- *Corresponding Author:
- P. Kumbhar
Department of Pharmaceutics, Tatyasaheb Kore College of Pharmacy, Warananagar, Panhala, Kolhapur, Maharashtra 416113,India
E-mail: johnsir4u@gmail.com
| Date of Received | 10 March 2020 |
| Date of Revision | 07 December 2021 |
| Date of Acceptance | 06 September 2022 |
| Indian J Pharm Sci 2022;84(5):1105-1115 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Emulgel is a promising drug delivery strategy that has gained popularity in recent years for topical delivery of hydrophobic drugs. Emulgel is an emulsion that is gelled by mixing it with gelling agents. It is an interesting topical drug delivery system as it has a dual release control system, i.e., gel and emulsion. Emulgel has several favorable properties for dermatological use such as being thixotropic, greaseless, easily spreadable, easily removable, emollient, nonstaining, long shelf life, transparent and pleasing appearance. Despite, many advantages of the emulgel, the disadvantages include low permeability, poor pharmacokinetics and pharmacodynamics performance; therefore an advanced concept nanoemulgel came into the existence. Regardless of having few limitations, nanoemulgel formulation can be considered as impending and promising candidates for topical delivery of lipophilic drugs in the future. To know the potential of emulgel as a delivery vehicle, the present review offers a comprehensive overview on the rationale behind the use of emulgel, formulation considerations and characterization of emulgel and advanced research in the emulgel. Furthermore, we have summarized and discussed the outcome of different in vitro and in vivo studies of emulgel or nanoemulgel. Finally, the major challenges of nanoemulgel drug delivery system, patented and marketed emulgel or nanoemulgel formulations have been discussed. Based on the studies covered in this manuscript, it was understood that emulgel or nanoemulgel has emerged as an optimistic approach in the topical delivery of hydrophobic drugs to improve solubility, permeability and bioavailability, and reduce toxicities.
Keywords
Emulgel, nanoemulgel, topical drug delivery, incompatibility, evaluations, patents, therapeutic window
Topical drug administration is a localized drug delivery system, through various routes such as ophthalmic, rectal, vaginal and skin, anywhere in the body. Topical preparations are chiefly utilized topically to achieve localized effects at the site of their application[1]. This route is most preferred in the treatment of a variety of skin disorders such as acne, eczema, psoriasis, etc. The topical formulations offer an array of benefits such as prevention of pre-systemic metabolism, augmented bioavailability and therapeutic response, improved patient compliance, ease of administration, reduced gastrointestinal tract incompatibility[2]. Moreover, topical formulations are highly appropriate for the delivery of cargo with a narrow therapeutic window[3].
The different kinds of topical drug delivery systems used for the treatment of skin-related diseases include emulsions, creams, ointments, lotions, powders, etc.[4,5]. These delivery systems are associated with many disadvantages such as sticky nature, lack of spreadability, stability issues, etc., ultimately leading to patient non-compliance. The aforementioned drawbacks have encouraged the use of transparent gels or hydrogels in cosmetics and pharmaceutical preparations due to their less sticky nature, better spreading coefficient and dissolution, greater patient compliance and improved efficacy. Gels are a relatively new class of dosage form created by trapping large amounts of aqueous or hydroalcoholic liquid in a colloidal solid particle network that may consist of inorganic substances, such as aluminum salts or natural or synthetic organic polymers[6]. However, beneficial gels show a significant disadvantage in the delivery of hydrophobic therapeutics. It is reported that approximately 40 % of new chemical entities are hydrophobic and the delivery of these poorly water- soluble drugs is a big challenge. Therefore, to cover up this deficiency, emulgel a new approach that came into existence. Emulgel is a mixture of emulsion and gel base.
Most of the naturally obtained or synthesized drugs are Biopharmaceutical Classification System (BCS) class II drugs[7,8]. They possess low solubility and high permeability. The low solubility of the drug affects the dissolution rate and extent of drug absorption and their permeation through the membrane and bioavailability. Therefore, Nanoemulgel (NEG) is one of the important strategies that can be used for the topical delivery of a hydrophobic drug. In the NEG, nanoemulsion is incorporated into the gel base. The nanoemulsion causes improved solubility of the hydrophobic drugs. In addition, the nanoemulsion of NEG is accountable for augmenting the skin permeability of cargo owing to finely distributed droplets of nanoemulsion. Thus, the NEG offers easy permeation of cargo through the skin to deliver it into the blood thereby improving the pharmacokinetic and pharmacodynamics performance of the drugs. The hydrophobic drug is commonly entrapped in oil-in-water (o/w) emulsion whereas hydrophilic drugs are entrapped in the water-in-oil (w/o) emulsion[9].
Through this manuscript, an attempt has been made to broadly review the emulgel as an efficient approach in topical drug delivery. The areas covered in this review include the rationale behind the use of emulgel, formulation considerations, manufacturing and characterization of emulgel. Furthermore, NEG as an advanced topical delivery approach for hydrophobic drug, packaging of emulgel, patented and marketed formulations are also discussed. We conclude by outlining future perspectives for the development of emulgel.
Factors affecting Topical Absorption of the Drug
The physiological and physicochemical factors play an imperative role in the topical absorption of the drug. The physiological factors that show influence on retention of the topically applied drug include skin pH and thickness, hydration of the skin, the sweat glands density, amount of blood flow and inflammation. Similarly, the physicochemical factors like molecular weight, partition coefficient and degree of ionization of drug also have a great impact on topical absorption of the drug[10].
Emulgel
Emulgel is just a mixture of gels and emulsions. Emulgel is emulsions, either o/w or w/o, which are gelled by combining with a gelling agent. Emulgel is more effective in curative aspects than regular gel. The conversion of an emulsion into the gel is accountable for improved stability and penetrability of emulsion[11]. Moreover, this system exhibits dual control release which is attributed to both emulsion and gel. However, the stability and release of incorporated drugs in emulgel are affected by the type and concentration of gel-forming polymer. Emulgel also prolongs the contact period of medication over the skin owing to its mucoadhesive property. In addition, this system is capable to prevent a dangerous inconvenience that arises from intravenous administration of drugs and variations in the absorption of the drug at different physiological conditions when administered via the oral route. Other various advantages of emulgel include easy incorporation of hydrophobic drugs, augmented drug loading capacity, production feasibility (simple and short processing steps) and low preparation cost, avoidance of first-pass metabolism and gastrointestinal incompatibility, improved patient compliance, suitability for self-medication, narrow therapeutic window and selective to a specific site. Despite the aforementioned advantages, emulgel allied with certain disadvantages like difficulty to absorb large size particle through the skin, poor permeability of drugs, bubble formation during the emulgel preparation and skin irritation or allergic reactions[12].
