- *Corresponding Author:
- Delian Kong
Department of Neurology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu 221004, China
E-mail: xykdl@163.com
| This article was originally published in a special issue, “Current Trends in Pharmaceutical and Biomedical Sciences” |
| Indian J Pharm Sci 2022:84(5) Spl Issue “1-9” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study was to investigate the effects of toll-like receptor 4 on transplanted bone marrow stem cells in the ischemic brain. Focal cerebral ischemia was induced by permanent occlusion of middle cerebral artery in toll-like receptor 4 gene knockout mice and wild type control mice. Bone marrow stem cells were injected into lateral cerebral ventricle. The cylinder tests, in vivo image, polymerase chain reaction array, etc. were applied to evaluate the neurological function and the underlying mechanisms. On the 10th d after permanent occlusion of middle cerebral artery, the ratio of right limb movement in group 1 was significantly higher than that in wild type and toll-like receptor 4 gene knockout mice without injection of bone marrow stem cells (p<0.05). In vivo imaging showed that on the 3rd d after injection of bone marrow stem cells, the fluorescence intensities in group 3 and group 1 were significantly enhanced compared with that on the 1st d and 7th d. There were more green fluorescent protein positive cells in group 3 and group 1 compared with sham controls (p<0.05). Amount of green fluorescent protein positive cells in group 1 was significantly higher compared with group 3. In toll-like receptor 4 gene knockout mice, bone morphogenetic protein 2 messenger ribonucleic acid and fibroblast growth factor 9 messenger ribonucleic acid were up-regulated and interleukin-1-beta protein was decreased, compared with wild type mice. The present study firstly demonstrated that toll-like receptor 4 deficiency further increased the amount of intracerebroventricularly injected bone marrow stem cells in ischemic brain areas, thereby improved neurological function after focal cerebral infarction. The increased expression of bone morphogenetic protein 2 and fibroblast growth factor 9 in the environment of toll-like receptor 4 deficiency might be one of the molecular mechanisms.
Keywords
Cerebral ischemia, bone marrow stem cells, toll-like receptor 4, inflammation, gene knockout
Ischemic stroke caused by occlusion of cerebral arteries supplying blood to brain tissue is a major cause of death and permanent disability in the world[1,2]. Although some new approaches, such as thrombolysis and arterial embolectomy, are available for acute phase of ischemic stroke, there is no effective treatment for ischemic stroke after acute stage at present. Cell therapy is providing a promising new treatment for reducing stroke-related disability. The stem cells used for cell therapy mainly include Embryonic Stem (ES) cells, Neural Stem Cells (NSCs), Bone Marrow Stem Cells (BMSCs), etc., in which, the BMSCs are considered to be the most promising therapeutic method[3]. For the past decades, numerous studies have indicated that the transplanted BMSCs significantly enhance the functional recovery after ischemic stroke in animal models[4]. However, many problems needed to be resolved before stem cell therapy which is widely used in clinic, such as how to improve the survival of BMSCs and their differentiation into nerve cells. In addition, the mechanisms underlying the regulation of the function and maturation process of BMSCs remains unclear. Previous researches have demonstrated that the maturation process, biological activities and functions of BMSCs are modulated by several mechanisms, in which, immune inflammatory responses play a leading role[5]. Immune inflammatory responses play a dual role on the regulation of stem cells. It is necessary to understand the mechanism underlying the effect of inflammatory responses on the regulation of BMSCs and the interaction between them in order to optimize the BMSCs maturation, differentiation and migration.
Toll-Like Receptor (TLR) is a type I transmembrane receptor, which plays an important role in the induction and regulation of immune inflammatory response. At present, at least 13 TLRs have been found. Toll-Like Receptor 4 (TLR4), a member of TLR family, has been extensively investigated and demonstrated to be involved in the inflammatory reactions in ischemic brain injury[6]. TLR4 was reported to play a role in the regulation of genesis and neurite outgrowth, proliferation and differentiation of stem cells[7]. In addition, many different results about the role of TLR4 in stem cells were also observed in the stem cells from different tissue, including bone marrow mesenchymal stem cells[8-10]. However, there is little study about the effect of TLR4 on the proliferation, migration, differentiation and survival of transplanted BMSCs in ischemic brain tissue at present. Based on the previous results, we hypothesize that TLR4 mediated signaling pathways regulates the growth process of exogenous BMSCs. In order to verify the hypothesis, in the present study, the model of permanent Occlusion of Middle Cerebral Artery (MCAO) was performed on TLR4 gene Knockout mice (TLR4KO) and Wild Type (WT) control mice. Exogenous BMSCs were injected intracerebroventricularly (i.c.v.). Neurological function testing, small animal in vivo imaging, histological analysis, Polymerase Chain Reaction (PCR) array and Enzyme-Linked Immunosorbent Assay (ELISA) were used to evaluate the effect of TLR4 on the BMSCs in ischemic brain tissues and the underlying mechanisms.
