qPCR (RT-qPCR):
The total RNA extracted using the Trizol reagent was measured, and the A260/A280 values on qualified RNA samples were between 1.8 and 2.0. Taking 2 ng of total RNA, miRNA reverse transcription kit was used to synthesize complementary Deoxyribonucleic Acid (cDNA). RT-qPCR was performed using cDNA as a template using the miRNA fluorescence quantitative PCR kit following the procedure described below. The reaction system was composed of cDNA template (2 μl), reverse primer (0.5 μl, 10 μM), forward primer (0.5 μl, 10 μM), 2× miRNA qPCR master mix (10 μl), ROX reference dye (1 μl), and double distilled Water (ddH2O) (6 μl). 2-ΔΔCt method was utilized for the calculation of miR-494 expression (forward 5’-CGCTGAAACATACACACGGGAA-3’, reverse 5’-CAGT GCAGGGTCCGAGGTAT-3’), with U6 (forward 5’-CTCGCTTCGGCAGCACA-3’ and reverse 5’-AACGCTTC ACGAATTTGCGT-3’) as an internal reference.
Statistical analysis:
All data are presented as mean±standard deviation. Statistical differences between groups were assessed by analysis of variance. Data were statistically analyzed using Statistical Package for the Social Sciences (SPSS) 22.0 (IBM Japan, Tokyo, Japan). Variation was deemed statistically significant when p<0.05.
Results and Discussion
Stimulation with LPS caused a remarkable increase in MDA levels, as well as an obvious reduction in SOD and GSH-Px activities in cardiomyocytes H9C2. Strikingly, TFEL treatment eroded LPS-mediated effects on Oxidative Stress (OxS) markers MDA levels, as well as GSH-Px and SOD activities in a dose-dependent model (Table 1). A counteracting effect of TFEL on LPS-induced OxS in cardiomyocytes was demonstrated by these results.
| Group | MDA (μmol/g) | SOD (U/mg) | GSH-Px (U/g) |
|---|---|---|---|
| Control | 4.13±0.42 | 72.77±6.88 | 226.60±18.69 |
| LPS | 50.31±4.51* | 18.54±1.17* | 50.37±4.22* |
| LPS+TFEL-low | 38.01±3.09# | 33.12±3.24# | 81.25±6.87# |
