- *Corresponding Author:
- M. Rabiha
Laboratory Antibiotics Antifungals, Physicochemical, Synthesis and Biological Activity (LAPSAB), Department of Biology, University of Tlemcen, Tlemcen-13000, Algeria
E-mail: rabiha.mezerai@gmail.com
| Date of Submission | 15 June 2016 |
| Date of Revision | 06 November 2016 |
| Date of Acceptance | 11 January 2017 |
| Indian J Pharm Sci 2017;79(1): 72-78 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of this work is to study the effect of the physicochemical environment on in vivo distribution of amphotericin B, which was prepared in acetic acid/acetate buffer pH 5.4 at a concentration of 10-4 M. Rats were injected intraperitoneally with this antifungal solution at the doses of 1, 2 or 5 mg/kg body weight. At predefined time, amphotericin B from tissues and plasma was extracted and analysed using the second derivative spectrophotometry methodology. The level of amphotericin B in tissues and plasma depended on the administered dose and decreased over a period of time. The highest concentrations were found in the spleen, followed by the liver, but were lower in kidneys and plasma. Amphotericin B was accumulated in the intraperitoneal cavity (the peritoneal adipose tissue) in large quantities proportional to the dose administered intraperitoneally. This formulation has the advantage of being easy to prepare and inexpensive, it seems very promising for the treatment of fungal infections of spleen and peritoneal cavity.
Keywords
Amphotericin B, physicochemical environment, pH, intraperitoneal rout, acetic acid/acetate buffer solution, in vivo distribution, fungal peritonitis
Amphotericin B remains the reference treatment for invasive fungal infections, recommended by guidelines for the treatment of haematogenous candidiasis at least till 2000 [1]. The conventional amphotericin B deoxycholate formulation has been available since 1958, but this preparation has a wide variety of acute and chronic side effects; nephrotoxicity being the main effect limiting its administration to 1 mg/kg/d [2]. This toxicity is suggested to be related to its low solubility in water, its interactions with cholesterol of the plasma membrane and its oxidative activity on human cells and on blood proteins [3,4]. In liquid media, amphotericin B molecules can adopt several different dispositions. They can stay freely separated forming true dissolutions (amphotericin B is presented in monomeric form), or assemble to macromolecular structures known as oligomers and poly-aggregates [5].
Both the therapeutic action and the toxic side effects of this drug are dependent on its molecular association [6]. The proportion of each association form has been shown to depend on different factors, such as amphotericin B concentration [7], the variations on the pH conditions during the formula preparation procedure [6], or even the temperatures they have been exposed too [8]. Amphotericin B prepared in an acetic acid/acetate buffer (100 mM) pH 5.4 at a concentration of 10-4 M, showed an improvement of the therapeutic index of this polyene, this has been proven in vitro on fungal cells (Candida albicans), on human cells (red blood cells), and in vivo in rats [9,10]. This strategy has the advantage of being easy to access, inexpensive, and provided more effective and less toxic formulations for intensive use in poor countries. For these reasons, it is important to study the effect of these conditions during the formula preparation on in vivo distribution of amphotericin B, because the antimycotic activity in vivo probably depends also on the amphotericin B concentration at the site of infection. In this study, in addition to determining amphotericin B levels in plasma and peritoneal adipose tissue, kidneys, liver, and spleen levels have also been included because these tissues are often the target organs of C. albicans infection [11]. Kidneys have been the principal organs where toxic effects of amphotericin B are expressed [11].
Materials and Methods
Our study was done using UV/Vis spectrophotometer (Specord® 200 plus, Analytik Jena), 10 mm pathlength quartz cells, a sonicator (Sonics,Vibra-Cell), a Polytron® homogenizer (PT1600 E, Kinematica) and a refrigerated table-top centrifuge (2-16PK, Sigma®), Ultra water purification system (aquaMAX™- Ultra 370 series, Young Lin, Korea). The reagents used were acetonitrile (Prolabo: analytical grades), dimethyl sulfoxide (DMSO, Guaranteed analysis Assay GC Sigma Aldrich), methanol (Laboratory Reagent ≥99.6%, Sigma Aldrich), acetic acid, sodium acetate trihydrate (Guaranteed analysis Riedel-de Haën) and amphotericin B (Sigma Aldrich).