Rationale Behind use of Emulgel
The commonly used topical formulations such as ointment, cream, lotion, etc., have many drawbacks, including stickiness causing patient irritation when applied, lower coefficient of spreading and need to be applied with rubbing. They also exhibit a stabilization problem. Because of the aforementioned disadvantages of the large group of semisolid preparations, the use of transparent gels has increased in cosmetics as well as in pharmaceutical preparations. The gel is colloid, which is normally 99 % liquid, which is immobilized by the surface tension between the gel and the macromolecular fiber network created by a small amount of gelling material present. In the present scenario, more than 40 % of therapeutically active compounds are hydrophobic and the handling of hydrophobic drugs is a major limitation of gel. The emulsion-based gel is an approach that can successfully incorporate and deliver a hydrophobic therapeutic moiety with improved solubility and penetrability through the skin. Moreover, the emulgel can cause substantial improvement in the pharmacological action and reduction in the dose of the drug due to significant penetration of emulgel globules in soft tissues[13]. There has been a great deal of interest in recent years in the use of novel polymers with complex functions as emulsifiers and thickeners. The gelling ability of these compounds allows stable emulsions and creams to be produced by reducing the surface and interfacial stress, as well as increasing the aqueous phase viscosity[13,14].
Formulation Considerations
In the formulation of topical emulgel, it is essential to evaluate emulgel for its non-toxic, non-irritating, non-comedogenic and non-sensitizing properties. Furthermore, formulating cosmetically elegant and biocompatible emulgel is of vital importance. The aforementioned properties of emulgel are chiefly associated with formulation excipients used. Thus, the formulation considerations assume an imperative role in the emulgel[15-20].
Drug:
The absorption of the drug through the skin is chiefly influenced by the properties of the drug. These kind of physicochemical and biological properties of drugs play an imperative role to formulate them into emulgel for topical or transdermal applications. The drug candidate suitable to formulate as an emulgel should possess’ high pKa value, Half-life (t1/2) of less than 10 h, less molecular size, the molecular mass of 500 daltons or less, partition coefficient (logP) value of 0.8 to 5 and less polarity. In addition, the drug candidate should be non-irritating with a skin permeability coefficient of equal to or more than 0.5×10-3 cm/h[3].
Vehicle:
The vehicle used in the formulation of emulgel also assumes a vital role in the absorption of the drug through the skin. The vehicle used for the preparation of emulgel should possess properties like efficient deposition of the medication with even distribution on the skin; deliver and release the drug at the site of operation; sustaining a level of therapeutics in the target tissue for a sufficient period. Furthermore, it should be compatible with the skin of the patient[3].
Aqueous material:
This forms the aqueous phase of the emulsion. In the presence of the gelling agent, this aqueous phase is accountable for the conversion of emulsion form into the emulgel. Commonly used aqueous materials are water and alcohols[21].
Oils:
An emulsion is the primary part of the emulgel. The selection of type and quantity of oil as one of the phases of the emulsion is mainly allied with the eventual use of emulgel. Moreover, this oil phases chiefly influence the viscosity, permeability and stability of the emulsion. In the selection of oil phase, it is essential to ensure that the oil is pure and free of unpleasant and unsaponifiable constituents like free radicals, peroxides, sterols and polymers. Numerous such types of unwanted constituents can be produced during storage causing the deterioration of the oil phase and that results in unstable formulation[12,21].
Mineral oils alone or mixed with soft or hard paraffin, are commonly used as the carrier as well as for its occlusive and sensory properties, for externally applied emulsions. Non-biodegradable mineral and castor oils, which have a local laxative effect are widely used oils in oral preparations. The fish liver oils or various fixed oils of vegetable origin (e.g., Arachis, cotton and maize oils) are used as nutritional complements[12].
Emulsifiers:
Emulgel is a gelled emulsion prepared by using a suitable gelling agent. An emulsion is a thermodynamically unstable system that can be made stable by the addition of appropriate emulsifying agents. The emulsifying agents are chiefly accountable for reducing the interfacial tension that causes augment in the stability of the emulsion. The emulsifying agent selected should possess good Hydrophilic-Lipophilic Balance (HLB) and yield stable emulsion. Furthermore, the stability of emulsion is mainly allied with type and quantity of emulsifying agent used to make emulsion. Generally, the emulsifying agents with HLB of less than 8 are utilized to prepare w/o type of emulsion while those with HLB of more than 8 are used to make o/w type of emulsion[12].
Emulsifying agents are used both at manufacturing time to facilitate emulsification and during shelf-life to maintain stability. Polyethylene glycol 40 stearate, sorbitan monooleate (Span 80), polyoxyethylene sorbitan monooleate (Tween 80), stearic acid and sodium stearate are widely used as emulsifiers[3,12].
Gelling agents:
Gelling (cross-linking) agents are key components of the emulgel used to make a system thixotropic. They are primarily used as a thickening agent to improve the texture as well as dosage form quality. The sort of gelling agent used and its concentration has great influence on the drug release and stability of emulgel. For instance, the emulgel prepared using Hydroxy Propyl Methyl Cellulose (HPMC) as a gelling agent have been reported to show better drug release when compared to emulgel prepared using Carbopol polymers[8]. Furthermore, various studies have reported inversely proportional relationship between the concentration of gelling agent and drug release from the emulgel. The combination of gelling agents was also found to augment the stability of emulgel[22].
Various kinds of gelling agents used in the emulgel preparation include natural, semi-synthetic and synthetic. However, the chief shortcoming associated with natural gelling agents is their high susceptibility towards microbial degradation. Therefore, semi- synthetic and synthetic gelling agents are found to be widely used nowadays in emulgel preparation[12]. The extensively used gelling agents in the preparation of emulgel are Carbopol 934, Carbopol 940, HPMC 2910, HPMC, Carboxy Methyl Cellulose (CMC) sodium and poloxamer 407[3].