| LPS+TFEL-middle | 22.86±2.09#& | 49.36±4.89#& | 127.62±11.53#& |
| LPS+TFEL-high | 11.96±1.07#&$ | 64.03±5.39#&$ | 196.31±14.71#&$ |
| F | 448.703 | 196.657 | 327.712 |
| p | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs. control, #p<0.05 vs. LPS, &p<0.05 vs. LPS+TFEL-low, and $p<0.05 vs. LPS+TFEL-middle
Table 1: TFEL decreased LPS-induced OxS in cardiomyocytes.
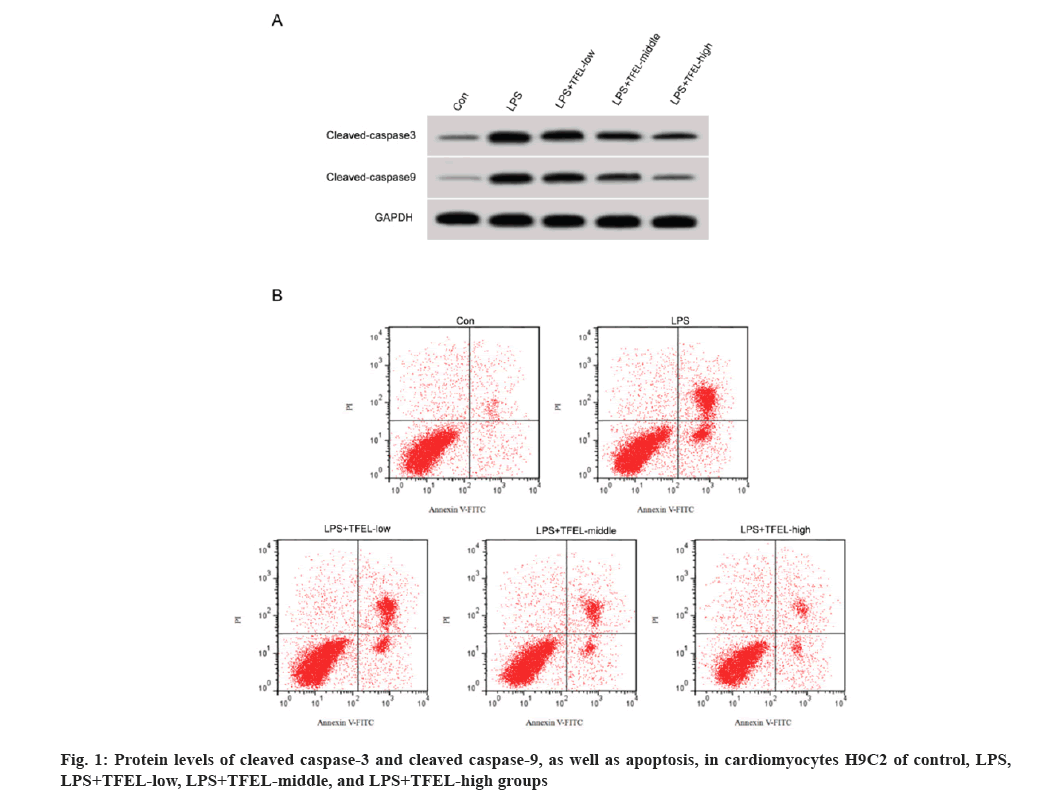
For apoptosis, we noted a significant increase in apoptosis, as well as cleaved caspase-3 and cleaved caspase-9 protein levels in cardiomyocytes H9C2 upon LPS stimulation. The above changes in cardiomyocytes evoked by LPS were abrogated following TFEL treatment (fig. 1A and fig. 1B, and Table 2).
| Group | Apoptotic rate (%) | Cleaved caspase-3 protein | Cleaved caspase-9 protein |
|---|---|---|---|
| Control | 7.14±0.59 | 0.23±0.02 | 0.11±0.02 |
| LPS | 35.73±3.02* | 0.77±0.05* | 0.66±0.04* |
| LPS+TFEL-low | 26.73±2.03# | 0.62±0.05# | 0.51±0.04# |
| LPS+TFEL-middle | 17.91±1.59#& | 0.47±0.04#& | 0.38±0.03#& |
| LPS+TFEL-high | 10.75±0.99#&$ | 0.31±0.03#&$ | 0.22±0.02#&$ |
| F | 360.202 | 277.975 | 444.765 |
| p | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs. control, #p<0.05 vs. LPS, &p<0.05 vs. LPS+TFEL-low, and $p<0.05 vs. LPS+TFEL-middle
Table 2: TFEL reduced LPS-induced cardiomyocyte apoptosis.
MiR-494 expression was significantly lower in cardiomyocytes H9C2 in the LPS group, but TFEL dose-dependently overrode the LPS-mediated influence on miR-494 expression as shown in Table 3.
| Group | miR-494 |
|---|---|
| Control | 1.00±0.00 |
| LPS | 0.29±0.03* |
| LPS+TFEL-low | 0.41±0.03# |
| LPS+TFEL-middle | 0.58±0.05#& |
| LPS+TFEL-high | 0.75±0.05#&$ |
| F | 521.007 |
| p | 0.000 |
Note: *p<0.05 vs. control, #p<0.05 vs. LPS, &p<0.05 vs. LPS+TFEL-low, and $p<0.05 vs. LPS+TFEL-middle
Table 3: TFEL dose-dependently reversed LPS-mediated influence on miR-494 expression in cardiomyocytes.