Healthy adult male and female Wistar rats obtained from the Pasteur Institute of Algeria (200-300 g body weight), were used. They were housed under conventional conditions, at a temperature of 24±2°, relative humidity of 50-60% under a 12 h light and dark cycle. A standard commercial rodent diet and tap water were given ad libitum. The animals were handled according to the ethical recommendations for use of laboratory animals universally recognized. The rats were given amphotericin B buffered solution as a single intraperitoneal dose of 1 or 2 or 5 mg/kg. Rats were sacrificed by chloroform inhalation on days 1, 2, 7 or 14 after drug administration.
Antifungal solution
Amphotericin B was dissolved in DMSO to obtain a stock solution of the concentration 10-2 M, which was further diluted to 10-4 M in acetic acid/acetate buffer (100 mM, pH 5.4). All antifungal solutions were kept at ambient temperature and away from light until injection.
Determination of amphotericin B concentration in tissue and plasma
Amphotericin B from tissue samples was extracted using a method described by Mayhew et al. [12], and from plasma using the method described by Monteil et al. [13] with some modifications. Animals were sacrificed, liver, spleen and kidneys were removed and weighed. Two milliliters of pure methanol was added to 1 g of tissue and the mixture was vortexed for 2 min, subsequently homogenized for 2 min at high speed and sonicated under 90% amplitude for 15 s, all in a glacial water bath. The homogenate was then centrifuged at 3500 g for 30 min. After re-extraction of the pellet, the supernatants were pooled and the final volume was adjusted to 5 ml with methanol. Aliquots of the extracts were further clarified by centrifugation at 12 800 g for 15 min just before the spectrophotometric analysis. Methanol was used as the blank.
Blood was collected in heparinized tubes. After centrifugation the assay was performed on plasma. The standards and rats samples were both subjected to the deproteinization procedure which was as follows; 1 ml plasma sample was combined with 2 ml of acetonitrile and vortexed for 30 s. After centrifugation at 2000 g for 1 min at 10°, the supernatant was used for spectrophotometric analysis. A reagent blank was prepared by addition of 2 ml of acetonitrile to 1 ml of ultra-pure water. All tubes were protected from light.
A stock standard solution (500 mg/l) was prepared in a mixture of DMSO and methanol (1:1 v/v). Immediately before use, this solution was diluted in ultra-pure water to yield a working solution at 25 mg/l. The calibration standards containing (0, 1.25, 2.50, and 5.0 mg/l) of amphotericin B were prepared by diluting the working solution of amphotericin B with free rats plasma, and (0, 0.75, 1.5, 2. 25 and 3 mg/l) with tissue extract. The recovery was studied in tissues to which known quantities of the drug were added before homogenization (before extraction) and in which the drug was added to methanol extracts of tissue homogenates (after extraction).
Spectrophotometric analysis
Absorption spectra were measured by a spectrophotometer in the wavelength range 320 to 450 nm, at a speed of 2 nm/s and Δλ of 0.5 nm. The second order derivative spectra were traced using Analytik Jena WinAspect Plus 4.0.0.0 software, according to Savitzky-Golay with a value 25 as base points, respectively. The amplitudes were calculated in each spectrum by reading the minimum value between 404 and 409 nm.
Statistical analysis
Statistics for comparisons of tissue drug concentrations were performed using Kruskal-Wallis followed by the two-tailed Mann-Whitney test if significant. P<0.05 was considered significant. The bar in the graphs indicates the median value.
