- *Corresponding Author:
- Zhenlin Hu
Department of Chinese Medicine, Shanghai University, Shanghai 200444, China
E-mail: zhenlinhu@hotmail.com
| Date of Received | 20 December 2023 |
| Date of Revision | 06 May 2024 |
| Date of Acceptance | 14 August 2024 |
| Indian J Pharm Sci 2024;86(4):1375-1382 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study investigates the impact of Saussurea involucrata polysaccharide on glucocorticoid-induced epidermal atrophy and barrier dysfunction. The topical application of Saussurea involucrata polysaccharide to the shaved dorsal skin of mice, administered 30 min after clobetasol propionate treatment twice daily for 6 d, significantly prevented clobetasol propionate-induced transepidermal water loss elevation and reduction in stratum corneum hydration. This was accompanied by an increase in epidermal thickness. Nile red staining demonstrated Saussurea involucrata polysaccharide augmentation of the overall epidermal lipid amount. Molecular analyses, including real-time quantitative polymerase chain reaction and immunohistochemical analysis, unveiled Saussurea involucrata polysaccharide influence on the upregulation of major lipid-synthesizing enzymes (serine-palmitoyl transferase, 3-hydroxy-3-methylglutaryl-coenzyme A reductase and fatty acid synthase) and epidermal differentiation-related proteins (filaggrin and involucrin). In conclusion, these findings suggest that Saussurea involucrata polysaccharide effectively prevents glucocorticoid-induced epidermal atrophy and barrier impairment by enhancing epidermal lipid synthesis and stimulating keratinocyte differentiation.
Keywords
Saussurea involucrata polysaccharide, epidermal atrophy, epidermal permeability barrier, glucocorticoid
Topical Glucocorticoids (GCs) are among the most widely used drugs in dermatologic therapy. Unfortunately, long-term treatment with topical GCs causes numerous adverse effects, including skin atrophy, rosacea, perioral dermatitis, pigmentation alterations, delayed wound healing, and exacerbation of skin infections[1,2]. Even short-term (3 d), topical GC treatment can compromise epidermal permeability barrier homeostasis[3]. Thus, there is a significant need for prevention of GC-induced adverse effects. The epidermal permeability barrier primarily resides within the Stratum Corneum (SC), the outermost layer of the epidermis. The SC is comprised of corneocytes derived from the terminal differentiation of keratinocytes, embedded in a lipid-enriched intercellular matrix, that together confers the major barrier function of the skin[4]. To form SC, keratinocytes pass through a tightly regulated differentiation process, in which they sequentially express specific proteins including keratins, filaggrin, involucrin and loricrin that serve as important structural scaffolds for the intercellular lipid matrix[5,6]. The intercellular lipids, composed primarily of ceramides, cholesterol, and free fatty acids, are initially synthesized in the keratinocytes during epidermal differentiation. They are stored in lamellar bodies and then extruded into the extracellular spaces of the SC, where they are arranged into intercorneocyte lipid lamellae that are indispensable for the skin's permeability barrier function. Thus, the keratinocyte differentiation process is extremely important for normal epidermal turnover and function[7].
Dysregulations in epidermal differentiation and lipid synthesis can result in perturbation of skin barrier function[8]. Accumulating evidence supports that the GC-induced abnormalities in epidermal barrier function can be attributed to an inhibition of keratinocyte differentiation and epidermal lipid synthesis[3,9-13]. Hence, modulations in keratinocyte differentiation and epidermal lipid synthesis represent an important treatment strategy for prevention of GC-induced adverse effects. Saussurea involucrata is a valuable herb known as “Tianshan snow lotus” that is frequently used in traditional medicine[14]. Polysaccharide is the major bioactive component of Saussurea involucrate. Saussurea involucrata Polysaccharide (SIP) has been demonstrated to have various biological properties including anti-inflammation[15], anti- oxidation[16-18], and anti-melanogenesis[19]. In a previous study, we showed that SIP could alleviate Ultraviolet B (UVB)-induced skin dryness by enhancing epidermal lipid synthesis and stimulating keratinocyte differentiation[20]. In the present study, we further tested whether topical applications of SIP could prevent GC-induced epidermal barrier impairment.
Materials and Methods
Materials:
SIP was provided by Infinitus Co., Ltd., (Guangzhou, China). Its molecular weight and monosaccharide compositions have been determined in our previous study[20]. According to the results, the molecular weight of SIP was approximately 120 kDa, and SIP was composed of galacturonic acid, arabinose, glucose, rhamnose, and galactose (44.21: 16.37: 12.19: 10.06: 9.70).