Materials and Methods
Animals:
TLR4 Knockout mice (TLR4KO, B6.B10ScN-Tlr4lps- del/JthJ, Stock No: 007227) and WT control mice (WT, C57 Black 6J (C57BL/6J), Stock No. 000664) were purchased from Jackson laboratory (USA), and bred in the animal facility of Xuzhou Medical University. The experiments outlined in this manuscript conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication No. 85-23, revised 1996). Animal care and experimental protocols were approved by the Committee on Animal Care at Xuzhou Medical University. 96 mice (48 TLR4KO and 48 WT, male, age: 8-12 w, body weight: 25-30 g) were divided into 8 groups. Group 1 include TLR4KO mice subjected to MCAO and cerebral ventricular i.c.v. injection of BMSCs (T4MC, n=12); group 2 include TLR4KO mice subjected to MCAO and i.c.v. injection of Phosphate-Buffered Saline (PBS) (T4MP, n=12); group 3 include WT mice subjected to MCAO and i.c.v. injection of BMSCs (WTMC, n=12), group 4 include WT mice subjected to MCAO and i.c.v. injection of PBS (WTMP, n=12); group 5 include TLR4KO mice subjected to sham operation and i.c.v. injection of BMSCs (T4SC, n=12); group 6 include TLR4KO mice subjected to sham operation and i.c.v. injection of PBS (T4SP, n=12), group 7 include WT mice subjected to sham operation and i.c.v. injection of BMSCs (WTSC, n=12), group 8 include WT mice subjected to sham operation and i.c.v. injection of PBS (WTSP, n=12). All mice were subjected to i.c.v. injection of BMSCs or PBS, 3 d after MCAO or sham operation. In each group, 6 mice were killed on the 21st d after MCAO or sham operation by transcardial perfusion with normal saline followed by 30 ml of 4 % buffered paraformaldehyde (pH=7.4). The brains were removed, post-fixed and cut into sections (10 μm) for histological studies. Other 6 mice in each group were killed 3 d after the i.c.v. injection of BMSCs or PBS. The brain tissues were harvested for molecular studies.
Induction of MCAO:
Permanent focal cerebral ischemia was induced by occlusion of the left Middle Cerebral Artery (MCA) [11,12]. Briefly, mice were subjected to anesthesia by inhalation of isoflurane (4 % induction and maintain 1.5 %). A 1 cm skin incision between the left ear and eye was made by scissors, and the skin and temporal muscles were separated. The left MCA was coagulated with electro coagulation forceps on the distal and two bifurcations after the bone above the artery was removed. The mice in sham operation groups underwent the same processes on left side without MCAO.
Culture of BMSCs:
BMSCs were purchased from Cyagen Bioscience, which were isolated from the bone marrow in C57BL/6J mice and transduced with lentiviral vector expressing Green Fluorescent Protein (GFP). The bacteria, fungi, mycoplasma and endotoxin were detected in the cultured cells, which were also confirmed for positive Cluster of Differentiation (CD) 29, CD44, Stem cell antigen-1 (Sca-1) (>70 %) and negative CD117 (<5 %) by flow cytometry. The BMSCs were passaged and prepared for i.c.v. injection.
Intracerebroventricularly injection of BMSCs:
3 d after MCAO or sham operation, mice were anesthetized and fixed on the stereotaxic apparatus. The three-dimensional coordinates of left lateral ventricle i.c.v. injection were: Longitudinal: Bregma 0.3 mm, transverse: 1.00 mm to the left of middle line and depth: 2.50 mm from brain surface[13]. The i.c.v. injection site was disinfected and a hole was drilled through the skull with a 26 Gauge (26 G) needle. Then 3 μl cell suspension (5×104 cells/μl) was injected into left lateral cerebral ventricle with a 10 μl Hamilton syringe at a constant rate of 0.50 μl/min. The needle was retained on site for 5 min after injection.
Cylinder test:
Behavioral tests (cylinder test) were performed 1 d before MCAO or sham operation and 1, 3, 7 and 21 d after MCAO or sham operation[14,15]. All behavioral tests were performed by two researchers blinded to the animal groups. The number of times, the wall was contacted by forelimbs was counted. The types of the contact included Contralateral Forelimb (CF, right forelimb) contact, Ipsilateral Forelimb (IF, left forelimb) contact and Both Forelimbs (BF) contact. The mice with the total number of contact less than five would be excluded. The percentage of the usage of CF (the ratio of right limb movement) in the total number of contacts was calculated as follows. Total percentage=(CF+BF/2)/(CF+BF+IF)×100 %.
Small animal in vivo imaging test:
Small animal in vivo imaging tests were performed on the 1st, 3rd and 7th d after lateral i.c.v. injection in WTMC and T4MC groups. After being anesthetized, the mice were epilated from head to the back of the neck and were put into the in vivo imaging system (LB983 NightOWL, Berthold Company, Germany) for 3-5 min. The testing process was operated by a specialist technician. Image acquisition and analysis were conducted by indigo software (Version 2.0.3.0). The fluorescence intensity was expressed by cps (count per second). Representing GFP concentration was analyzed with the system software.
BMSCs histological analysis:
On the 21st d after MCAO, mice were anesthetized and transcardially perfused with normal saline followed by 30 ml of 4 % buffered paraformaldehyde (pH=7.4). The brains were removed, post-fixed and cut into sections (10 μm). The brain sections were mounted with anti- decay sealing agents (including 4',6-Diamidino-2- Phenylindole (DAPI)) and observed with fluorescence microscope. The number of BMSCs (GFP positive cells) in the infarct area was counted by Image Pro software. The exposure time was adjusted to 1 s to observe BMSCs and 400 ms to observe the nuclei.