Penetration enhancers:
These agents are primarily used to advance the transdermal delivery of the drug. The penetration of a drug from an emulgel can be significantly influenced by the type and concentration of the penetration enhancer. Therefore, there is a necessity to optimize the type and concentration of these agents to achieve better transdermal delivery of the drug. The penetration enhancers used in the emulgel should possess low irritancy, toxicity and better penetrability. These agents facilitate drug absorption through different mechanisms such as temporarily interrupting the skin barrier, fluidizing the lipid channels between corneocytes; altering the partitioning of the drug into skin structures, etc.,[12].
As a penetration enhancer, oleic acid, lecithin, isopropyl myristate, linoleic acid, clove oil, menthol and eucalyptus oil, Myrj™, Transcutol® P, cineol, etc., can be used[23].
Methods of Preparation
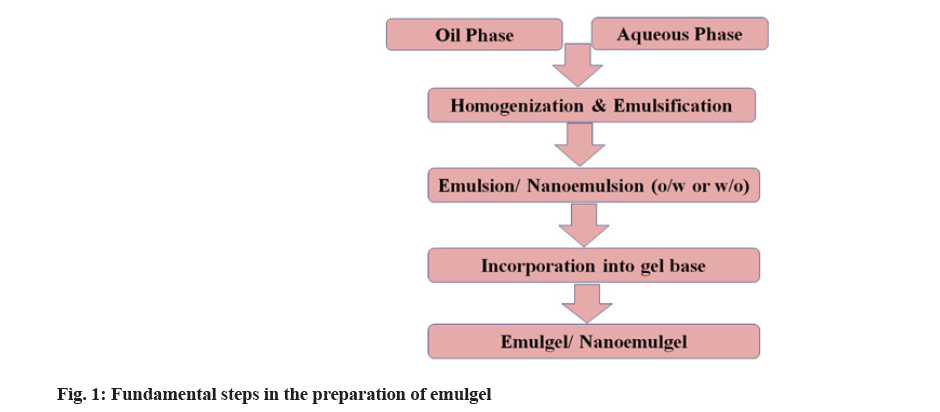
The simple method used for the preparation of emulgel includes three main steps. The first two steps are the formulation of emulsion and gel base separately followed by incorporation of an emulsion into gel base that leads to the formation of emulgel. The fundamental steps in the emulgel preparation are shown in fig. 1. In the emulsion formulation, initially, aqueous phase is prepared by dissolving hydrophilic surfactant or emulsifying agents like Tween 20 into distilled water. Similarly, the oil phase is prepared by dissolving lipophilic surfactant or emulsifying agents like Span 20 into oil (liquid paraffin). Both aqueous and oil phases are heated separately at a temperature of 70°-80° and then both phases are blended together with constant stirring, resulting in an emulsion. The gel phase is prepared by dispersing gelling agents like Carbopol or HPMC) into distilled water. Finally, the emulsion and gel phase are mixed together in the ratio of 1:1 with gentle stirring that leads to the formation of emulgel[24].
Another method described for emulgel formulation involves several steps such as dispersion of polymer in aqueous phase and its neutralization and oil phase emulsification. Initially, the polymer is dispersed in deionized water and stirred continuously at suitable speed and duration at room temperature. The resulting dispersion is then neutralized by the addition of Sodium hydroxide (NaOH) solution that leads to the formation of stable gel via polymer chains distension. The gel is then stored at 4° for 24 h that causes the entire hydration of polymer gels. Finally, the oil phase is added into the polymer gel with continuous stirring that result in the formation of emulgel[25].
Evaluation of Emulgel
Physical appearance:
The prepared emulgel formulations are visually inspected for color, homogeneity, consistency and pH. A pH meter (Digital pH meter 115 pm) is used to calculate the pH values of 1 % aqueous solutions of the prepared gellified emulsion[26-28].
Spreading coefficient:
The spreading coefficient is determined by the apparatus suggested by Mutimer. It consists of a wooden block, which is attached to a pulley at one end. The spreading coefficient is measured based on ‘Slip’ and ‘Drag’ characteristics of emulgel. A ground glass slide is fixed on the wooden block. An excess of emulgel (about 2 g) under study is placed on this ground slide. The emulgel preparation is then sandwiched between this slide and the second glass slide having the same dimension as that of the fixed ground slide. The second glass slide is provided with the hook. A weight of 500 mg is placed on the top of the two slides for 5 min to expel air and to provide a uniform film of the emulgel between the two slides. The measured quantity of weight is placed in the pan attached to the pulley with the help of a hook. The time (s) required by the top slide to cover a distance of 5 cm is noted. A shorter interval indicates a better spreading coefficient[26-28]. It is calculated using the below formula
S=M×L/T
Where, M=Weight tied to upper slide; L=Length of glass slides and T=Time taken to separate the slides.
Rheological study:
The viscosity of the developed emulgel formulations is determined by using a cone and plate type of Brookfield viscometer. The maximum shear rate used is 100 rpm while a minimum shear rate is 10 rpm[26-28].
Globule size and size distribution in emulgel:
Globule size and size distribution are determined by the Malvern Zetasizer. 1.0 g sample is dissolved in purified water and agitated to get homogeneous dispersion. The sample is then injected into the photocell of Zetasizer[26-28].
Drug content determination:
The drug content in emulgel is measured by dissolving the known quantity of emulgel in a suitable solvent like ethanol by sonication. The resulting solution is then filtered and absorbance is measured after suitable dilution at the maximum wavelength of drug-using Ultraviolet-Visible (UV/Vis) spectrophotometer[26-28].
Swelling index:
The swelling index is determined by taking 1 g of gel on porous aluminum foil and then placed separately in a 50 ml beaker containing 10 ml 0.1 N NaOH. Then the samples are removed from beakers at different time intervals and put on dry place for some time after it reweighed[26-28]. Swelling index is calculated using the following formula
Swelling Index (SW) %=[(Wt–Wo)/Wo]×100
Where, (SW) %=Equilibrium percent swelling; Wo=Original weight of emulgel at zero time after time t and Wt=Weight of swollen emulgel.