Results and Discussion
The low toxicity observed with amphotericin B buffered formulation (acetic acid/acetate buffer, pH 5.4, 10-4 M) was due to the antibiotic screening by the acetate ions of the buffer, without reorganization of amphotericin B self-association [9]. This in vitro study should be extended to in vivo studies, such as monitoring this preparation in tissues and plasma after its injection in rats. The initial step in our study was to create a calibration curve and to determine the recovery of the antifungal in plasma and tissue extracts of rats spiked with amphotericin B at different concentrations. Table 1 showed the linear regression equation, correlation coefficient (r2), and the recovery (%).
| Biological material | Equation | Correlation coefficient (r2) | The recovery±SD % |
|---|---|---|---|
| Liver | y=0.006528133 x | 0.991 | 88.29±5.71 |
| Spleen | y=0.007740068 x | 0.999 | 90.74±5.91 |
| Kidney | y=0.008370961 x | 0.998 | 81.96±3.29 |
| Plasma | y=0.002787844 x | 0.999 | 100 |
Table 1: Linear Regression Data for Calibration Curves
The correlation coefficient for each biological material was found to be close to 99%, which confirm the linearity of the method over the concentration range considered. The recovery of amphotericin B was more than 80% these values show that the method of amphotericin B extraction from tissue and plasma was effective. These results are in agreement with those of previous studies using the UV/Vis methods for the determination of amphotericin B in biological material [13,14].
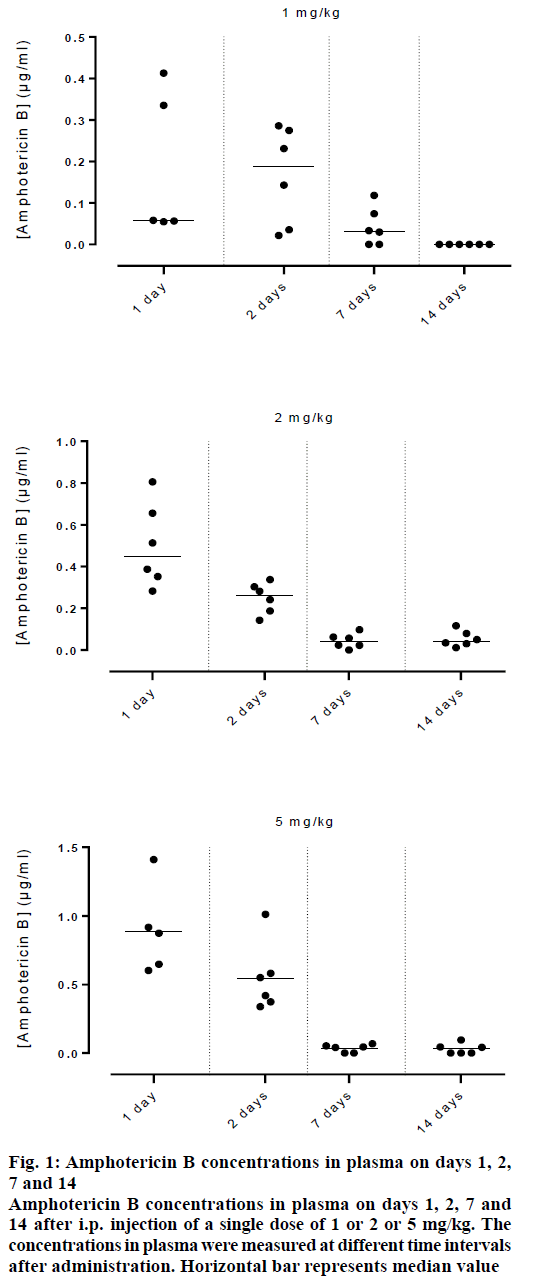
Figure 1 showed the data of amphotericin B concentrations in plasma of rats injected intraperitoneally with 1 or 2 or 5 mg/kg of amphotericin B prepared in the buffered system, at various time intervals post injection. Amphotericin B concentrations were lower and decreased faster in plasma. This was attributed to its poor solubility in an aqueous solution and to the screening of the positively charged aggregated antibiotic by the negatively charged acetate ions of the buffer. The pharmacokinetics and tissue distribution of amphotericin B-laden liposomes are strongly affected by their physico-chemical properties, such as the surface electrical charge [15,16]. Positively charged and neutral liposomes have a slower circulation in blood, compared to those, which are negatively charged [17].