SIP hydrogel preparation:
To prepare the hydrogel for topical application, 0.6 g carbopol, 5 g glycerinum, and 3 g propylene glycol were first mixed in 20 g hot water and stirred slowly to achieve a uniform mixture, then 0.6 g triethanolamine was added to the mixture and stirred slowly to form the hydrogel matrix. To prepare the hydrogel containing SIP, 0.3 g SIP was dissolved in 80 g water, and the SIP solution was slowly added to the hydrogel matrix in a batch-wise manner and stirred to obtain a uniform mixture. These preparations of hydrogel containing 0.3 % SIP was referred to as SIP hydrogel.
Animal model and treatments:
All animal experiments were conducted according to International Ethical Guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and under protocols approved by the Animal Care and Use Committee of Shanghai University. Healthy female C57BL/6 mice (7 w old, 18-22 g body weight) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd., (Shanghai, China). The mice were housed at 22°±1° with 50 %-55 % relative humidity and a 12 h light/dark cycle. They were fed with a standard laboratory diet, and had ad libitum access to food and water. After 1 w of acclimatization, the hairs on the dorsal skin were shaved and the mice were then randomly divided into three groups with 6 mice per group. The normal control group received no treatment, whereas the other two groups were topically treated with 0.05 % Clobetasol Propionate (CP) cream (Guilin Huaxin Pharmaceutical Co., Ltd., Guangxi, China) twice daily for 6 d. 30 min after each CP application, either SIP hydrogel or hydrogel vehicle were applied to the clobetasol treated area. The skin was photographed daily with a camera before CP application.
Assessment of epidermal permeability barrier function:
To assess the epidermal permeability barrier function, Transepidermal Water Loss (TEWL) and SC hydration were measured daily immediately before each application of CP using a VapoMeter (Delfin Technologies, Finland) and MoistureMeterEpid (Delfin Technologies, Finland), respectively.
Histological examination:
Histological examination of murine dorsal skin was performed as previously described[20]. Skin biopsies were collected from each mouse on 6th d, 18 h after the last hydrogel application, and fixed in 4 % neutral buffered formalin. The skin samples were then embedded in paraffin and cut into 6 μm slices. After that, the skin slices were stained with Hematoxylin and Eosin (H&E) (Solarbio, Beijing, China). For immunohistochemical analysis, the fixed slices were treated with 3 % H2O2 and then blocked with 5 % Bovine Serum Albumin (BSA) for 4 h. The specimens were then incubated with anti-filaggrin (1:500; Santa Cruz, California, United States of America (USA) or anti-involucrin (1:500; Absin, Shanghai, China) antibody overnight at 4°, and then incubated with horseradish peroxidase-conjugated secondary antibody at 37° for 4 h followed by reaction with 3,3-Diaminobenzidine (DAB). Finally, DAB (a chromogen) was added to the specimen followed by counterstaining with hematoxylin. The stained specimens were examined under a DM3000 microscope (Leica, Wetzlar, Germany) to assess the histological changes.
Nile red fluorescence staining:
The epidermal lipids were visualized and quantified by nile red staining of frozen sections of skin tissue. The skin samples were embedded in Tissue-Tek® OCT medium (Sakura, Tokyo, Japan) and stored at -80° until completely solidified, then the samples were cut into 10 μm slices. The slices were incubated with Hoechst solution (Dojindo, Tokyo, Japan) and then in nile red fluorescent dye working solution (Glpbio, Montclair, CA, USA). Finally, the samples were placed in glycerin gelatin and imaged using a fluorescence microscope.
Real-Time quantitative Polymerase Chain Reaction (RT-qPCR):
Total RNA from a skin sample was extracted with TRIzol® reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. For each sample, 1 μg total Ribonucleic Acid (RNA) was reverse transcribed using PrimeScript RT reagent kit (Accurate Biotechnology Co., Ltd., Hunan, China). qPCR was performed on the QuantStudio 6 RT-PCR system (Thermo Fisher Scientific, Inc., Waltham, MA, USA) using SYBR green master mix (Accurate Biotechnology Co., Ltd., Hunan, China). The following primers were used in qPCR: SPT-forward: 5′- AGGGTTCTATGGCACATTTGATGT-3′, reverse: 5′-TGGCTTCTTCGGTCTTC ATAAAC-3′; Hydroxymethylglutaryl (HMG)- Coenzyme A (CoA) reductase-forward: 5′- GCCGTGAACTGGGTCGA-3′, reverse: 5′- GCATATATAGCAATGTCTCCTGC-3′; FAS-forward: 5′-GCTGCGGAAACTTCAGGAAAT-3′, reverse: 5′-AGAGACGTGTCACTCCTGGACT T-3’; Filaggrin-forward: 5′-ATGTCCGCTCTCCTGGAAAG-3′, and reverse: 5′- TGGATTCTTCAAGACTGCCTGTA-3′; Involucrin-forward: 5′-ATGTCCCATCAACACACACTG-3′, and reverse: 5′-TGGAGTTGGTTGCTTTGCTTG-3′; Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH)- forward: 5′-TTAAGAGGGATGCTGCCCTTACCC-3′ and reverse: 5′-TTGTCTACGGGACGAGGAAACAC-3′. The relative messenger RNA (mRNA) level of each target gene was quantified using the 2−ΔΔCT method and normalized to that of GAPDH.