Real-time PCR:
Total Ribonucleic Acid (RNA) was isolated from ischemic hemisphere (left) harvested 3 d after BMSCs i.c.v. injection using a standard protocol. Takara reverse transcription kit was used to synthesize complementary Deoxyribonucleic Acid (cDNA). The PCR array kit was provided from the Biomedical Company (Shanghai, China). Amplification, data acquisition and the melting curve were carried out by the Applied Biosystems ABI 7900 Real Time PCR system (Applied Biosystems, Foster city, California). The PCR cycling program was set as follows. Stage 1: 95° for 10 min; stage 2: 95° for 15 s followed by 60° for 1 min repeated for 40 cycles and stage 3: 95° for 15 s, 60° for 15 s and 95° for 15 s. The delta Ct (ΔCt) method was used for PCR array data analysis.
ELISA test:
Proteins were isolated from ischemic hemispheres harvested 3 d after BMSCs injection. The protein levels of Tumor Necrosis Factor alpha (TNF-α) and Interleukin-1 beta (IL-1β) were examined by using ELISA kits (Mouse TNF-α ELISA kit, eBioscience; IL-1β ELISA kit, eBioscience). The concentration of the detected protein was calculated according to the standard curve[16].
Statistical analysis:
Continuous scale measurements were expressed by the mean and Standard Error of the Mean (SEM) for each group. All data were analyzed by Statistical Package for the Social Sciences (SPSS) 22.0 software. The data from cylinder tests and animal in vivo image were analyzed by Analysis of Variance (ANOVA) of repeated measurements. Comparison of GFP positive cells and the levels of proteins and messenger RNA (mRNA) among groups were analyzed by one-way ANOVA. Probability levels of 0.05 or smaller were considered significant.
Results and Discussion
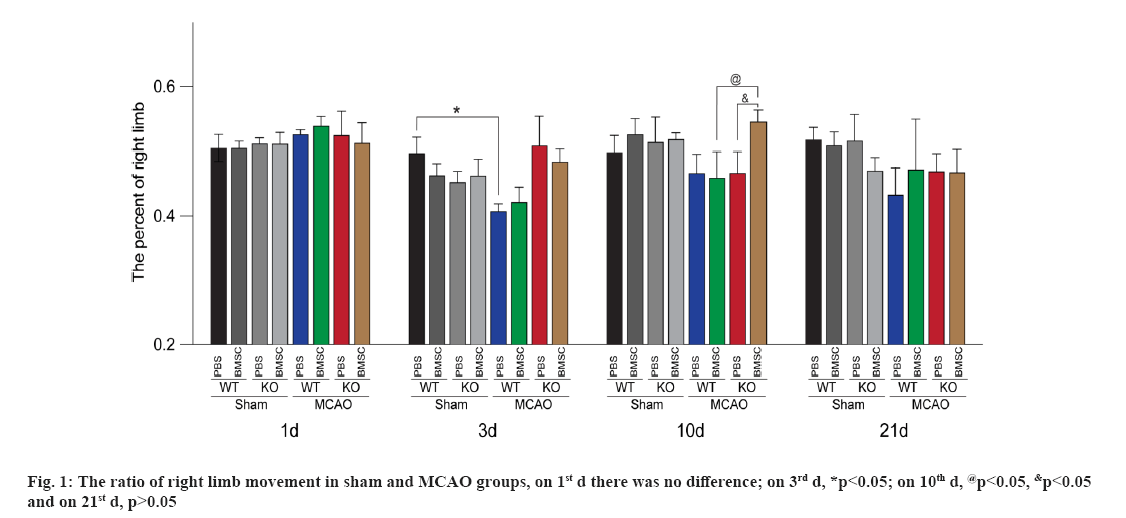
The results from cylinder test showed that, there was no difference among the groups of MCAO mice before operation. On the 3rd d after MCAO or sham operation, the percentage of right forelimb usage in the mice from WTMP (WT+MCAO+PBS) was lower than that from WTSP group (WT+Sham+PBS) (p<0.05), which indicated that the ischemic models were successfully established. On the 10th d after ischemia (7th d after i.c.v. injection), compared with the WT mice subjected to MCAO with i.c.v. injection of BMSCs (WT+MCAO+BMSC, WTMC) group, the percentage of right forelimb usage in TLR4KO mice subjected to MCAO with i.c.v. injection of BMSCs (T4+MCAO+BMSC, T4MC) group was significantly increased (p<0.05), which was also higher than that in the TLR4KO mice subjected to MCAO with i.c.v. injection of PBS (T4+MCAO+PBS, T4MP) (p<0.05). There was no difference among the groups of MCAO mice on the 21st d after MCAO (p>0.05) (fig. 1).