Figure 1: Amphotericin B concentrations in plasma on days 1, 2, 7 and 14
Amphotericin B concentrations in plasma on days 1, 2, 7 and 14 after i.p. injection of a single dose of 1 or 2 or 5 mg/kg. The concentrations in plasma were measured at different time intervals after administration. Horizontal bar represents median value
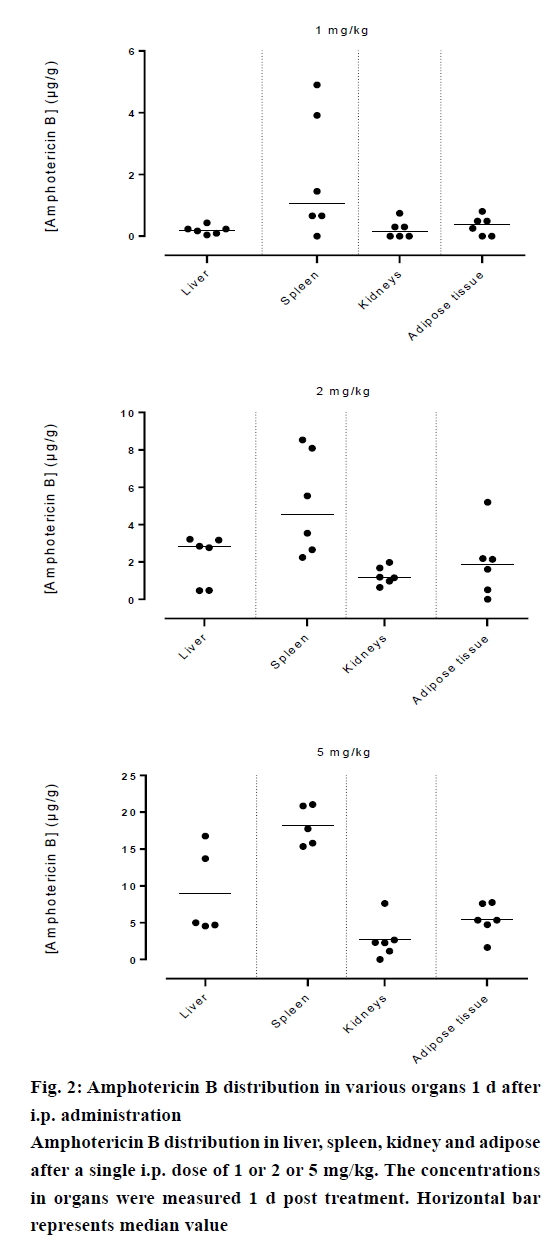
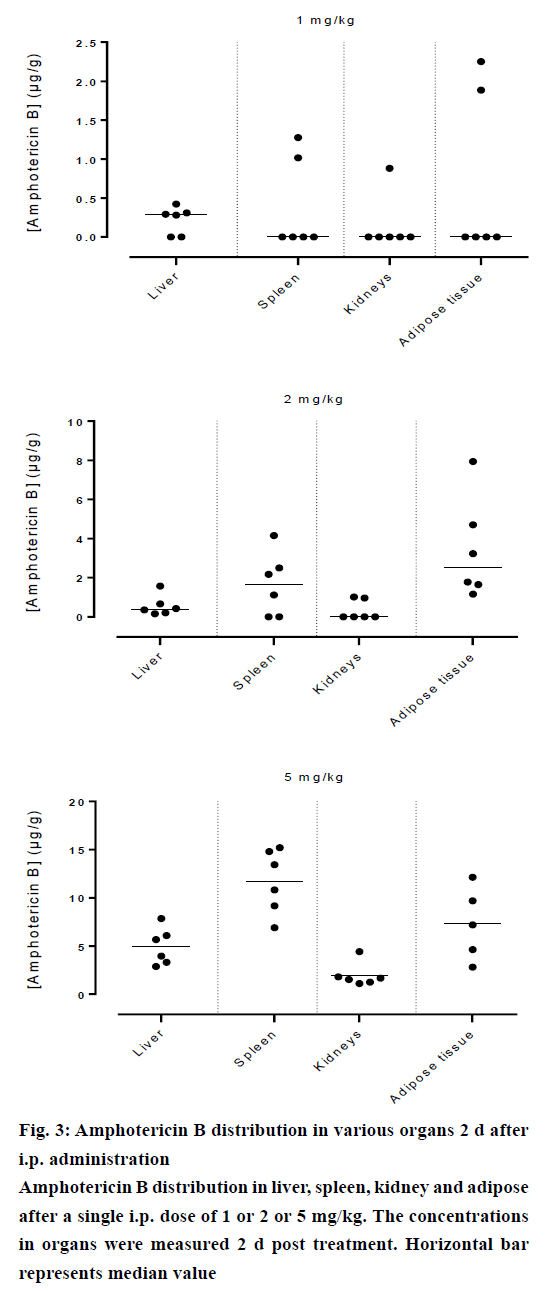
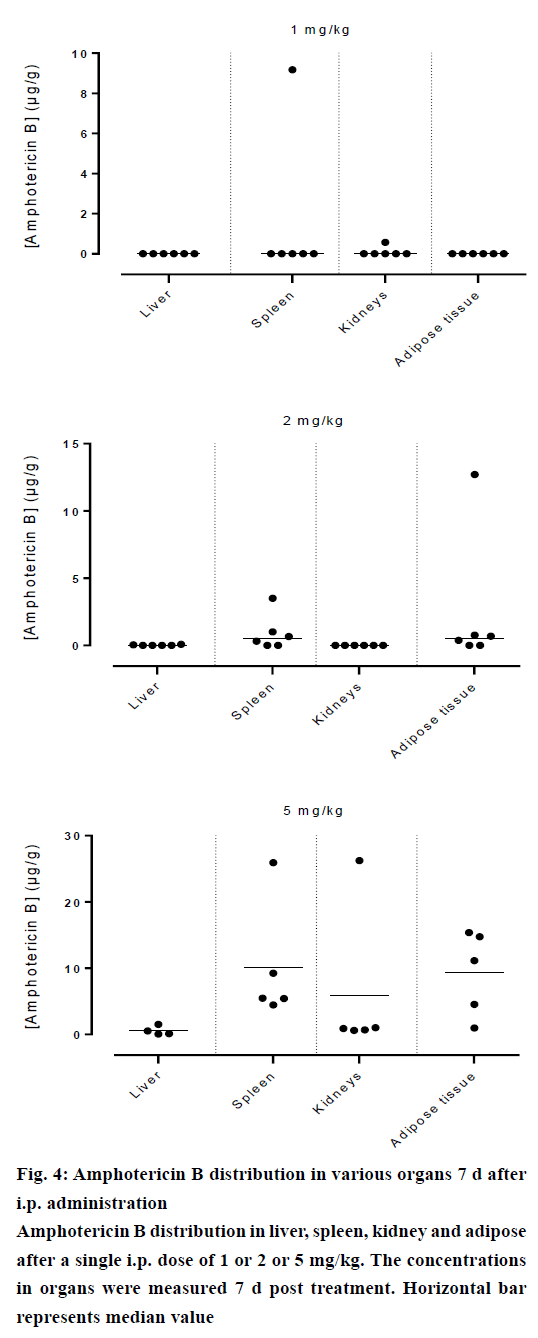
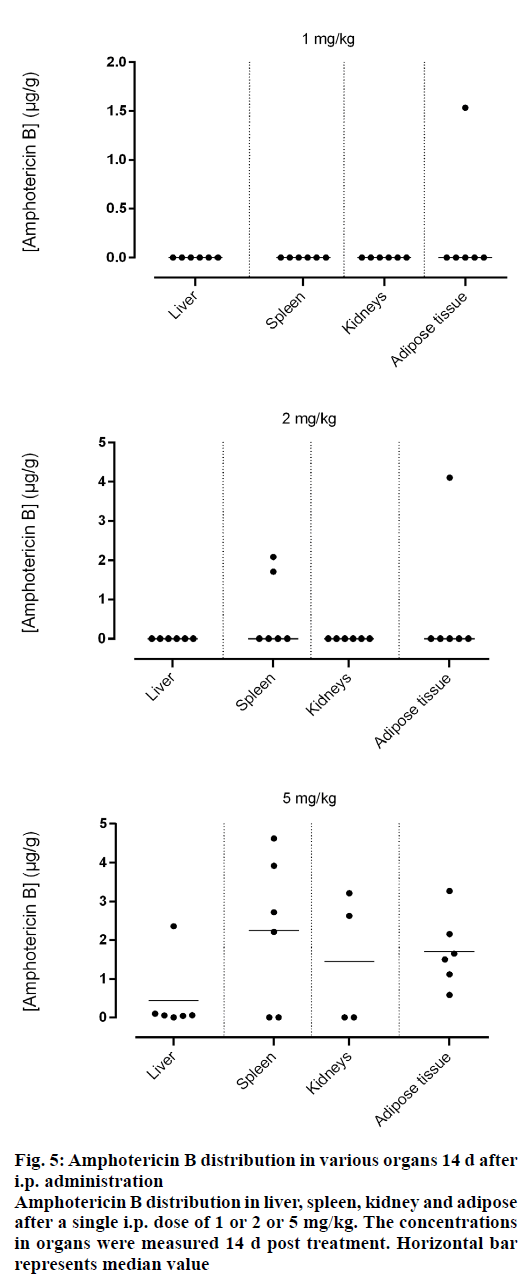
Amphotericin B was found in all compartments (liver, spleen, kidneys and the peritoneal adipose tissue), the highest levels of amphotericin B were found at the first day post injection, followed by a gradual decrease over the time. After