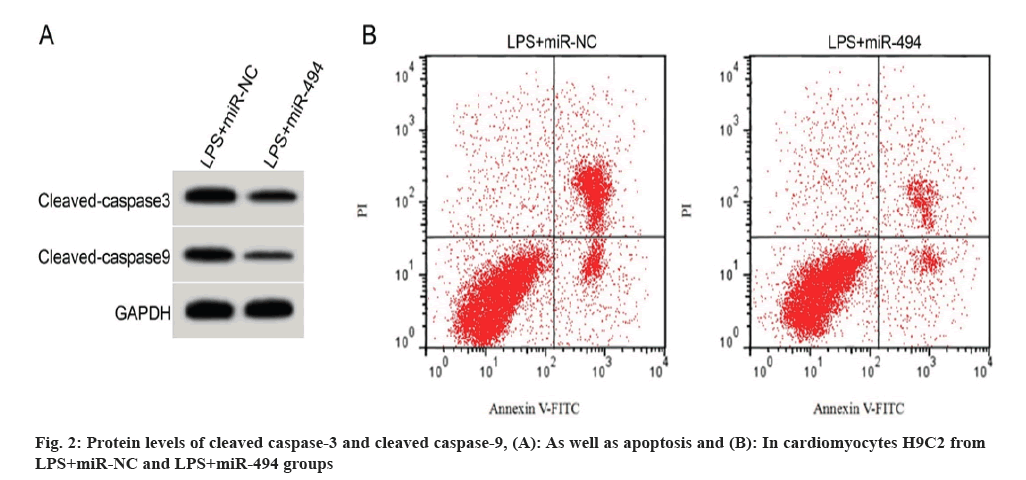
When compared with the LPS+miR-NC group, miR-494 expression, as well as SOD and GSH-Px activities of cardiomyocytes H9C2 in the LPS+miR-494 group were solidly higher, but MDA levels were strongly lower (Table 4). On the contrary, the apoptotic rate, as well as cleaved caspase-3 and cleaved caspase-9 protein expression were significantly lower in H9C2 cells in the LPS+miR-494 group vs. the LPS+miR-NC group (fig. 2A and fig. 2B, and Table 5).
| Group | miR-494 | MDA (μmol/g) | SOD (U/mg) | GSH-Px (U/g) |
|---|---|---|---|---|
| LPS+miR-NC | 1.00±0.00 | 51.12±4.93 | 17.05±1.57 | 46.61±4.16 |
| LPS+miR-494 | 3.29±0.27* | 19.03±1.69* | 55.31±4.71* | 150.78±12.52* |
| t | 25.444 | 18.472 | 23.119 | 23.688 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs. LPS+miR-NC
Table 4: miR-494 overexpression reversed LPS-forced cardiomyocyte OxS.
| Group | Apoptotic rate (%) | Cleaved caspase-3 protein | Cleaved caspase-9 protein |
|---|---|---|---|
| LPS+miR-NC | 36.61±3.12 | 0.79±0.06 | 0.67±0.05 |
| LPS+miR-494 | 13.96±1.07* | 0.39±0.03* | 0.28±0.03* |
| t | 20.601 | 17.889 | 20.065 |
| p | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs. LPS+miR-NC
Table 5: LPS-forced cardiomyocyte apoptosis was weakened by miR-494 overexpression.
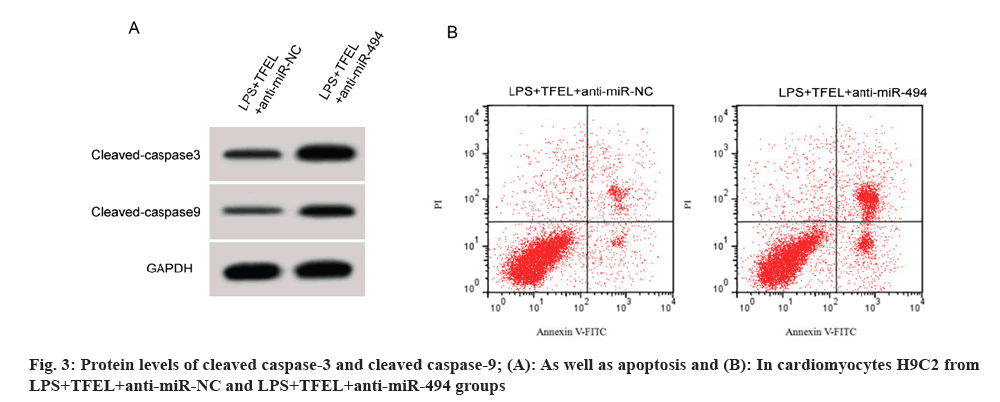
We observed that miR-494 expression, as well as GSH-Px and SOD activities, were markedly reduced in cardiomyocytes H9C2 in the LPS+TFEL+anti-miR-494 group relative to the specified control group, while MDA levels, apoptotic rate, as well as, cleaved caspase-3 and cleaved caspase-9 protein levels were significantly elevated (fig. 3A and fig. 3B, and Table 6).