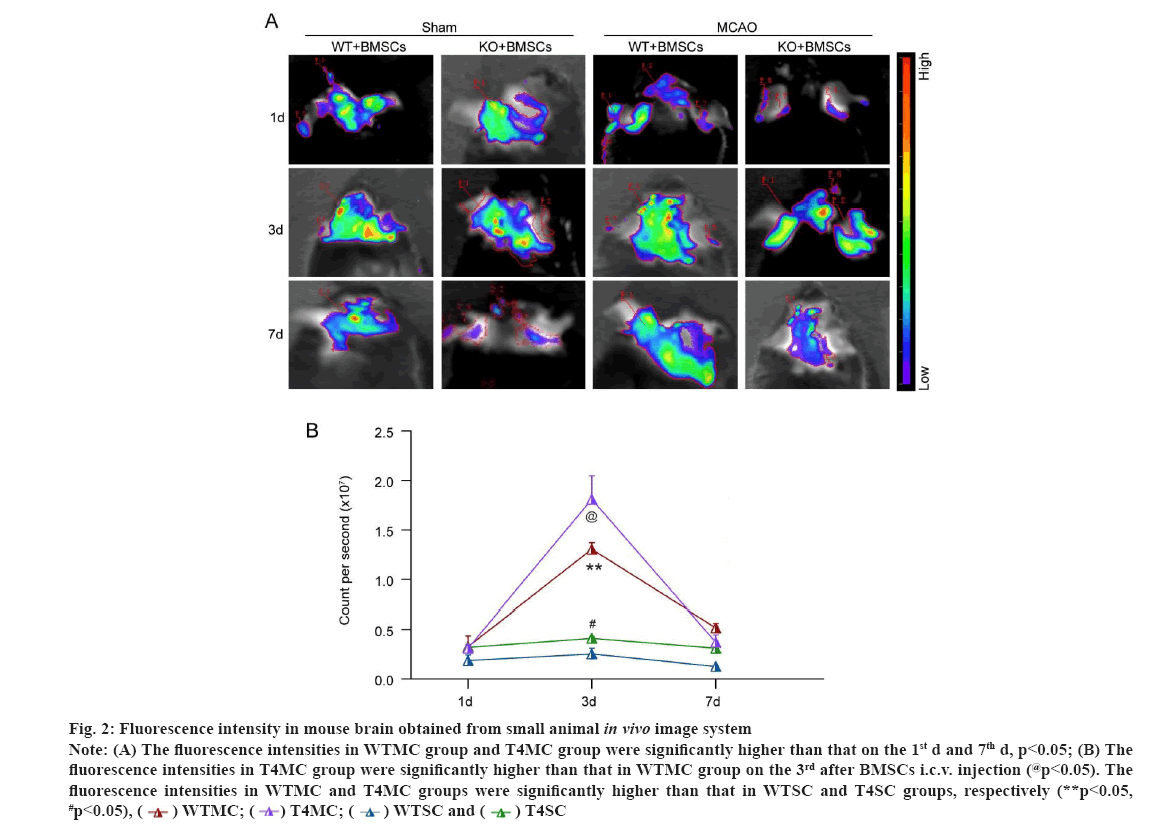
The results of in vivo imaging showed that (fig. 2) on the 1st d after BMSCs injection, there was no significant difference in fluorescence intensity between WTMC (WT+MCAO+BMSC) and T4MC (TLR4+MCAO+BMSC) group. On the 3rd d after i.c.v. injection, the fluorescence intensities in WTMC (WT+MCAO+BMSC) group and T4MC (TLR4+MCAO+BMSC) group were significantly enhanced compared with that on the 1st d (p<0.05), and on the 7th d after BMSCs injection, the fluorescence intensity of the two groups decreased compared to that on the 3rd d (p<0.05) as shown in fig. 2A. Interestingly, on the 3rd d after BMSCs injection, the fluorescence intensities in T4MC (TLR4+MCAO+BMSC) group were significantly higher than that in WTMC (WT+MCAO+BMSC) group (p<0.05) and when the groups of WT/ TLR4KO mice that were injected with BMSCs were compared, the fluorescence intensities in MCAO group (WT+MCAO+BMSC/TLR4+MCAO+BMSC) were significantly higher than that in sham group (WT+sham+BMSC/TLR4+sham+BMSC), respectively (p<0.05) as shown in fig. 2B.
Figure 2: Fluorescence intensity in mouse brain obtained from small animal in vivo image system Note: (A) The fluorescence intensities in WTMC group and T4MC group were significantly higher than that on the 1st d and 7th d, p<0.05; (B) The fluorescence intensities in T4MC group were significantly higher than that in WTMC group on the 3rd after BMSCs i.c.v. injection (@p<0.05). The fluorescence intensities in WTMC and T4MC groups were significantly higher than that in WTSC and T4SC groups, respectively (**p<0.05, #p<0.05), 
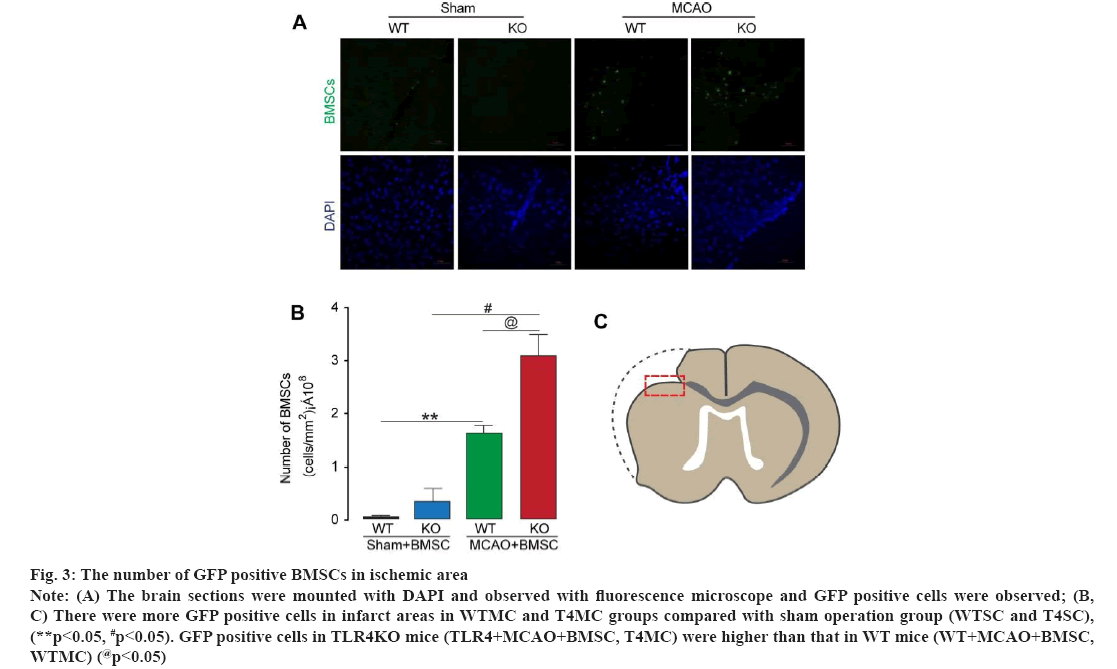
The number of GFP positive BMSCs in ischemic area was observed in fig. 3. The brain sections were mounted with DAPI and observed with fluorescence microscope. The number of BMSCs (GFP positive cells) in the infarct area was counted by Image Pro software (fig. 3A). Results from analysis for GFP positive cells showed that, there were more GFP positive cells in infarct areas in WTMC (WT+MCAO+BMSC) and T4MC (TLR4+MCAO+BMSC) groups compared with sham operation groups (WT+sham+BMSC, WTSC and TLR4+sham+BMSC, T4SC), respectively (p<0.05). In addition, there were more GFP positive cells in TLR4KO mice (TLR4+MCAO+BMSC, T4MC) compared with WT mice (WT+MCAO+BMSC, WTMC) (p<0.05) as shown in fig. 3B and fig. 3C.