day 1, a homogeneous distribution of amphotericin B in the organs of rats injected with the lowest dose of 1 mg/kg was observed while a significant differences in organ distribution were found in animals given 2 mg/kg (P<0.05) and 5 mg/kg (P<0.05), where the vast majority of amphotericin B was concentrated in the spleen followed by liver while very little in the kidneys (Figure 2). This distribution pattern was maintained even at 2 d post injection, after which the level of amphotericin B started to decrease (Figure 3). After 7 d, the amount of amphotericin B in the organs reached a no detectable level in animals administered with 1 or 2 mg/kg, but in animals injected with the high dose of 5 mg/kg, the antifungal remained in the spleen even after 14 d post treatment (2.46 μg/g). The level in the kidney however, remained constant throughout the study (day 1 median=2.282 μg/g, the day 14 median=2.625 μg/g, Figures 4 and 5). In the intraperitoneal cavity (adipose tissue) amphotericin B showed a similar pattern as found in the spleen, that it was trapped in this compartment at considerable quantities, which were proportional to the dose administered intraperitoneally. The amount of amphotericin B increased on day 2 but was not statistically significant in rats treated with 1 or 2 mg/kg, but in the rats, which received 5 mg/kg significantly higher levels were accumulated (P<0.05) and these levels lasted up to 7 d post treatment (Figures 2-5).