Statistical analysis:
All quantitative data were expressed as mean±Standard Deviation (SD). The statistical differences between groups were performed using one way Analysis of Variance (ANOVA) followed by a Dunnett’s post-hoc test using GraphPad Prism 6.0 software (Graphpad Software, Inc., San Diego, CA, USA). p values of <0.05 were considered significant difference.
Results and Discussion
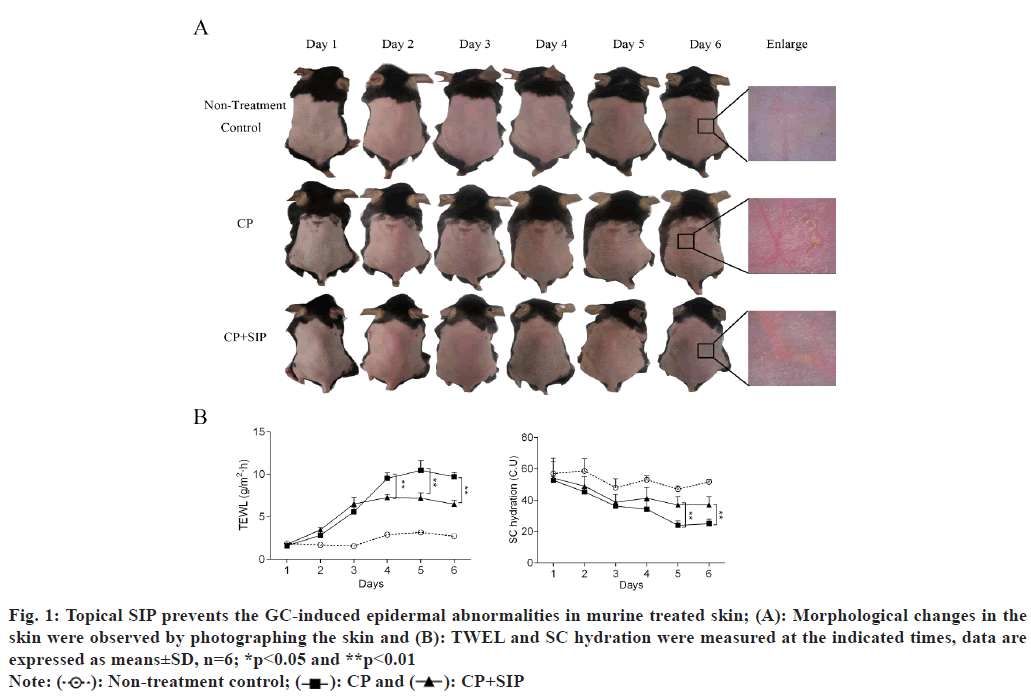
To determine the protective effect of topical SIP on GC-induced epidermal barrier impairment, the shaved dorsal skin of mice was treated topically twice a day for 6 d with CP, a super-potent GC. After 30 min each CP treatment, the animals were topically treated at the same site with an application of SIP hydrogel or with hydrogel vehicle. The shaved dorsal skin of C57BL/6J mice was topically treated 0.05 % CP cream or CP plus SIP hydrogel twice daily for 6 d. After 6 d of topical CP treatments, the treated skin appeared dry, erythematous and atrophic as compared with normal skin, while co-applications of SIP hydrogel with CP largely prevented the emergence of these changes in skin appearance (fig. 1A). The epidermal permeability barrier functions were assessed by measuring TEWL and SC hydration. The results showed that the TEWL value increased after topical CP treatment and this was partially prevented by co-application of SIP hydrogel. In addition, CP treatment also resulted in a significant reduction of SC hydration, which was significantly mitigated by co-application of SIP hydrogel (fig. 1B). These results showed that co-applied topical SIP alleviated epidermal barrier impairment induced by topical GC.