In vitro release study:
Franz diffusion cell is used for drug release studies. Gellified emulsion approximately (200 mg) is applied onto the surface of the egg membrane evenly. The egg membrane is clamped between the donor and the receptor chamber of the diffusion cell. The receptor chamber is filled with freshly prepared Phosphate Buffered Saline (PBS) (pH 5.5) solution to solubilize the drug. The receptor chamber is stirred by a magnetic stirrer. The samples (1.0 ml aliquots) are collected at a suitable time interval and are analyzed for drug content by UV visible Spectrophotometer after appropriate dilutions. The cumulative amount of drug released across the egg membrane is determined as a function of time[26-28].
Antimicrobial assay:
The antimicrobial assay is used for both qualitative as well as quantitative estimation of antimicrobial agents using the microorganisms. This assay can be carried out by using the agar well diffusion or ditch plate technique. In the agar well diffusion technique, initially, the agar plates are prepared using a sterile nutrient agar medium. These plates are then inoculated with a specific quantity of 24 h broth culture. Then the cavities of 8 mm in diameter are prepared on the agar plates via a sterile borer. The test formulations (fixed volume) are then added into each cavity separately. Finally, all the plates are incubated for 24 h-48 h at 37° and the diameter of the zone of inhibition in mm is measured[24].
In the case of the ditch plate technique, the ditch is prepared in the plate containing media and then the test formulation is placed in a ditch. The loopful of fresh culture is then streaked across the agar at a right angle from the ditch to the edge of the plate. Finally, the plates are incubated at 25° for 18 h-24 h and the percentage inhibition is calculated using the following formula[29] % Inhibition=L2/L1 ×100
Where, L1=Total length of the streaked culture and L2=Length of inhibition
Skin irritation test:
Skin irritation studies are performed by both in vitro and in vivo methods. This study is chiefly carried out to evaluate the tolerability of the emulgel components after the topical application. In the case of in vitro skin irritation study, Hen’s Egg-Chorioallantoic Membrane (HET-CAM), an Organisation for Economic Co- operation and Development (OECD) recommended test is used. In this technique, freshly layed hen eggs with developed chick embryos are used and the irritation behavior of test formulation on chick embryo is studied[30].
On the other hand, various studies reported the use of rabbits or rats to perform the in vivo skin irritation test. The skin of the rat or rabbit (4 cm2) is shaven prior to beginning of the study. Then developed emulgel formulation (specific dose) is applied on the shaven dorsal side at a definite area of the animal skin. At the end of 24 h, animals are examined for any signs of irritation. Any skin irritation, such as erythema or edema is noted in the animals and a score is assigned[24].
Various other in vivo animal studies performed for emulgel may relate to the category of drug incorporated into the emulgel and ultimate use of the developed system. This test may include anti-inflammatory activity, anti-fungal activity, etc.,[24].
Pharmacokinetic study:
The pharmacokinetic study is performed for those emulgel formulations which show systemic absorption on transdermal applications. The animals like rats are used to assess the various pharmacokinetic parameters such as peak plasma concentration (Cmax), the time to reach Cmax (Tmax), the total Area Under the Curve (AUC0-∞). To estimate the aforementioned parameters, the blood sample is collected from the animal via the retro-orbital vein after a specific time interval on topical administration. The samples are then centrifuged at 15 000 rpm for 10 min at 4° temperature. The separated plasma (100 μl) is then mixed with acetonitrile (1 ml) which causes protein precipitation. Further, the samples are centrifuged again at 15 000 rpm, 4° for 5 min and the supernatant (20 μl) is collected. Finally, the sample is analyzed using High-Performance Liquid Chromatography (HPLC)[31].
Stability study:
The stability study of emulgel is performed according to International Council on Harmonization (ICH) guidelines. Briefly, the emulgel formulations are packed in collapsible tubes made up of aluminum. Then these tubes are stored at different temperatures and relative humidity such as 5°, 25°/60 % RH, 30°/65
% RH and 40°/75 % RH for 3 mo. During the storage, the formulations are withdrawn after a particular time interval (15, 30, 60 and 90 d) and can be subjected for evaluation of physical appearance, viscosity, pH, drug content and in vitro drug release, etc.,[29].
NEG as an Advanced Approach in Topical Drug Delivery
NEG is an advanced approach employed in the topical delivery of hydrophobic drugs. In the NEG, the nanoemulsion is mixed with a gelling agent. Nowadays, most of the researches are intensified on NEG-based transdermal delivery of hydrophobic drugs. The clinical applications of various conventional dosage forms such as creams, ointments, gels, emulsion and emulgel are limited because of poor drug permeability through the skin due to large particle size. Therefore, to overcome the permeability problem, the NEG concept came into existence.
Nanoemulsion in NEG is a nano sized solvent droplet stabilized with the use of surfactants that do not require penetration enhancers[32]. A large number of studies reported improved skin permeation of drugs from nanoemulsions than conventional emulsions, gels, creams and ointments[33,34]. The improved permeability of drugs from nanosized topical formulations might be due to lipid bilayer disruption which is evident from the distinct void and empty spaces in the nanoemulsion treated skin samples and drug extent retention at the site of action[35,36]. Despite, the many advantages of nanoemulsion, its topical applications are limited due to its low viscosity and spreadability and stability[37].
The aforementioned drawbacks of nanoemulsion can be conquered by the incorporation of nanoemulsion into a gelling system. NEG is chiefly an emulsion-based topical gel formulation, where nanosized emulsion globules are further converted into NEG by adding a suitable gelling agent. Some biocompatible gelling agents such as Carbomer 934, Carbomer 940, Carbomer 980, pluronic, xanthan gum and carrageenan have been identified which is compatible with surfactants and can modify the viscosity of nanoemulsion[38]. The various advantages of NEG include improved patient compliance due to their non-greasy and non- irritant property, better drug release, high drug-loading capacity, increased permeability and low skin irritation. Currently, formulation scientists are developing NEG for delivery via different routes such as transdermal, dental, vaginal, ocular, nose to the brain for the treatment of various local as well as systemic ailments[38,39]. The drug loaded emulgel and NEG prepared using different gelling agents exhibiting significant improvement in the physicochemical properties and, in vitro and in vivo performance are summarized in Table 1[17,40-53].