Figure 3: The number of GFP positive BMSCs in ischemic area Note: (A) The brain sections were mounted with DAPI and observed with fluorescence microscope and GFP positive cells were observed; (B, C) There were more GFP positive cells in infarct areas in WTMC and T4MC groups compared with sham operation group (WTSC and T4SC), (**p<0.05, #p<0.05). GFP positive cells in TLR4KO mice (TLR4+MCAO+BMSC, T4MC) were higher than that in WT mice (WT+MCAO+BMSC, WTMC) (@p<0.05)
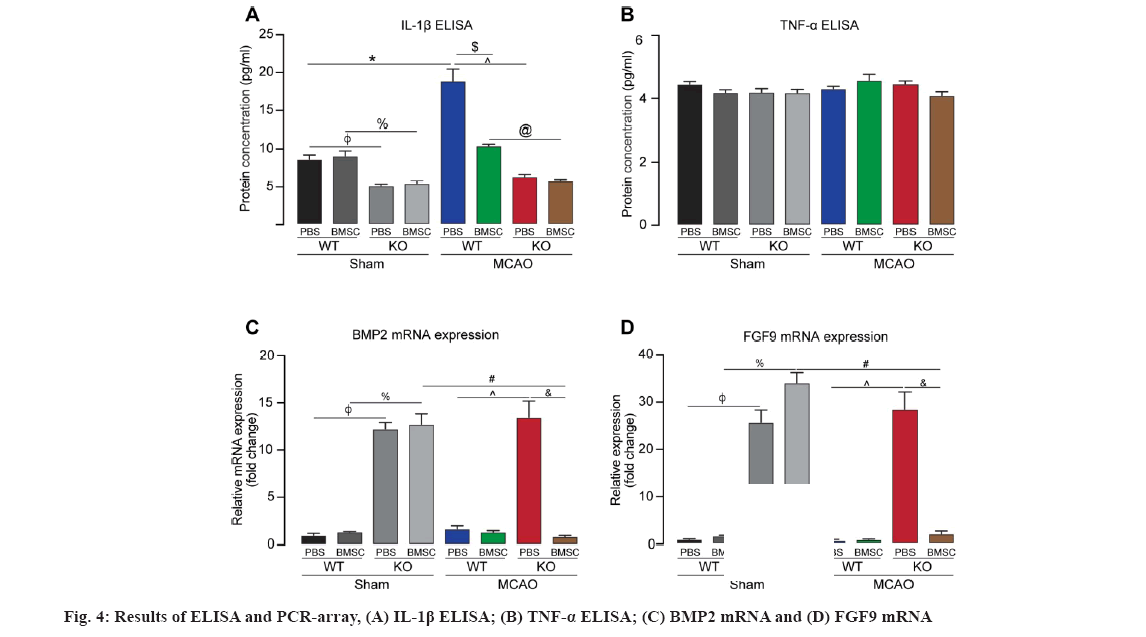
The results of ELISA showed that the level of IL- 1β protein in brain tissue of WTMP group was higher than that of WTSP group and WTMC group (*p<0.05, $p<0.05). The level of IL-1β in T4MC/ T4MP groups was lower than that in WTMC/WTMP groups, respectively(@p<0.05, ^p<0.05). There were no statistical difference in the level of IL-1β protein in brain tissue of WTSP group and WTSC group (p>0.05) as shown in fig. 4A. There were no statistical difference in the level of TNF-α among each groups (p>0.05) as shown in fig. 4B. The mRNA of 22 cytokines related to stem cell regulation was screened by PCR array. The results showed that there were more than 2 folds difference in the mRNAs of Bone Morphogenetic Protein 2 (BMP2) (fig. 4C) and Fibroblast Growth Factor 9 (FGF9) (fig. 4D).