Fig. 1: Topical SIP prevents the GC-induced epidermal abnormalities in murine treated skin; (A): Morphological changes in the skin were observed by photographing the skin and (B): TWEL and SC hydration were measured at the indicated times, data are expressed as means±SD, n=6; *p<0.05 and **p<0.01
Note:  Non-treatment control;
Non-treatment control;  CP+SIP
CP+SIP
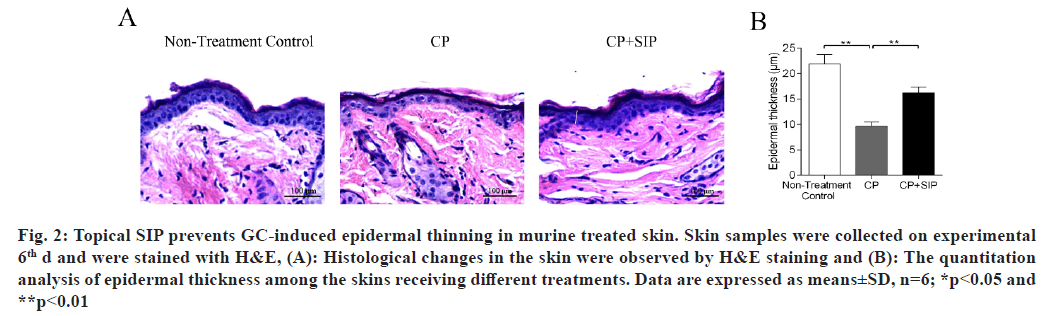
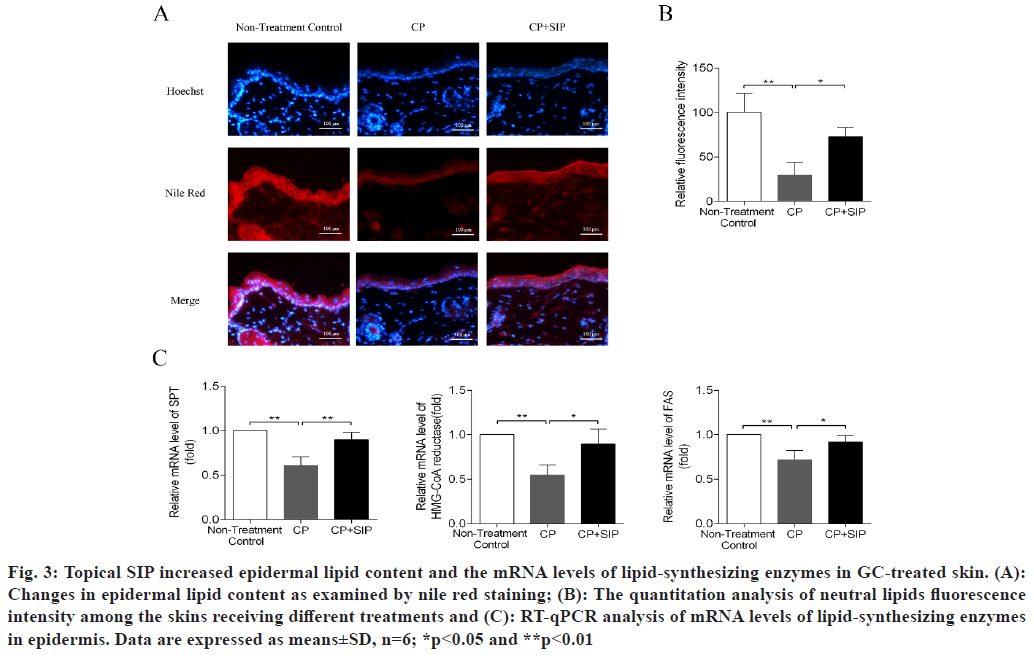
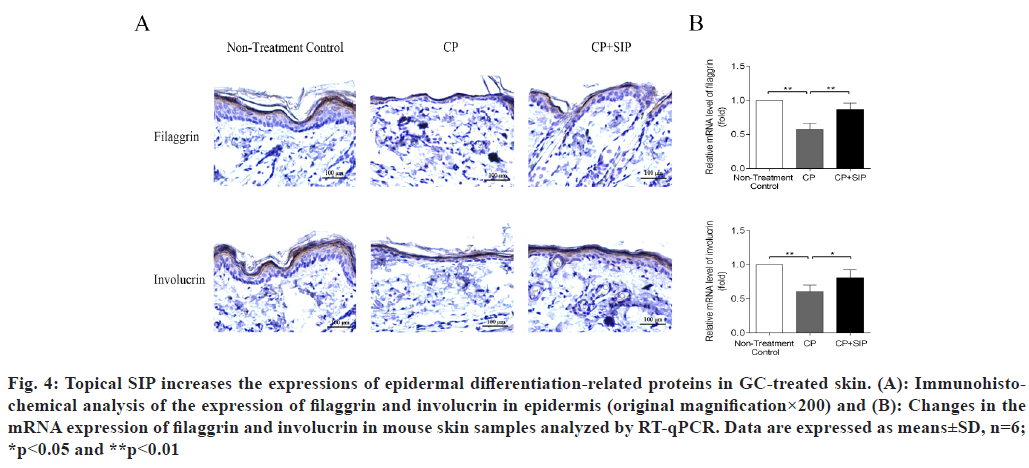
H&E staining of the skin samples taken from the CP group revealed the development of severe epidermal atrophy after 6 d of topical treatment with CP, as seen by the decreased thickness of the epidermis as compared with normal skin, while in the case of SIP hydrogel application, the epidermis thickness became significantly increased as compared with CP-treated skin, indicating that SIP could alleviate epidermal atrophy caused by topical GC (fig. 2). The neutral lipids existing in the intercellular spaces of SC is essential for the epidermal permeability barrier[21,22]. Topical GCs are known to reduce skin barrier integrity by impeding epidermal lipid synthesis[3,11,13]. Nile red is a sensitive lipid probe for visualization of neutral lipid depositions in tissue sections[23]. Here, to elucidate the potential mechanisms underlining the beneficial effects of SIP, we further investigated whether topical SIP increased epidermal lipid production in CP-treated skin by nile red staining. A lower content of neutral lipids was found in the epidermis of the CP treatment group relative to that of the normal skin control, whereas a more intense fluorescence caused by nile red staining throughout the epidermis was observed when topical SIP hydrogel was co- applied, indicating an increase in the amount of epidermal lipids (fig. 3A and fig. 3B). To determine whether co-applied SIP increased epidermal lipid synthesis, we measured the mRNA levels of lipid- synthesizing enzymes in GC-treated skin, including Serine-Palmitoyltransferase (SPT), HMG-CoA reductase and Fatty Acid Synthase (FAS), the key enzymes involved in the synthesis of ceramides, cholesterol and fatty acids. The results showed that CP treatment decreased the expression level of SPT, HMG-CoA reductase and FAS, whereas co-applied SIP hydrogel significantly increased the expression level of these mRNAs compared with vehicle (fig. 3C). Together, these results demonstrated that topical SIP prevent GC-induced defects in skin barrier by increasing epidermal lipid synthesis. As previous studies showed that topical GCs impair epidermal permeability barrier homeostasis in part by reducing the expression of epidermal differentiation-related proteins such as filaggrin and involucrin (fig. 3C)[9,10], we therefore