| Drug | Gelling agent | Significance |
|---|---|---|
| Amphotericin B | Carbopol | NEG showed significantly higher cumulative amphotericin B permeation (999.81±7.3 mg) than a solution (254.161±1.45 mg) and nanoemulsion (870.42±4.2 mg) after 24 h[38] |
| Atorvastatin | CMC | The skin permeation potential of atorvastatin was significantly (p<0.05) augmented by NEG. In vivo wound healing studies in rats exhibited the highest percent of wound contraction by atorvastatin from NEG [40] |
| Chlorphenesin | HPMC | Remarkably improved drug release and antifungal activity[41] |
| Curcumin | Carbopol 940 | NEG exhibited significantly improved anti-inflammatory activity by inhibiting the phosphorylation of IKK-α[37] |
| Cyclosporine | Guar gum | Significantly (p<0.05) augmented permeation of cyclosporine from NEG than gel[42] |
| Eprinomectin | Carbomer 940 | Significantly enhanced (p<0.01) permeability of eprinomectin from NEG and emulgel by 8.07 fold and 5.57 fold respectively than suspension[43] |
| Itraconazole | Carbopol 934 | J flux and permeability coefficient of emulgel is found to be 0.2301(mg/cm2/h) and 0.001151(Kp) which is similar to marketed formulation[44]. |
| Itraconazole | Xanthan gum | Emulgel showed promising results of spreadability; extrudability, itraconazole content and release[45] |
| Ketoconazole | Carbopol 934 | The emulgel showed good spreadability, homogeneity, stability and smoothening effect. It also exhibited improved skin penetration of ketoconazole[46] |
| Ketoconazole | Carbopol 934 | Emulgel showed sustained ketoconazole release in a controlled manner than cream[47] |
| Ketoprofen | Carbopol 934P | NEG demonstrated sustained release of ketoprofen into the periodontal pocket[17] |
| Luliconazole | Carbopol 934 | Exhibited acceptable physical properties, pH, drug content, viscosity and stability. It also displayed momentous antifungal activity[48] |
| Mentha essential oil | Carbopol 940 | Carbopol 940 gives a smooth texture and acceptable viscosity for mucosal administration. The emulgel showed better stability over 90 d and improved the effectiveness of mentha essential oil against candidiasis like condition[49] |
| Piroxicam | Carbomer 934 | NEG displayed a significantly (p<0.05) augmented permeation and flux of piroxicam than marketed formulation[35] |
| Tolnaftate | Carbopol 940 | Emulgel showed substantially augmented penetration of tolnaftate in fungi cells that result in remarkable antifungal activity[50] |
| Terbinafine | Carbopol 934 | NEG exhibited substantially improved permeability of terbinafine through the skin, and increased physical stability[51] |
| Terbinafine HCl | Carbopol 940 | Significantly (p<0.05) improved skin permeation from formulated emulgel than the commercial emulgel[52] |
| Tenofovir | HPMC | Ex vivo permeation studies demonstrated that the NEG significantly enhanced the tenofovir permeation by 39.65-fold, with a cumulative amount of 1866.54±108.62 μg.cm-2[53] |
Table 1: Emulgel And NEG With Improved In Vitro And In Vivo Performance
An ample of studies proved the improved pharmacokinetic and pharmacodynamics performance of drugs from NEG. Aparna et al.[31], have fabricated telmisartan-loaded NEG with improved solubility and permeability via topical administration. They found substantial improvement in the bioavailability of telmisartan from a NEG when compared to the normal gel in vivo in rats. The telmisartan NEG has displayed significant (p<0.01) improvement in the area under the plasma concentration-time curve to infinity time (AUC -1) value (334.37 mg.h/ml) when compared to the normal gel (221.08 mg.h/ml). Furthermore, the peak plasma concentration of telmisartan was found to be more from NEG (6.2 µg/ml) than normal gel (5.7 µg/ml). They concluded that substantial improvement in the bioavailability of telmisartan from the NEG might be due to the augment in permeability via disturbing the cell membrane. Furthermore, augment in the bioavailability might be attributed to the improved lymphatic absorption owing to the presence of long- chain oils[36]. Jeengar et al. investigated curcumin- loaded NEG using emu oil to improve the permeability and anti-inflammatory activity in the treatment of rheumatoid arthritis. The NEG displayed momentous (higher in terms of inhibition of rat paw edema 66 %) anti-inflammatory activity of curcumin NEG when compared to the normal curcumin gel (14.22 %)[37]. Similarly, Srivastava et al. have developed ketoprofen- loaded in situ NEG for the treatment of periodontitis. This NEG demonstrated sustained in vitro release and reduced toxicity of ketoprofen[54].
In another study, Mohamed et al. have developed NEG for topical delivery of atorvastatin against wound healing. This NEG exhibited significant (p<0.05) permeation of atorvastatin through the skin. In addition, the NEG displayed remarkable wound healing potential in vivo in the Wistar rats[40]. Begur et al. have designed cyclosporine-loaded NEG with improved permeability via transdermal application. The NEG demonstrated significantly (p<0.05) augmented permeation and flux in vivo in rats[42]. Furthermore, Yujuan et al. investigated NEG for transdermal delivery of eprinomectin against endoparasites and ectoparasites. They studied the permeability behavior of eprinomectin-loaded NEG and emulgel and were compared with suspension. Both NEG and emulgel loaded with eprinomectin exhibited momentous permeability 8.07 and 5.57 folds respectively than eprinomectin suspension revealing better absorption of cargo from NEG[43]. In another study, Dhawan et al. have fabricated NEG for transdermal delivery of piroxicam with improved permeability and reduced skin irritation. This NEG displayed significantly (p<0.05) improved permeability of piroxicam from NEG[35].
NEG Drug Delivery Challenges
Despite distinct advantages, some challenges associated with the NEG drug delivery system are the high energy requirement for nanoemulsion preparation and stability of nanoemulsion. Though, low-energy methods exist, but are not perfectly appropriate for large-scale manufacturing and normally need higher amounts of surfactants. However, a high amount of surfactant use causes skin irritation and contact dermatitis. Sometimes, NEG was reported to show allergic reactions on the skin. Moreover, the critical step to provide adequate stability of the NEG formulation is a selection of the proper type and amount of surfactants and co-surfactants[21]. Another important concern is the availability of inadequate types of surfactants and co- surfactant for nanoemulsion preparation. Furthermore, NEG formulations are unable to deliver large size drug molecules with an average molecular weight of more than 400 Da through the skin. Most of the gelling agents used in NEG preparations are highly pH and temperature-sensitive. In addition, another challenge allied with NEG preparation is the incorporation of nanoemulsion into the gel. Low stirring homogenization speed cannot produce a uniform or homogenate mixture, whereas, excess stirring speed cause cracking of the gel[21,38].