The levels of BMP2 mRNA and FGF9 mRNA in TLR4KO mice (T4SP and T4MP) were significant higher than that in WT mice (SP and WTMP), respectively (φp<0.05, ^p<0.05). Moreover, FGF9 mRNA and BMP2 mRNA in the mice from T4SC group was significantly up-regulated compared with the mice from WTSC group (p<0.05). However, the levels of BMP2 and FGF9 mRNA did not show significant up-regulation in TLR4KO mice subjected to MCAO 3 d after i.c.v. injection of BMSCs. There were no statistical difference in the level of BMP2 mRNA and FGF9 mRNA in brain tissue of WTSP group and WTSC group, WTSP group and WTMP group, WTMC group and WTMP group, respectively (p>0.05).
Stroke is the main cause of disability and death in the world within neurological diseases. Despite such a huge impact, enzymatic and mechanical recanalization is the only effective treatments available so far for ischemic stroke, but only <20 % of patients can benefit from them. Stem cells, as a possible cell therapy in stroke, had attracted the attention of many researchers. Although many questions remain unanswered, Mesenchymal Stem Cells (MSCs) still are promising candidates for cell therapy-based tissue repair and disease treatment.
TLRs are a family of transmembrane receptors responsible for recognition and initiation of a response to invading microbes by the immune system. TLRs recognize molecules from injured cells or pathogens acting as the first line of the immune defense system.
MSCs express TLR2, TLR3, TLR4, TLR7 and TLR9. The expression levels of these TLRs vary significantly based on their tissue origin[17]. TLR activation can further stimulate immune cells and MSCs[18]. Activated MSCs respond to TLR ligands and release anti-inflammatory factors. It has been found that TLR4 is involved in nervous system development and regulates the differentiation and proliferation of adult neuronal precursor cells and enhanced or silenced expression of TLR4 affected Schwann cells proliferation, migration, apoptosis and relative gene expression[19]. In the present study, by using TLR4KO mice to establish phosphorylated MCAO (p-MCAO) model, after BMSCs were injected into the lateral ventricle, we obtained the following novel findings.
The result showed that, 10 d after cerebral ischemia (7 d after i.c.v. injection of BMSCs), the percentage of right forelimb usage in T4MC group was significantly higher compared to WTMC group and T4MP group, which indicated that TLR4KO could enhance the effect of transplanted exogenous BMSCs on functional recovery after cerebral ischemia. However, there was no significant difference among the groups of MCAO mice on the 21st d after MCAO. Maybe it was because that the artery we blocked was the distal MCA, which results in the local cortical small infarction. Furthermore, the functional defect of each group may be repaired obviously on the 21st d, which counteracted the possible large difference.
It has been found that inflammatory responses in the ischemic brain tissue can induce BMSCs to migrate into ischemic areas and affect its proliferation[20]. LPS-induced upregulation of the TLR4 signaling pathway inhibits osteogenic differentiation of human periodontal ligament stem cells under inflammatory conditions[21]. However, whether TLR4-mediated signaling takes part in the proliferation of BMSCs and its migration to ischemic areas remain uninvestigated. The present study applied in vivo image system to evaluate the fluorescence intensity of GFP labeled BMSCs, which represented the proliferation and survival of BMSCs in brain tissue. Our results from in vivo imaging showed that exogenous BMSCs that proliferate in ischemic brain tissue, will reach the peak on the 3rd d after i.c.v. injection and started to decrease from the 3rd to 7th d after injection. It is interesting to note that on the 3rd d after BMSCs injection, the fluorescence intensities in T4MC group were significantly higher than that in WTMC group (p<0.05), which suggesting that TLR4 deficiency (gene knockout) may improve the proliferation and survival of exogenous BMSCs in ischemic brain.
GFP positive cells were counted in ischemic areas in brain section. The results showed that there were more GFP positive cells in infarct areas in WTMC and T4MC groups compared with sham operation groups (WTSC and T4SC), respectively (p<0.05), which indicated that cerebral ischemia can promote the migration and survival of exogenous BMSCs in WT mice. In addition, the number of GFP positive cells in T4MC group significantly increased compared with WTMC. Since the BMSCs were injected into lateral cerebral ventricle, the GFP positive cells in ischemic areas away from later cerebral ventricle partly reflected the migration of BMSCs to ischemic areas, which, nevertheless, also indicated the amount of proliferation and survival of migrated BMSCs. Although the proportion of migration, proliferation and survival of cells in the counted GFP positive cells was not identified, the results at least showed that the amount of BMSCs were increased in ischemic areas, which indicated that TLR4 deficiency (gene knockout) the increase strength of BMSCs in ischemic areas after i.c.v. injection of BMSCs.
IL-1β and TNF-α were also detected by the method of ELISA in our study. IL-1β was involved in brain injury by binding to the Interleukin type-1 Receptor (IL-R1) and activating the IL-1R1-Myeloid Differentiation Primary Response Protein 88 (MyD88)-Interleukin-1 Receptor Associated Kinases (IRAK) pathway to mediate immune inflammatory response[22]. The expression of IL-1β increases rapidly in acute brain injury, however, lower expression of IL-1β plays neuroprotective role in focal cerebral ischemia. Previous studies have shown that transplantation of BMSCs inhibited the expression of IL-1β, reduced cerebral edema and infarct size after cerebral ischemia. TNF-α is a subtype of TNF, which induces the expression of inducible Nitric Oxide Synthase (iNOS) in microglia and astrocytes, promotes leukocyte infiltration into brain tissue, and exacerbates ischemic brain damage[23]. TNF-α also induce the expression of adhesion molecules on the surface of MSCs and further compulsively induce the cellular contact-dependent immunosuppressive function. On the other hand, BMSCs was shown to alleviate the inflammation in damaged spinal cord by inhibiting TLR4-mediated signaling pathways and reduced IL-1β and TNF-α. Our data showed that cerebral ischemia increased the expression of IL-1β, which was inhibited by transplantation of BMCSs via lateral cerebral ventricle (WTMP vs. WTSP, p<0.05; WTMC vs. WTMP, p<0.05) and TLR4KO reduced the expression of IL-1β (T4MC vs. WTMC, p<0.05). However, we failed to find the further decreased expression of TNF-α in TLR4KO mice subjected to cerebral ischemia 3 d after BMSCs transplantation. The possible reason is that TNF-α expressed earlier before 72 h, after ischemia in brain tissue. Some animal studies have found that the expression of TNF-α gradually increased after 1 h of cerebral ischemia-reperfusion. TNF-α serum levels and protein levels in brain tissue reached its peak at 2 h after MCAO. The time of the present experiment that detects the expression of TNF-α was 6 d after operation, which may have missed the peak of TNF-α expression.