Figure 5: Amphotericin B distribution in various organs 14 d after i.p. administration
Amphotericin B distribution in liver, spleen, kidney and adipose after a single i.p. dose of 1 or 2 or 5 mg/kg. The concentrations in organs were measured 14 d post treatment. Horizontal bar represents median value
Amphotericin B prepared in acetic acid/acetate buffer, pH 5.4 displayed higher penetration into the spleen, which would likely serve to reduce the risk of hepatic cell damage that could have caused by higher hepatic levels of amphotericin B because some experimental data suggested that it might interfere with the hepatic cytochrome P450 and may have an influence on the metabolic capacity of the liver [18], these results are compatible with the those of NIKI and collaborators [19], the highest concentrations were found in the spleen and liver; and lower in kidneys and in serum, after administration of amphotericin B-deoxycholate by similar route. However, the bulk of amphotericin B of lipid formulations (liposomal and colloidal preparation) was found in the liver [20,21].
The results of Belkherroubi et al. [10] showed that urea and creatinine levels in the blood are not disturbed, indicating that the renal function was not affected when amphotericin B prepared in 10-4 M acetic acid/ acetate buffer, pH 5.4 was administered. According to our data this could be due to the fact that lower levels were found in the kidneys. Boswell et al. [20] reported that treatment with liposomal formulations resulted in lower concentrations in kidneys with significantly less nephrotoxicity than conventional amphotericin B therapy.
The retention of amphotericin B in the spleen, in the peritoneal cavity and in the kidneys after administration of a single dose of 5 mg/kg intraperitoneally can be considered as a way for the treatment of fungal infection in these tissues and avoid the injection of multiple doses for up to 2 w, more especially for the treatment of fungal peritonitis which is a potentially fatal complication of chronic peritoneal dialysis, associated with high morbidity and mortality ranging between 20 and 30% [22]. Furthermore the peritoneal concentrations of amphotericin B after administration of conventional or lipid formulations were similar or lower than the minimum inhibitory concentration (MIC) for the most relevant pathogens. This may increase the risk of treatment failure in fungal peritonitis [23].
Amphotericin B is the gold standard for the treatment of systemic fungal infections. This study attempts to provide more clarity about the influence of the physicochemical environment on in vivo distribution of amphotericin B prepared in acetic acid/acetate buffer at pH 5.4. The improvement in the therapeutic index of this drug by simple modification of the molecular composition, pH of the environment surrounding amphotericin B with peritoneal injection, resulting in a greater affinity to spleen cells, reduced levels in the liver and especially in the kidneys with lower renal and hepatic toxicity. Amphotericin B’s remarkable highest concentrations in the peritoneal cavity, appears highly appropriate for treating fungal peritonitis.
Conflict of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Rex JH, Walsh TJ, Sobel JD, Filler SG, Pappas PG, Dismukes WE, et al. Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin Infect Dis 2000;30:662-78.
- Espada R, Valdespina S, Dea MA, Molero G, Ballesteros MP, Bolas F, et al. In vivodistribution and therapeutic efficacy of a novel amphotericin B poly-aggregated formulation. J AntimicrobChemother2008;61:1125-31.
- Wilcock BC, Endo MM, Uno BE, Burke MD. C2'-OH of amphotericin B plays an important role in binding the primary sterol of human cells but not yeast cells. J Am ChemSoc 2013;135:8488-91.
- Brajtburg J, Elberg S, Bolard J, Kobayashi GS, Levy RA, Ostlund RE Jr., et al. Interaction of plasma proteins and lipoproteins with amphotericin B. Clin Infect Dis 1984;149:986-97.
- Sanchez-Brunete JA, Dea MA, Rama S, Bolas F, Alunda JM, Torrado-Santiago S, et al.Amphotericin B molecular organization as an essential factor to improve activity/toxicity ratio in the treatment of visceral leishmaniasis. J Drug Target 2004;12:453-60.