assessed whether topical SIP reversed GC-induced reduction in expression of filaggrin and involucrin by immunohistochemistry and RT-qPCR. As expected, CP treatments decreased the protein and mRNA levels of filaggrin and involucrin in epidermis in comparison to normal controls, whereas co-application of SIP hydrogel increased the expression levels of these proteins (fig. 4). These results indicated that topical SIP prevent GC-induced epidermal dysfunction by promoting keratinocyte differentiation.
Fig. 2: Topical SIP prevents GC-induced epidermal thinning in murine treated skin. Skin samples were collected on experimental 6th d and were stained with H&E, (A): Histological changes in the skin were observed by H&E staining and (B): The quantitation analysis of epidermal thickness among the skins receiving different treatments. Data are expressed as means±SD, n=6; *p<0.05 and **p<0.01
Fig. 3: Topical SIP increased epidermal lipid content and the mRNA levels of lipid-synthesizing enzymes in GC-treated skin. (A): Changes in epidermal lipid content as examined by nile red staining; (B): The quantitation analysis of neutral lipids fluorescence intensity among the skins receiving different treatments and (C): RT-qPCR analysis of mRNA levels of lipid-synthesizing enzymes in epidermis. Data are expressed as means±SD, n=6; *p<0.05 and **p<0.01
Fig. 4: Topical SIP increases the expressions of epidermal differentiation-related proteins in GC-treated skin. (A): Immunohistochemical analysis of the expression of filaggrin and involucrin in epidermis (original magnification×200) and (B): Changes in the mRNA expression of filaggrin and involucrin in mouse skin samples analyzed by RT-qPCR. Data are expressed as means±SD, n=6; *p<0.05 and **p<0.01
Skin atrophy and barrier impairment are common adverse effects of topical GC[1,2,24]. The mouse model has been widely used to examine the cutaneous side effects of topical GCs[3]. In this study, we chose CP to induce skin atrophy in mice, since this drug is a super-potent GC that has been commonly used in clinic and has strong atrophogenic properties both in man and in mice[25]. Topical treatment with CP induced epidermal atrophy, which was characterized by epidermis thinning as determined by histological examinations. We also found the increased TEWL and decreased SC hydration in mouse skin following topical CP treatment, which indicated disruption in epidermal permeability barrier function. Notably, co-application of SIP hydrogel significantly increased epidermis thickness in CP- treated animals, and effectively prevented CP- induced elevation in TEWL and decrease in SC hydration. These results demonstrated that topical SIP can attenuate the deleterious effects of the GC on epidermal structure and function.