Packaging of Emulgels
Packaging of emulgel is usually done in membrane- sealed lacquered aluminum tube with an inner coating of a phenoxy-epoxy based lacquer closed with a propylene screw cap or aluminum laminated tubes closed by a molded seal, with a propylene screw cap (Public Assessment Report of Voltaren Emulgel). These laminate tubes give the benefit of aluminum tube properties with the appearance of plastic. The new generation of laminate tubes uses modern technology to produce the tube with maximum space for graphics. Laminate material prevents the transfer of light, air and moisture. It consists of two layers, an aluminum layer providing integrity and shelf appealing plastic tubes. The protective barrier serves various functions as they provide high gloss protective lacquer, a resistant barrier for products requiring maximum compatibility along with the flavor and fragrance protection with the reduced absorption [55].
Material for laminates tubes:
Foil laminates: Foil laminates provide a barrier against light, air and moisture. It reduces the absorption of aroma (flavor and fragrance). Besides, it has aluminum properties with the look and feel of plastic.
All plastic laminates: It has a chemical-resistant barrier. It helps to retain the shape and form, and offers the plastic like appearance and feel. It has both an opaque and transparent appearance.
Patented and Marketed Emulgel Formulation
Various patented emulgel and NEG formulations loaded with different drugs for the treatment of diverse type of diseases via the transdermal route are presented in Table 2. Furthermore, some commercially available emulgel formulations in the market are represented in Table 3. Voltren emulgel is composed of a diclofenac diethyl ammonium which is used to relieve back, neck and shoulder pain and reduce swelling. Miconaz-H- emulgel is marketed by Medical Union Pharmaceutical which contains miconazole nitrate and hydrocortisone as Active Pharmaceutical Ingredients (APIs). It is chiefly employed in the treatment of fungal and bacterial infections, skin rashes, skin inflammation and pains. In addition, it is also recommended in hormonal conditions, immune problems, arthritis, etc. Diclomax emulgel is composed of diclofenac sodium and is used in the treatment variety of conditions such as rheumatism, osteoarthritis of the spine and peripheral joints. Kleraderm Gaia 480 emulgel is employed as a vaginal antiseptic formulation. Moreover, Isofen emulgel is loaded with Nonsteroidal Anti-Inflammatory Drug (NSAID) ibuprofen. It is found to be useful for the treatment of rheumatic and other muscular pains. Cataflam emulgel marketed by Novartis Alcon is composed of diclofenac diethyl ammonium and is used in the treatment of inflammation and swelling in the joints and muscles, pain, osteoarthritis, etc.,[3,12].
| Patent no | Application no | Title of patent | Inventors | Year |
|---|---|---|---|---|
| EP2214642 | A1 EP20080844931 | Topical composition Fabienne | Caillet-Bois, Isabelle Rault and Michel Steiger | 2010 |
| EP2019666 | A2 EP20070734379 | Pharmaceutical preparations for transdermal use | Cristina Cavallari, Barbara Luppi, Pietra Anna Maria Di and Lorenzo Rodirguez | 2009 |
| US 6004566 | A US 08/036, 116 | Topical and transdermal delivery system utilizing submicron oil spheres | Doron Friedman, Joeph Schwartz and Haim Aviv | 2007 |
| WO2002017905 | A2 PCT/EP2001/010041 | Treatment of burns | Ancerewicz Jacek, Kienzler Jean-Luc, SallinDominique and Schumann Phyllis | 2002 |
| 2007129162 | PCT/IB2007/001061 | Pharmaceutical preparations for transdermal use | Cristina Cavallari, Barbara Luppi, Pietra Anna Maria Di and Lorenzo Rodirguez | 1999 |
| US 6113921 | A US 09/006, 446 | Topical and transdermal delivery system utilizing submicron oil spheres | Doron Friedman, Joseph Schwartz and Haim Aviv | 1993 |
Table 2: Patented Emulgel Formulations
| Product Name | API | Manufacturer |
|---|---|---|
| Voltren emulgel | Diclofenac diethyl ammonium | Novartis Pharma |
| Miconaz-H-emulgel | Miconazole nitrate and hydrocortisone | Medical Union Pharmaceuticals |
| Diclomax emulgel | Diclofenac sodium | Torrent Pharma |
| Kleraderm Gaia 480 emulgel | Bidens pilosa extract | Kleraderm Pharma |
| Isofen emulgel | Ibuprofen | Beit Jala Pharmaceuticals |
| Cataflam emulgel | Diclofenac diethyl ammonium | Novartis Alcon |
Table 3: Various Marketed Emulgel Formulations with their Manufacturers
Conclusions and Future Perspectives
The main problem in front of the formulation scientist is the development of a new formulation for the delivery of hydrophobic drugs due to their poor water solubility which ultimately affects the bioavailability of drugs. 40 % of the drugs are hydrophobic and their delivery to the biological system has been challenging. Therefore, topical drug administration is one of the significant approaches to conquer the shortcomings associated with oral administration including solubility and bioavailability of drugs. Amongst the various topical formulation approaches, emulgel was reported to be crucial in the improvement of the topical delivery of such types of hydrophobic drugs. The incorporation of an emulsion into gel makes it a dual control release system. Furthermore, problems such as phase separation, creaming associated with emulsion gets resolved and its stability is improved. However, the drug permeability is the chief problem of emulgel due to large particle size which can be solved by NEG system where nanoemulsion is incorporated into gel base.
NEG contains different constituents and the selection of type and quantity of such components requires skilled knowledge because the properties of NEG are varying from component to component. Furthermore, the development of a thermodynamically stable nanoemulsion and NEG is exclusively dependent on the suitable selection of its components and the primary methodology. The selection of surfactants plays an imperative role in the safety of emulgel. Therefore, proper screening of the type of surfactants and their concentration is of vital importance to reduce skin irritation and other allergic reactions. Also, there is a necessity to use biosurfactants as a substitute for synthetic surfactants in emulsion preparation. The biosurfactants possess high biodegradability and biocompatibility, minimum toxicity and significant stability at extremes of temperature and pH. In addition, they are economic when compared to synthetic surfactants. Thus, the use of biosurfactants in the preparation of emulgel can help to reduce the cost of formulation and improve the safety of emulgel formulations towards the patients.