- Gagos M, Herec M, Arczewska M, Czernel G, Dalla Serra M, Gruszecki WI. Anomalously high aggregation level of the polyene antibiotic amphotericin B in acidic medium: implications for the biological action. BiophysChem 2008;136:44-9.
- Legrand P, Romero EA, Cohen BE, Bolard J. Effects of aggregation and solvent on the toxicity of amphotericin B to human erythrocytes. Antimicrob Agents Chemother 1992;36(11):2518-22.
- Gaboriau F, Cheron M, Petit C, Bolard J. Heat-induced superaggregation of amphotericin B reduces its in vitrotoxicity: a new way to improve its therapeutic index. J AntimicrobChemother 1997;41:2345-51.
- Belkherroubi-Sari L, Boucherit Z, Cheron M, Boucherit K, Benyoucef M, Belbraouet S. Modulation of the polyene antibiotic amphotericin B selective toxicity by pH change of the stock solutions. Afr J Microbiol Res 2008;2:242-6.
- Belkherroubi-Sari L, Boucherit Z, Boucherit K, Belbraouet S. Study of Renal Toxicity in Wistar Rats Following the Action of Amphotericin B Solution Prepared under Extreme pH Conditions. Food NutrSci 2011;2:731-5.
- Fanos V, Cataldi L. Amphotericin B-induced nephrotoxicity: a review. J Chemother 2000;12:463-70.
- Mayhew JW, Fiore C, Murray T, Barza M. An internally-standardized assay for amphotericin B in tissues and plasma. J Chromatogr 1983;274:271-9.
- Ganiere-Monteil C, Kergueris MF, Iooss P, Thomas L, Larousse C. Quantitation of amphotericin B in plasma by second-derivative spectrophotometry. J Pharm Biomed Anal 1998;17:481-5.
- Casaccia P, Ladogana A, Xi YG, Ingrosso L, Pocchiari M, Silvestrini MC, et al. Measurement of the concentration of amphotericin B in brain tissue of scrapie-infected hamsters with a simple and sensitive method. J AntimicrobChemother 1991;35:1486-8.
- Gregoriadis G. Overview of liposomes. J AntimicrobChemother 1991;28:39-48.
- Janknegt R, de Marie S, Bakker-Woudenberg IA, Crommelin DJ. Liposomal and lipid formulations of amphotericin B. Clinical pharmacokinetics. ClinPharmacokinet 1992;23:279-91.
- Wong-Beringer A, Jacobs RA, Guglielmo BJ. Lipid formulations of amphotericin B: clinical efficacy and toxicities. Clin Infect Dis 1998;27:603-18.
- Inselmann G, Inselmann U, Heidemann HT. Amphotericin B and liver function. Eur J Intern Med 2002;13:288-92.
- Niki Y, Bernard EM, Schmitt HJ, Tong WP, Edwards FF, Armstrong D. Pharmacokinetics of aerosol amphotericin B in rats. J AntimicrobChemother 1990;34:29-32.
- Boswell GW, Bekersky I, Buell D, Hiles R, Walsh TJ. Toxicological profile and pharmacokinetics of a unilamellar liposomal vesicle formulation of amphotericin B in rats. J AntimicrobChemother 1998;42:263-8.
- Vogelsinger H, Weiler S, Djanani A, Kountchev J, Bellmann-Weiler R, Wiedermann CJ, et al.Amphotericin B tissue distribution in autopsy material after treatment with liposomal amphotericin B and amphotericin B colloidal dispersion. J AntimicrobChemother 2006;57:1153-60.
- Matuszkiewicz?Rowinska J. update on fungal peritonitis and its treatment. International Society for Peritoneal Dialysis 2009;29:S161-S5.
- Weiler S, Bellmann-Weiler R, Dunzendorfer S, Joannidis M, Bellmann R. Levels of amphotericin B lipid formulations in ascites. JAntimicrobChemother 2008;62:1163-4.