It has been demonstrated that the deleterious effects of GCs on epidermis are in large part attributable to a profound inhibition of epidermal lipid synthesis by GCs[3,11,13]. Accordingly, either topical replenishing physiologic lipids or enhancing epidermal lipid synthesis reverses the GC-induced abnormalities in epidermal barrier function[3,26,27], further evidence that lipid deficiency accounts for GC-induced functional abnormalities of epidermis. We recently found that SIP could increase lipid synthesis in UVB-irradiated HaCaT cells and murine epidermis, and consequently alleviate UVB-induced skin dryness[20]. Therefore, we postulated that SIP could blunts the harmful effects of GC on epidermis through enhancing epidermal lipid synthesis. As expected, co-applied topical SIP increased the overall epidermal lipid content in the CP-treated epidermis, as determined by nile red staining. Furthermore, topical co-applications of SIP also upregulated the expression of lipid- synthesizing enzymes in CP-treated murine epidermis, including SPT (the rate-limiting enzyme of ceramide synthesis), HMG-CoA reductase (the key enzyme of cholesterol synthesis) and FAS (the key enzyme of fatty acid), as measured by RT-qPCR. These results further confirmed that topical co-application of SIP prevent GC-induced defects in skin barrier by enhancing epidermal lipid synthesis.
Besides epidermal lipid synthesis, topical GC therapy also perturbs keratinocyte differentiation, resulting in a reduced expression of various protein markers of epidermal differentiation, leading to structural defects in the epidermis, especially SC[10]. Keratinocyte differentiation is a multistep process that is required for the formation of a functional SC[28,29]. As keratinocytes differentiate to form the SC, they sequentially express specific proteins including keratins, filaggrin, involucrin and loricrin, which are cross-linked together underneath the plasma membrane by the action of transglutaminases, generating a rigid insoluble layer called the cornified Cell Envelope (CE) that not only provides strength and chemical resistance for SC but also serve as important structural scaffolds for the extracellular lipid[5,30]. Both filaggrin and involucrin are essential components of CE and therefore important for the structural integrity of the SC. The degradation products of filaggrin on the other side are involved in the maintenance of skin hydration and acidic milieu, both crucial for the optimal activity of enzymes involved in epidermal lipid synthesis[31-33]. A large amount of research has shown that topical GCs significantly reduced the expression of filaggrin and involucrin in epidermis[9,10,34-36], suggesting inhibition on keratinocyte differentiation play an important part in the pathogenesis of barrier dysfunction after topical use of GCs. In line with the previous studies, we found a significant decrease in the expression of involucrin and filaggrin in murine epidermis following topical CP application. Yet, topical co-applications of SIP blocked the CP-induced decrease in the expression of these differentiation-related proteins, indicating SIP can prevent the GC-induced decrease in keratinocyte differentiation, which could also contribute to the improvements in epidermal permeability barrier function.
In the present study, we demonstrated that topical application of SIP reduces the adverse effect of topical GC on epidermal structure and function, likely due to the stimulating activity of SIP on the epidermal lipid synthesis and differentiation, thus providing a proof of principle for using SIP to prevent adverse effects induced by applied GCs in skin. Further experiments are needed to assess the molecular mechanisms by which SIP protect from the adverse effects of GC. In addition to skin atrophy, chronic treatment with GCs results in other adverse effects including delayed wound healing, pigment alteration and increased risk of skin infections. It will be important to test whether SIP can ameliorate these cutaneous side effects of GCs.
Funding:
This study was financially supported by Shanghai Fengxian District Science and Technology Commission Social Science and Technology Development Fund Project (No. 20221204).
Author’s contributions:
Conception and design was done by Zhenlin Hu and Zhenliang Sun; administrative support and provision of study materials was done by Jie Shen; collection and assembly of data was done by Dandan Yang, Yong Wang, Qi Zhao and Zhiwei Liu; data analysis and interpretation was done by Dandan Yang and Yong Wang; manuscript writing and final approval of manuscript was done by all authors.
Conflict of interests:
The authors declared no conflicts of interest.
References
- Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol 2006;54(1):1-5.