The emulgel or NEG can be developed by incorporating active components effective against viral, bacterial, fungal infections or even for melanoma however more molecular assessment on the absorption process of the drugs should be necessary. Thus, emulgel or NEG could be a potential drug delivery carrier to target specific dermatological disorders and in the improvement of various systemic ailments.
Acknowledgement:
We are greatly thankful to our Head of Institute and Institute Management for supporting this research project.
Conflict of interests:
The authors declared no conflict of interests.
References
- Kshirsagar NA. Drug delivery systems. Indian J Pharmacol 2000;32(4):54-61.
- Wermeling DP, Banks SL, Hudson DA, Gill HS, Gupta J, Prausnitz MR, et al. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Natl Acad Sci 2008;105(6):2058-63.
[Crossref] [Google Scholar] [PubMed]
- Charyulu NR, Joshi P, Dubey A, Shetty A. Emulgel: A boon for enhanced topical drug delivery. J Young Pharm 2021;13(1):76-9.
- Kumar S, Singh N, Arora SC. Emulgel: An insight. EJPMR 2015;2(4):1168-86.
- Upadhyaya S, Chauhan B, Kothiyal P. Emulgel: A novel approach for topical delivery of hydrophobic drugs. Int J Univ Pharm Biosci 2014;3(2):176-89.
- Sonaje S, Gondkar S, Saudagar R. Gellified emulsion: A new born formulation for topical delivery of hydrophobic drugs. World J Pharm Pharm Sci 2013;3:233-51.
- Lachman L, Lieberman HA, Kanig JL. The theory and practice of industrial pharmacy. 3rd ed. Philadelphia: Lea and Febiger; 1976. p. 502-33.
- Mohamed MI. Optimization of chlorphenesin emulgel formulation. AAPS J 2004;6(3):81-7.
[Crossref] [Google Scholar] [PubMed]
- Pathan IB, Setty CM. Chemical penetration enhancers for transdermal drug delivery systems. Trop J Pharm Res 2009;8(2):173-9.
- Yassin GE. Formulation and evaluation of optimized clotrimazole emulgel formulations. J Pharm Res Int 2014;4(9):1014-30.
- Hardenia A, Jayronia S, Jain S. Emulgel: An emergent tool in topical drug delivery. Int J Pharm Sci Res 2014;5(5):1653-60.
- Alexander A, Khichariya A, Gupta S, Patel RJ, Giri TK, Tripathi DK. Recent expansions in an emergent novel drug delivery technology: Emulgel. J Control Release 2013 Oct 28;171(2):122-32.
[Crossref] [Google Scholar] [PubMed]
- Patel CJ, Tyagi S, Gupta AK, Sharma P, Prajapati PM, Potdar MB. Emulgel: A combination of emulsion and gel. J Drug Discov Ther 2013;1(6):72-6.
- Panwar A, Upadhyay N, Bairagi M, Gujar S, Darwhekar G, Jain D. Emulgel: A review. Asian J Pharm Life Sci 2011;1(3):333-43.
- Aher SD, Banerjee SK, Gadhave MV, Gaikawad DD. Emulgel: A new dosage form for topical drug delivery. Int J Inst Pharm Life Sci 2013;3(3):1-10.
- Alexander A, Khichariya A, Gupta S, Patel RJ, Giri TK, Tripathi DK. Recent expansions in an emergent novel drug delivery technology: Emulgel. J Control Release 2013;171(2):122-32.
[Crossref] [Google Scholar] [PubMed]
- Pant S, Badola A, Baluni S, Pant W. A review on emulgel novel approach for topical drug delivery system. World J Pharm Pharm Sci 2015;4(10):1728-43.
- Dev A, Chodankar R, Shelke O. Emulgels: A novel topical drug delivery system. Pharm Biol Eval 2015;2(4):64-75.
- Khullar R, Kumar D, Seth N, Saini S. Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi Pharm J 2012;20(1):63-7.
- Mulye SP, Wadkar KA, Kondawar MS. Formulation development and evaluation of indomethacin emulgel. Der Pharm Sinica 2013;4(5):31-45.
- Anand K, Ray S, Rahman M, Shaharyar A, Bhowmik R, Bera R, et al. Nano-emulgel: Emerging as a smarter topical lipidic emulsion-based nanocarrier for skin healthcare applications. Recent Pat Antiinfect Drug Discov 2019;14(1):16-35.
[Crossref] [Google Scholar] [PubMed]
- Shahin M, Abdel Hady S, Hammad M, Mortada N. Novel jojoba oil-based emulsion gel formulations for clotrimazole delivery. AAPS Pharm Sci Tech 2011;12(1):239-47.
[Crossref] [Google Scholar] [PubMed]
- Shokri J, Azarmi S, Fasihi Z, Hallaj-Nezhadi S, Nokhodchi A, Javadzadeh Y. Effects of various penetration enhancers on percutaneous absorption of piroxicam from emulgels. Res Pharm Sci 2012;7(4):225-34.
[Google Scholar] [PubMed]
- Malavi S, Kumbhar P, Manjappa A, Disouza J, Dwivedi J. Emulgel for improved topical delivery of Tretinoin: Formulation design and characterization. Ann Pharm Fr 2022;80(2):157-68.
[Crossref] [Google Scholar] [PubMed]
- Perioli L, Pagano C, Mazzitelli S, Rossi C, Nastruzzi C. Rheological and functional characterization of new antiinflammatory delivery systems designed for buccal administration. Int J Pharm 2008;356(1-2):19-28.
[Crossref] [Google Scholar] [PubMed]
- Azeem A, Ahmad FJ, Khar RK, Talegaonkar S. Nanocarrier for the transdermal delivery of an antiparkinsonian drug. AAPS PharmSci Tech 2009;10(4):1093-103.
[Crossref] [Google Scholar] [PubMed]
- Bolzinger MA, Briançon S, Pelletier J, Fessi H, Chevalier Y. Percutaneous release of caffeine from microemulsion, emulsion and gel dosage forms.Eur J Pharm Biopharm 2008;68(2):446-51.
[Crossref] [Google Scholar] [PubMed]
- Fini A, Bergamante V, Ceschel GC, Ronchi C, de Moraes CA. Control of transdermal permeation of hydrocortisone acetate from hydrophilic and lipophilic formulations. AAPS PharmSciTech 2008;9(3):762-8.