| Group | miR-494 | MDA(μmol/g) | SOD(U/mg) | GSH-Px(U/g) | Apoptotic rate (%) | Cleaved caspase-3 protein | Cleaved caspase-9 protein |
|---|---|---|---|---|---|---|---|
| LPS+TFEL+anti-miR-NC | 1.00±0.00 | 10.69±1.18 | 67.47±5.34 | 201.87±16.13 | 9.76±0.90 | 0.29±0.03 | 0.20±0.02 |
| LPS+TFEL+anti-miR-494 | 0.41±0.04* | 39.66±3.14* | 29.26±2.47* | 62.51±5.86* | 23.22±2.22* | 0.67±0.05* | 0.54±0.05* |
| t | 44.25 | 25.909 | 19.483 | 24.362 | 16.857 | 19.551 | 18.941 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs. LPS+TFEL+anti-miR-NC
Table 6: Repression of miR-494 weakened TFEL-mediated influence on LPS-induced cardiomyocyte damage.
OxS has a major function in SIMI, which is reflected mainly in the overproduction of ROS and the consumption of antioxidants in the cardiac defense system, giving rise to an imbalance between oxidative and antioxidant activities[15]. SOD and GSH-Px activities, as well as MDA levels are the main parameters for evaluating the metabolism of free radicals[16]. GSH-Px and SOD are important myocardial antioxidant enzymes that scavenge oxygen radicals and convert superoxide radicals into oxygen and water ones[17]. Levels of MDA are routinely employed to estimate the extent of lipid peroxidation, which is a key indicator for cellular damage[17]. Findings of our study exhibited that TFEL was able to curb LPS-induced elevation of MDA levels, as well as enhancement of GSH-Px and SOD activities in a dose-dependent effect, implying that TFEL could restrain LPS-induced OxS in cardiomyocytes. Apart from OxS, apoptosis is another important event in LPS-induced myocardial injury[18]. Our findings demonstrated that TFEL repressed LPS-induced apoptosis and decreased the pro-apoptotic proteins cleaved caspase-3 and cleaved caspase-9 in cardiomyocytes H9C2, suggesting a protective effect of TFEL against LPS-induced apoptosis in cardiomyocytes. TFEL has been disclosed to improve sex hormone secretion, regulate disorders of glycolipid metabolism and inhibit ovarian hyperplasia by regulating Phosphoinositide 3-Kinase (PI3K)/Protein Kinase B (AKT) signaling[19]. Wang et al.[20] discovered that TFEL can activate the endogenous apoptotic pathway and make human glioblastoma cells re-sensitize to radiotherapy. Qiu et al.[21] exhibited that aucubin extracted from EUO resists acute lung injury in mouse models. Alsharif et al.[22] established that EUO-derived from protocatechuic acid reduces septic lung injury in mouse models, which may exert its effects by inhibiting OxS, inflammation and apoptosis. The above outcomes revealed that TFEL resisted LPS-induced cardiomyocyte injury.
Available studies have shown that miRNAs are important regulators of myocardial injury in sepsis[23]. A study published that miR-192-5p was significantly heightened in a sepsis-associated myocardial injury model, and miR-192-5p inhibited cardiomyocyte viability and induced apoptosis by targeting XIAP[24]. Moreover, miR-146a may ameliorate sepsis-induced myocardial inflammatory response and cardiac dysfunction by mediating the Toll-Like Receptor 4 (TLR4) signaling[25]. Also, miR-99a overexpression alleviated LPS-induced OxS and apoptosis in cardiomyocytes[26]. MiR-494 is a miRNA with protective effects against myocardial injury. It was reported that miR-494 has the ability to ameliorate ischemia-reperfusion-induced myocardial injury by targeting apoptosis-associated proteins[27]. Furthermore, miR-494 can inhibit hypoxia/reoxygenation-induced cardiomyocyte autophagy and apoptosis by targeting SIRT1[28]. In addition, miR-494 upregulation also mediates the protective effect of estrogen on cardiomyocytes against OxS[29]. Our study found that miR-494 expression in cardiomyocytes H9C2 was decreased after LPS treatment, and TFEL could counteract the LPS-induced downregulation of miR-494, implying that miR-494 may mediate the protective effect of TFEL against LPS-induced cardiomyocyte injury. Functional analysis showed that overexpression of miR-494 distinctly enhanced SOD and GSH-Px activities, as well as inhibited LPS-induced MDA production and apoptosis, which was in line with the protective effects of TFEL. Response experiments displayed that downregulation of miR-494 abrogated TFEL-mediated effects on LPS-induced apoptosis and oxidative damage in cardiomyocytes H9C2, which further confirmed that TFEL counteracted LPS-induced cardiomyocyte injury through upregulation of miR-494.