[Crossref] [Google Scholar] [PubMed]
- Niculet E, Bobeica C, Tatu AL. Glucocorticoid-induced skin atrophy: The old and the new. Clin Cosmet Investig Dermatol 2020:1041-50.
[Crossref] [Google Scholar] [PubMed]
- Kao JS, Fluhr JW, Man MQ, Fowler AJ, Hachem JP, Crumrine D, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: Inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol 2003;120(3):456-64.
[Crossref] [Google Scholar] [PubMed]
- del Rosso J, Zeichner J, Alexis A, Cohen D, Berson D. Understanding the epidermal barrier in healthy and compromised skin: Clinically relevant information for the dermatology practitioner: proceedings of an expert panel roundtable meeting. J Clin Aesthet Dermatol 2016;9:2.
[Google Scholar] [PubMed]
- Goleva E, Berdyshev E, Leung DY. Epithelial barrier repair and prevention of allergy. J Clin Invest 2019;129(4):1463-74.
[Crossref] [Google Scholar] [PubMed]
- Kypriotou M, Huber M, Hohl D. The human epidermal differentiation complex: cornified envelope precursors, S100 proteins and the ‘fused genes’ family. Exp Dermatol 2012;21(9):643-9.
[Crossref] [Google Scholar] [PubMed]
- Feingold KR, Elias PM. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim Biophys Acta 2014;1841(3):280-94.
[Crossref] [Google Scholar] [PubMed]
- Baroni A, Buommino E, de Gregorio V, Ruocco E, Ruocco V, Wolf R. Structure and function of the epidermis related to barrier properties. Clin Dermatol 2012;30(3):257-62.
[Crossref] [Google Scholar] [PubMed]
- Sheu HM, Chang CH. Alterations in water content of the stratum corneum following long-term topical corticosteroids. J Formos Med Assoc 1991;90(7):664-9.
[Google Scholar] [PubMed]
- Sheu HM, Tai CL, Kuo KW, Yu HS, Chai CY. Modulation of epidermal terminal differentiation in patients after long?term topical corticosteroids. J Dermatol 1991;18(8):454-64.
[Crossref] [Google Scholar] [PubMed]
- Sheu HM, Lee JY, Chai CY, Kuo KW. Depletion of stratum corneum intercellular lipid lamellae and barrier function abnormalities after long?term topical corticosteroids. Br J Dermatol 1997;136(6):884-90.
[Crossref] [Google Scholar] [PubMed]
- Radoja N, Komine M, Jho SH, Blumenberg M, Tomic-Canic M. Novel mechanism of steroid action in skin through glucocorticoid receptor monomers. Mol Cell Biol 2000;20:4328-39.
[Crossref] [Google Scholar] [PubMed]
- Ropke MA, Alonso C, Jung S, Norsgaard H, Richter C, Darvin ME, et al. Effects of glucocorticoids on stratum corneum lipids and function in human skin-A detailed lipidomic analysis. J Dermatol Sci 2017;88(3):330-8.
[Crossref] [Google Scholar] [PubMed]
- Chik WI, Zhu L, Fan LL, Yi T, Zhu GY, Gou XJ, et al. Saussurea involucrata: A review of the botany, phytochemistry and ethnopharmacology of a rare traditional herbal medicine. J Ethnopharmacol 2015;172:44-60.
[Crossref] [Google Scholar] [PubMed]
- Liu G, Kamilijiang M, Abuduwaili A, Zang D, Abudukelimu N, Liu G, et al. Isolation, structure elucidation, and biological activity of polysaccharides from Saussurea involucrata. Int J Biol Macromol 2022;222:154-66.
[Crossref] [Google Scholar] [PubMed]
- Mandal A, Meda V, Zhang WJ, Dalai AK. Spectroscopic investigation of collagen scaffolds impregnated with AgNPs coated by PEG/TX-100 mixed systems. Int J Biol 2012;50(3):603-12.
[Crossref] [Google Scholar] [PubMed]
- Chen W, Ma J, Gong F, Xi H, Zhan Q, Li X, et al. Two novel polysaccharides from the torus of Saussurea laniceps protect against AAPH-induced oxidative damage in human erythrocytes. Carbohydr Polym 2018;200:446-55.
[Crossref] [Google Scholar] [PubMed]
- Zheng RL, Liu GS, Xing GX, Jia ZJ, Du M, Tan LQ. Free radical scavenging and antifatigue activities of Saussurea involucrata polysaccharides. Zhongguo Yao Li Xue Bao 1993;14:S47-9.
[Google Scholar] [PubMed]
- Kamilijiang M, Zang D, Abudukelimu N, Aidarhan N, Liu G, Aisa HA. Anti-melanogenesis effect of polysaccharide from Saussurea involucrata on forskolin-induced melanogenesis in B16F10 melanoma cells. Nutrients 2022;14(23):5044.