In the present study, the mRNA of 22 cytokines associated with stem cell survival and differentiation were detected using PCR array. The results showed that the levels of BMP2 mRNA and FGF9 mRNA in TLR4 knockout mice (T4SP, T4SC and T4MP) were significant higher than that in WT mice (WTSP, WTSC and WTMP) (p<0.05), respectively.
BMP, a member of the transforming growth factor-β super family, is named for its ability to induce the formation of new bone, which can regulate the survival and differentiation of neuron as well as promote neurogenesis. It has been reported that bone marrow stromal cells transplantation after ischemia can increase the expression of BMP2 in astrocytes in ischemic areas. FGF9, a member of the FGF family, is widely distributed in various tissues and organs of the human body, participates in many pathophysiological processes such as angiogenesis, nerve regeneration and damage repair, etc[24]. Stem cells have the ability to promote nerve repair and regeneration. However, their effectiveness is limited by stem cells survival, proliferation and differentiation into appropriate neuronal cell types. BMP2 and FGF9 can regulate the process. Studies have found that BMP2 regulates progenitor and stem cell differentiation by binding to their receptors, and the upregulation of BMP2 has been shown to promote neuroprotection, glial hyperplasia and gliosis. FGF9 promotes the proliferation and survival of adult Neural Progenitor Cells (NPCs) and increase the number of neurons. Some studies predict that FGF9 can act as a nutrient factor to promote neuronal regeneration after injury to the middle nervous system.
We found that the effects of TLR4 on exogenous BMSCs in ischemic brain might be mediated through up-regulating the expression of BMP2 and FGF9. However, 3 d after i.c.v. injection of BMSCs, the levels of BMP2 and FGF9 mRNA were not up- regulated in TLR4KO mice subjected to MCAO. The phenomenon could be interpreted as that the peak time of the expression of BMP2 and FGF9 mRNA was changed (advanced or postponed) by the combination of TLR4KO, ischemia and i.c.v injection of BMSCs, which need to be further clarified by detecting the expression of BMP2 and FGF9 mRNA in different time points after ischemia and i.c.v. injection of BMSCs.
The lateral ventricle injection of exogenous BMSCs can improve the neurological function after cerebral infarction in mice in TLR4 blocked environment. TLR4 signaling pathways are involved in the regulation of exogenous BMSCs in ischemic brain tissue. And the survival, proliferation and migration to ischemic regions of BMSCs will be increased by blocking TLR4 (knockdown). TLR4 is involved in the regulation of inflammatory response and nerve regeneration. TLR4 knockout inhibits the expression of IL-1β caused by cerebral ischemia and upregulates the expression of BMP2 and FGF9 mRNA, which may be potential molecular mechanisms for the regulation of BMSCs in ischemic brain tissue by TLR4 signaling pathways.
In conclusion, the current research firstly explored that TLR4 deficiency (gene knockout) further increased the amount of BMSCs in ischemic brain areas following i.c.v. injection of BMSCs, thereby improved neurological function after cerebral ischemia. The increased expression of BMP2 and FGF9 in the environment of TLR4 deficiency might be one of the molecular mechanisms underlying the effect of TLR4 on the transplanted BMSCs. The study helps to elucidate the importance of TLR4 on cell therapy for stroke and may provide the basis for future therapeutic strategies to treat ischemic cerebrovascular disease.
Author’s contributions:
Delian Kong designed the topic and drafted the manuscript. Junrong Li helped to draft the manuscript. Delian Kong, Jie Wu, Yanan Wang, Xiaohua Wang and Shuyu Dong conducted concrete experimental operations. All authors read and approved the final manuscript.
Funding:
This work was supported by China National Nature Scientific Foundation (81571469 and 81271268).
Acknowledgements:
I would like to express my heartfelt thanks to my mentor for his unconditional trust and support. And I would like to thank Junrong Li, Jie Wu, Yanan Wang, Xiaohua Wang and Shuyu Dong for their work for the research.
Conflict of interests:
The authors declare that they have no conflict of interest.
References
- Heo JS, Choi SM, Kim HO, Kim EH, You J, Park T, et al. Neural transdifferentiation of human bone marrow mesenchymal stem cells on hydrophobic polymer-modified surface and therapeutic effects in an animal model of ischemic stroke. Neuroscience 2013;238:305-18.
[Crossref] [Google Scholar] [PubMed]
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019;50(12):e344-418.
[Crossref] [Google Scholar] [PubMed]
- DelaRosa O, Dalemans W, Lombardo E. Mesenchymal stem cells as therapeutic agents of inflammatory and autoimmune diseases. Curr Opin Biotechnol 2012;23(6):978-83.