[Crossref] [Google Scholar] [PubMed]
- Tanaji DN. Emulgel: A comprehensive review for topical delivery of hydrophobic drugs. Asian J Pharm 2018;12(2):S382.
- Sandeep DS. Development, characterization and in vitro evaluation of aceclofenac emulgel. Asian J Pharm 2020;14(3):330.
- Aparna C, Srinivas P, Patnaik KS. Enhanced transdermal permeability of telmisartan by a novel nanoemulsion gel. Int J Pharm Pharm Sci 2015;7(4):335-42.
- Shakeel F, Baboota S, Ahuja A, Ali J, Shafiq S. Skin permeation mechanism and bioavailability enhancement of celecoxib from transdermally applied nanoemulsion. J Nanobiotechnol 2008;6(8):1477-3155.
[Crossref] [Google Scholar] [PubMed]
- Abolmaali SS, Tamaddon AM, Farvadi FS, Daneshamuz S, Moghimi H. Pharmaceutical nanoemulsions and their potential topical and transdermal applications. Iran J Pharm Sci 2011;7(3):139-50.
- Khurana S, Jain NK, Bedi PM. Nanoemulsion based gel for transdermal delivery of meloxicam: Physico-chemical, mechanistic investigation. Life Sci 2013;92(6-7):383-92.
[Crossref] [Google Scholar] [PubMed]
- Dhawan B, Aggarwal G, Harikumar SL. Enhanced transdermal permeability of piroxicam through novel nanoemulgel formulation. Int J Pharm Investig 2014;4(2):65.
[Crossref] [Google Scholar] [PubMed]
- Aparna C, Srinivas P, Patnaik KS. Enhanced transdermal permeability of telmisartan by a novel nanoemulsion gel. Int J Pharm Pharm Sci 2015;7(4):335-42.
- Jeengar MK, Rompicharla SV, Shrivastava S, Chella N, Shastri NR, Naidu VG, et al. Emu oil based nano-emulgel for topical delivery of curcumin. Int J Pharm 2016;506(1-2):222-36.
[Crossref] [Google Scholar] [PubMed]
- Hussain A, Samad A, Singh SK, Ahsan MN, Haque MW, Faruk A, et al. Nanoemulsion gel-based topical delivery of an antifungal drug: In vitro activity and in vivo evaluation. Drug Deliv 2016;23(2):642-57.
[Crossref] [Google Scholar] [PubMed]
- Mirza MA, Ahmad S, Mallick MN, Manzoor N, Talegaonkar S, Iqbal Z. Development of a novel synergistic thermosensitive gel for vaginal candidiasis: An in vitro, in vivo evaluation. Colloids Surf B Biointerfaces 2013;103:275-82.
[Crossref] [Google Scholar] [PubMed]
- Morsy MA, Abdel-Latif RG, Nair AB, Venugopala KN, Ahmed AF, Elsewedy HS, et al. Preparation and evaluation of atorvastatin-loaded nanoemulgel on wound-healing efficacy. Pharmaceutics 2019;11(11):609.
[Crossref] [Google Scholar] [PubMed]
- Mohamed MI. Optimization of chlorphenesinemulgel formulation. AAPS J 2004;6(3):81-7.
[Crossref] [Google Scholar] [PubMed]
- Begur M. Enhanced permeability of cyclosporine from a transdermally applied nanoemulgel. Der Pharm Sinica 2015;6:69-79.
- Mao Y, Chen X, Xu B, Shen Y, Ye Z, Chaurasiya B, et al. Eprinomectinnanoemulgel for transdermal delivery against endoparasites and ectoparasites: Preparation, in vitro and in vivo evaluation. Drug Deliv 2019;26(1):1104-14.
[Crossref] [Google Scholar] [PubMed]
- Shah M, Modi D, Shah D. Formulation, design and evaluation of microemulsion and micro-emulgel of itraconazole for topical application. Int J Pharm Pharm Res 2017;9(2):124-59.
- Khule PK. Formulation and evaluation of itraconazoleemulgel for various fungal infections. Asian J Pharm 2019;13(1):19-22.
- Pranali S, Charushila S, Sayali C, Namrata M. Design and characterisation of emulgel of an antifungal drug. J Pharm Sci Res 2019;11(6):2357-61.
- Jain A, Gautam SP, Gupta Y, Khambete H, Jain S. Development and characterization of ketoconazole emulgel for topical drug delivery. Der Chem Sinica 2010;1(3):22131.
- Shankar D, Gajanan S, Suresh J, Dushyant G. Formulation and evaluation of luliconazole emulgel for topical drug delivery. Int Res J SciEng 2018;3:85-9.
- Srivastava N, Patel DK, Rai VK, Pal A, Yadav NP. Development of emulgel formulation for vaginal candidiasis: Pharmaceutical characterization, in vitro and in vivo evaluation. J Drug DelivSciTechnol 2018;48:490-8.
- Yadav S, Wairkar S, Invally M, Ranade S. Topical emulgel of tolnaftate with penetration enhancer: Development, characterisation and antifungal activity. Indian J Med Res Pharm Sci 2017;4(10):28-35.
- Paliwal S, Kaur G, Arya R. Formulation andi characterization of topical nanoemulgel of terbinafine. Univ J Pharm Res 2018;3(6):28-37.
- Elmataeeshy ME, Sokar MS, Bahey-El-Din M, Shaker DS. Enhanced transdermal permeability of terbinafine through novel nanoemulgel formulation; development, in vitro and in vivo characterization. Future J Pharm Sci 2018;4(1):18-28.
- Rambharose S, Kalhapure RS, Govender T. Nanoemulgel using a bicephalous hetero lipid as a novel approach to enhance transdermal permeation of tenofovir. Colloids Surf B Biointerfaces 2017;154:221-7.
[Crossref] [Google Scholar] [PubMed]
- Srivastava M, Kohli K, Ali M. Formulation development of novel in situ nanoemulgel (NEG) of ketoprofen for the treatment of periodontitis. Drug Deliv 2016;23(1):154-66.
[Crossref] [Google Scholar] [PubMed]
- Bhavesh S, Shah CN. Nanoemulgel: A comprehensive review on the recent advances in topical drug delivery. Pharm Sci Monit 2016;7(2):346-55.