[Crossref] [Google Scholar] [PubMed]
- Wang L, Yang K, Jing R, Zhao W, Guo K, Hu Z, et al. Protective effect of Saussurea involucrata polysaccharide against skin dryness induced by ultraviolet radiation. Front Pharmacol 2023;14:1089537.
[Crossref] [Google Scholar] [PubMed]
- Sahle FF, Gebre-Mariam T, Dobner B, Wohlrab J, Neubert RH. Skin diseases associated with the depletion of stratum corneum lipids and stratum corneum lipid substitution therapy. Skin Pharmacol Physiol 2015;28(1):42-55.
[Crossref] [Google Scholar] [PubMed]
- van Smeden J, Bouwstra JA. Stratum corneum lipids: Their roles for the skin barrier function in healthy subjects and atopic dermatitis patients. Curr Probl Dermatol 2016;49:8-26.
[Crossref] [Google Scholar] [PubMed]
- Sheu HM, Tsai JC, Lin TK, Wong TW, Lee JY. Modified Nile red staining method for improved visualization of neutral lipid depositions in stratum corneum. J Formos Med Assoc 2003;102(9):656-60.
[Google Scholar] [PubMed]
- Cain DW, Cidlowski JA. Specificity and sensitivity of glucocorticoid signaling in health and disease. Best Pract Res Clin Endocrinol Metab 2015;29(4):545-56.
[Crossref] [Google Scholar] [PubMed]
- Pels R, Sterry W, Lademann J. Clobetasol propionate--where, when, why? Drugs Today 2008;44:547-57.
[Crossref] [Google Scholar] [PubMed]
- Hong SP, Oh Y, Jung M, Lee S, Jeon H, Cho MY, et al. Topical calcitriol restores the impairment of epidermal permeability and antimicrobial barriers induced by corticosteroids. Br J Dermatol 2010;162(6):1251-60.
[Crossref] [Google Scholar] [PubMed]
- Wen S, Wu J, Ye L, Yang B, Hu L, Man MQ. Topical applications of a heparinoid-containing product attenuate glucocorticoid-induced alterations in epidermal permeability barrier in mice. Skin Pharmacol Physiol 2021;34(2):86-93.
[Crossref] [Google Scholar] [PubMed]
- Fuchs E. Epidermal differentiation: The bare essentials. J Cell Biol 1990;111(6 Pt 2):2807-14.
[Crossref] [Google Scholar] [PubMed]
- Melino G, de Laurenzi V, Catani MV, Terrinoni A, Ciani B, Candi E, et al. The cornified envelope: A model of cell death in the skin. Results Probl Cell Differ 1998:175-212.
[Crossref] [Google Scholar] [PubMed]
- Nemes Z, Steinert PM. Bricks and mortar of the epidermal barrier. Exp Mol Med 1999;31(1):5-19.
[Crossref] [Google Scholar] [PubMed]
- Steinert PM, Marekov LN. Initiation of assembly of the cell envelope barrier structure of stratified squamous epithelia. Mol Biol Cell 1999;10(12):4247-61.
[Crossref] [Google Scholar] [PubMed]
- Steinert PM, Marekov LN. Direct evidence that involucrin is a major early isopeptide cross-linked component of the keratinocyte cornified cell envelope. J Biol Chem 1997;272(3):2021-30.
[Crossref] [Google Scholar] [PubMed]
- Kezic S, Jakasa I. Filaggrin and skin barrier function. Curr Probl Dermatol 2016;49:1-7.
[Crossref] [Google Scholar] [PubMed]
- Demerjian M, Choi EH, Man MQ, Chang S, Elias PM, Feingold KR. Activators of PPARs and LXR decrease the adverse effects of exogenous glucocorticoids on the epidermis. Exp Dermatol 2009;18(7):643-9.
[Crossref] [Google Scholar] [PubMed]
- Hatano Y, Elias PM, Crumrine D, Feingold KR, Katagiri K, Fujiwara S. Efficacy of combined peroxisome proliferator-activated receptor-α ligand and glucocorticoid therapy in a murine model of atopic dermatitis. J Invest Dermatol 2011;131(9):1845-52.
[Crossref] [Google Scholar] [PubMed]
- Jensen JM, Scherer A, Wanke C, Bräutigam M, Bongiovanni S, Letzkus M, et al. Gene expression is differently affected by pimecrolimus and betamethasone in lesional skin of atopic dermatitis. Allergy 2012;67(3):413-23.
[Crossref] [Google Scholar] [PubMed]