[Crossref] [Google Scholar] [PubMed]
- Hess DC, Sila CA, Furlan AJ, Wechsler LR, Switzer JA, Mays RW. A double-blind placebo-controlled clinical evaluation of multistem for the treatment of ischemic stroke. Int J Stroke 2014;9(3):381-6.
[Crossref] [Google Scholar] [PubMed]
- Zhu J, Tang H, Zhang Z, Zhang Y, Qiu C, Zhang L, et al. Kaempferol slows intervertebral disc degeneration by modifying LPS-induced osteogenesis/adipogenesis imbalance and inflammation response in BMSCs. Int Immunopharmacol 2017;43:236-42.
[Crossref] [Google Scholar] [PubMed]
- Hua F, Wang J, Ishrat T, Wei W, Atif F, Sayeed I, et al. Genomic profile of toll-like receptor pathways in traumatically brain-injured mice: Effect of exogenous progesterone. J Neuroinflammation 2011;8(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Okun E, Griffioen KJ, Son TG, Lee JH, Roberts NJ, Mughal MR, et al. TLR2 activation inhibits embryonic neural progenitor cell proliferation. J Neurochem 2010;114(2):462-74.
[Crossref] [Google Scholar] [PubMed]
- Sun Y, Deng W, Yao G, Chen W, Tang X, Feng X, et al. Citrullinated fibrinogen impairs immunomodulatory function of bone marrow mesenchymal stem cells by triggering toll-like receptor. Clin Immunol 2018;193:38-45.
[Crossref] [Google Scholar] [PubMed]
- Xiu G, Sun J, Li X, Jin H, Zhu Y, Zhou X, et al. The role of HMGB1 in BMSC transplantation for treating MODS in rats. Cell Tissue Res 2018;373(2):395-406.
[Crossref] [Google Scholar] [PubMed]
- Ding H, Jin M, Liu D, Wang S, Zhang J, Song X, et al. Tenascin‑C promotes the migration of bone marrow stem cells viatoll‑like receptor 4‑mediated signaling pathways: MAPK, AKT and Wnt. Mol Med Rep 2018;17(6):7603-10.
[Crossref] [Google Scholar] [PubMed]
- Atif F, Yousuf S, Sayeed I, Ishrat T, Hua F, Stein DG. Combination treatment with progesterone and vitamin D hormone is more effective than monotherapy in ischemic stroke: The role of BDNF/TrkB/Erk1/2 signaling in neuroprotection. Neuropharmacology 2013;67:78-87.
[Crossref] [Google Scholar] [PubMed]
- Llovera G, Roth S, Plesnila N, Veltkamp R, Liesz A. Modeling stroke in mice: Permanent coagulation of the distal middle cerebral artery. J Vis Exp 2014;(89):1-8.
[Crossref] [Google Scholar] [PubMed]
- deVos SL, Miller TM. Direct intraventricular delivery of drugs to the rodent central nervous system. J Vis Exp 2013;(75):1-10.
[Crossref] [Google Scholar] [PubMed]
- Roome RB, Vanderluit JL. Paw-dragging: A novel, sensitive analysis of the mouse cylinder test. J Vis Exp 2015;(98):e52701.
[Crossref] [Google Scholar] [PubMed]
- Rattka M, Fluri F, Krstić M, Asan E, Volkmann J. A novel approach to assess motor outcome of deep brain stimulation effects in the hemiparkinsonian rat: Staircase and cylinder test. J Vis Exp 2016;(111):e53951.
[Crossref] [Google Scholar] [PubMed]
- Hua F, Tang H, Wang J, Prunty MC, Hua X, Sayeed I, et al. TAK-242, an antagonist for toll-like receptor 4, protects against acute cerebral ischemia/reperfusion injury in mice. J Cereb Blood Flow Metab 2015;35(4):536-42.
[Crossref] [Google Scholar] [PubMed]
- Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif 2020;53(1):e12712.
[Crossref] [Google Scholar] [PubMed]
- DelaRosa O, Dalemans W, Lombardo E. Toll-like receptors as modulators of mesenchymal stem cells. Front Immunol 2012;3:182.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Shao Z, Zhu Y, Shi L, Li Z, Hou R, et al. Toll-like receptor 4 (TLR4) expression affects Schwann cell behavior in vitro. Sci Rep 2018;8(1):1-2.
- Kuroda S. Bone marrow stromal cell transplantation for ischemic stroke-its multi-functional feature. Acta Neurobiol Exp 2013;73(1):57-65.
[Google Scholar] [PubMed]
- Yu B, Li Q, Zhou M. LPS‑induced upregulation of the TLR4 signaling pathway inhibits osteogenic differentiation of human periodontal ligament stem cells under inflammatory conditions. Int J Mol Med 2019;43(6):2341-51.
[Crossref] [Google Scholar] [PubMed]
- Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant 2007;40(7):609-19.
[Crossref] [Google Scholar] [PubMed]
- Yoon JS, Lee JH, Tweedie D, Mughal MR, Chigurupati S, Greig NH, et al. 3, 6′-dithiothalidomide improves experimental stroke outcome by suppressing neuroinflammation. J Neurosci Res 2013;91(5):671-80.
[Crossref] [Google Scholar] [PubMed]
- Falcone C, Filippis C, Granzotto M, Mallamaci A. Emx2 expression levels in NSC s modulate astrogenesis rates by regulating EgfR and Fgf9. Glia 2015;63(3):412-22.
[Crossref] [Google Scholar] [PubMed]