In conclusion, TFEL could alleviate cardiomyocyte oxidative damage and apoptosis caused by LPS, which was achieved by elevating miR-494 expression. This study preliminarily uncovered the possible mechanism of the protective effect of TFEL on cardiomyocyte injury, providing a theoretical basis for SIMI therapy.
Conflict of interests:
The authors declared no conflict of interests.
References
- Hollenberg SM, Singer M. Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol 2021;18(6):424-34.
[Crossref] [Google Scholar] [PubMed]
- Lanspa MJ, Cirulis MM, Wiley BM, Olsen TD, Wilson EL, Beesley SJ, et al. Right ventricular dysfunction in early sepsis and septic shock. Chest 2021;159(3):1055-63.
[Crossref] [Google Scholar] [PubMed]
- Bi CF, Liu J, Yang LS, Zhang JF. Research progress on the mechanism of sepsis induced myocardial injury. J Inflamm Res 2022;15:4275-90.
[Crossref] [Google Scholar] [PubMed]
- Khalid N, Patel PD, Alghareeb R, Hussain A, Maheshwari MV. The effect of sepsis on myocardial function: A review of pathophysiology, diagnostic criteria, and treatment. Cureus 2022;14(6):e26178.
[Crossref] [Google Scholar] [PubMed]
- Mongirdiene A, Skrodenis L, Varoneckaite L, Mierkyte G, Gerulis J. Reactive oxygen species induced pathways in heart failure pathogenesis and potential therapeutic strategies. Biomedicines 2022;10(3):602.
[Crossref] [Google Scholar] [PubMed]
- Sidheeque Hassan V, Hanifa M, Navik U, Bali A. Exogenous fetuin-A protects against sepsis-induced myocardial injury by inhibiting oxidative stress and inflammation in mice. Fundam Clin Pharmacol 2023;37(3):607-17.
[Crossref] [Google Scholar] [PubMed]
- Zhao L, Zhao H, Sun M, Chen M, Wu X, Deng C, et al. Kudzu celery decoction exerts protection against sepsis-induced myocardial injury. Oxid Med Cell Longev 2022;2022:2886932.
[Crossref] [Google Scholar] [PubMed]
- Wang CY, Tang L, He JW, Li J, Wang YZ. Ethnobotany, phytochemistry and pharmacological properties of Eucommia ulmoides: A review. Am J Chin Med 2019;47(2):259-300.
[Crossref] [Google Scholar] [PubMed]
- Fu G, Tong H, Zeng H, Zou B, Chai J, Zhang L, et al. Antioxidant and xanthine oxidase inhibitory activity of Eucommia ulmoides oliver leaf extracts. Pak J Pharm Sci 2018;31(4):1333-9.
[Google Scholar] [PubMed]
- Zhang Q, Su Y, Zhang J. Seasonal difference in antioxidant capacity and active compounds contents of Eucommia ulmoides Oliver leaf. Molecules 2013;18(2):1857-68.
[Crossref] [Google Scholar] [PubMed]
- Zhang C, Yuan D, Li L, Li, Y, Qin L, Cheng L, et al. Protective effects of total flavonoids of Eucommia ulmoides on the oxidative damage of IEC-6 cells induced by H2O2. Chin J Clin Pharmacol Ther 2015;50(10):4.
- Kansakar U, Varzideh F, Mone P, Jankauskas SS, Santulli G. Functional role of microRNAs in regulating cardiomyocyte death. Cells 2022;11(6):983.
[Crossref] [Google Scholar] [PubMed]
- Wu P, Kong L, Li J. MicroRNA-494-3p protects rat cardiomyocytes against septic shock via PTEN. Exp Ther Med 2019;17(3):1706-16.
[Crossref] [Google Scholar] [PubMed]
- Perez-Hernández EG, Delgado-Coello B, Luna-Reyes I, Mas-Oliva J. New insights into lipopolysaccharide inactivation mechanisms in sepsis. Biomed Pharmacother 2021;141:111890.
[Crossref] [Google Scholar] [PubMed]
- Joffre J, Hellman J. Oxidative stress and endothelial dysfunction in sepsis and acute inflammation. Antioxid Redox Signal 2021;35(15):1291-307.
[Crossref] [Google Scholar] [PubMed]
- Chen L, Liu P, Feng X, Ma C. Salidroside suppressing LPS-induced myocardial injury by inhibiting ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J Cell Mol Med 2017;21(12):3178-89.
[Crossref] [Google Scholar] [PubMed]
- Xing PC, An P, Hu GY, Wang DL, Zhou MJ. LncRNA MIAT promotes inflammation and oxidative stress in sepsis-induced cardiac injury by targeting miR-330-5p/TRAF6/NF-κB axis. Biochem Genet 2020;58(5):783-800.
[Crossref] [Google Scholar] [PubMed]
- Xu P, Zhang WQ, Xie J, Wen YS, Zhang GX, Lu SQ. Shenfu injection prevents sepsis-induced myocardial injury by inhibiting mitochondrial apoptosis. J Ethnopharmacol 2020;261:113068.
[Crossref] [Google Scholar] [PubMed]
- Peng MF, Tian S, Song YG, Li CX, Miao MS, Ren Z, et al. Effects of total flavonoids from Eucommia ulmoides oliv. leaves on polycystic ovary syndrome with insulin resistance model rats induced by letrozole combined with a high-fat diet. J Ethnopharmacol 2021;273:113947.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Tan X, Li S, Yang S. The total flavonoid of Eucommia ulmoides sensitizes human glioblastoma cells to radiotherapy via HIF-α/MMP-2 pathway and activates intrinsic apoptosis pathway. Onco Targets Ther 2019;12:5515-24.
[Crossref] [Google Scholar] [PubMed]
- Qiu YL, Cheng XN, Bai F, Fang LY, Hu HZ, Sun DQ. Aucubin protects against lipopolysaccharide-induced acute pulmonary injury through regulating Nrf2 and AMPK pathways. Biomed Pharmacother 2018;106:192-9.
[Crossref] [Google Scholar] [PubMed]
- Alsharif KF, Almalki AA, Alsanie WF, Alzahrani KJ, Kabrah SM, Elshopakey GE, et al. Protocatechuic acid attenuates lipopolysaccharide-induced septic lung injury in mice: The possible role through suppressing oxidative stress, inflammation and apoptosis. J Food Biochem 2021;45(10):e13915.
[Crossref] [Google Scholar] [PubMed]
- Ge C, Liu J, Dong S. miRNA-214 protects sepsis-induced myocardial injury. Shock 2018;50(1):112-8.
[Crossref] [Google Scholar] [PubMed]
- Sun F, Yuan W, Wu H, Chen G, Sun Y, Yuan L, et al. LncRNA KCNQ1OT1 attenuates sepsis-induced myocardial injury via regulating miR-192-5p/XIAP axis. Exp Biol Med (Maywood) 2020;245(7):620-30.
[Crossref] [Google Scholar] [PubMed]
- Xie J, Zhang L, Fan X, Dong X, Zhang Z, Fan W. MicroRNA-146a improves sepsis-induced cardiomyopathy by regulating the TLR-4/NF-κB signaling pathway. Exp Ther Med 2019;18(1):779-85.
[Crossref] [Google Scholar] [PubMed]
- Jing R, Zhou Z, Kuang F, Huang L, Li C. MicroRNA-99a reduces lipopolysaccharide-induced oxidative injury by activating notch pathway in H9c2 cells. Int Heart J 2017;58(3):422-7.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Zhang X, Ren XP, Chen J, Liu H, Yang J, et al. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation 2010;122(13):1308-18.
[Crossref] [Google Scholar] [PubMed]
- Ning S, Li Z, Ji Z, Fan D, Wang K, Wang Q, et al. MicroRNA-494 suppresses hypoxia/reoxygenation-induced cardiomyocyte apoptosis and autophagy via the PI3K/AKT/mTOR signaling pathway by targeting SIRT1. Mol Med Rep 2020;22(6):5231-42.
[Crossref] [Google Scholar] [PubMed]
- Tang ZP, Zhao W, Du JK, Ni X, Zhu XY, Lu JQ. miR-494 contributes to estrogen protection of cardiomyocytes against oxidative stress via targeting (NF-κB) repressing factor. Front Endocrinol 2018;9:215.
[Crossref] [Google Scholar] [PubMed]