- *Corresponding Author:
- Donghua Li
Department of Neurosurgery, Panzhihua Central Hospital, Panzhihua, Sichuan Province 617000, China
E-mail: 18096306988@163.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “171-177” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In this study, the therapeutic role of edaravone dexborneol injection in acute ischemic stroke and its influence on nerve function were analyzed. We selected 123 acute ischemic stroke individuals admitted from December 2018 to December 2022 and grouped them based on different therapies into control and research groups. The control group (n=56) received conventional (alteplase) treatment while the research group (n=67) received edaravone dexborneol injection. The two groups were comparatively analysed for therapeutic effects, side effects of medication such as nausea, vomiting, chest tightness, fever, dizziness and headache. Similarly, the nerve function, disability degree were assessed according to the National Institutes of Health Stroke Scale and modified Rankin Scale respectively. Activities of daily living ability was evaluated using Barthel index and serum indices such as nitric oxide, endothelin-1 and matrix metalloproteinase were analysed. After analysis, the research group was found to have an evidently higher total effective rate than the control group, with equivalent side effects of medication. Marked reductions in National Institutes of Health Stroke Scale, modified Rankin Scale, endothelin-1 and matrix metalloproteinase were observed in the research group after treatment, which were notably lower than the pre-treatment levels in the control group; while the Barthel index score and nitric oxide level were statistically elevated after treatment, higher than the pre-treatment level and the control group. Conclusively, edaravone dexborneol injection is conducive to enhance the therapeutic effects with mild side effects of medication, which helps to improve patients’ nerve function, reduce the risk of disability and enhance their daily life activities, the mechanism of which may be partly associated with the regulation of serum nitric oxide, endothelin-1 and matrix metalloproteinase levels.
Keywords
Edaravone dexborneol injection, ischemic stroke, nitric oxide, matrix metalloproteinase, endothelin-1
Stroke or apoplexy, is related to insufficient blood supply to the brain, which may induce neurological dysfunction and various neurodegenerative diseases, causing varying degrees of negative impacts on patient’s normal life[1,2]. According to epidemiological data, the global number of stroke cases is as high as 13.7 million and ischemic stroke occupies 87 %[3]. Acute Ischemic Stroke (AIS) is a common type of stroke with high risk of morbidity, disability and death[4]. Effective treatment strategy for AIS is to restore sufficient cerebral blood perfusion and rescue ischemic penumbra in a timely manner[5]. Routine intravenous thrombolysis given within 3-4.5 h after the episode of AIS is the primary choice for treating AIS. However, its clinical application is restricted by limited time window and complex contraindications[6]. Therefore, effective treatment strategies are still needed to improve the nerve function and efficacy in patients with AIS.
Edaravone dexborneol injection is a neuroprotective preparation composed of edaravone and dexborneol. It can be used for the treatment of AIS, which has a significant inhibitory effect on lipid peroxidation of nerve cells and plays an important role in brain protection[7,8]. Edaravone which is a free radical scavenger, can relieve brain edema, inhibit delayed neuronal death and disease progression by scavenging Hydroxyl (OH•), Nitric oxide (NO•) radical and peroxynitrite (ONOO-) anion[9-11]. Dexborneol, on the other hand, is capable of preventing brain injury by downregulating the secretion of inflammation-related proteins and thus exert therapeutic effects[12]. It has been reported that edaravone dexborneol injection can cooperate with edaravone and dexborneol to play a better therapeutic role in AIS, with good tolerance[13].
This study attempts to analyze the effect of edaravone dexborneol injection in the treatment of AIS and its influence on nerve function, so as to provide a better choice for the treatment of AIS patients.
Materials and Methods
General information:
From December 2018 to December 2022, 123 AIS patients who received treatment in Panzhihua Central Hospital were selected as the research participants. Among them, 53 cases in the control group received conventional (alteplase) treatment, and 67 cases in the research group received edaravone dexborneol injection. All participants signed informed consent after the study was approved by the hospital’s Ethics Committee. The two groups were clinically comparable with no statistical inter-group difference in general data (p>0.05).
Inclusion criteria:
Patients who were diagnosed with AIS[14] within 4.5 h of onset; patients who did not use the intravascular interventional therapy and patients who provided intact clinical data and cooperated throughout the study, were included.
Exclusion criteria:
Patients with the presence of other malignant tumors, severe hypertension, serious heart disease and mental illness; patients having allergy to the drugs used in this study; presence of intracranial hemorrhage confirmed by Computed Tomography (CT) within 24 h after intravenous thrombolysis and pregnant or lactating women were excluded from the study.
Treatment methods:
The control group was given routine treatment of 10 % 0.9 mg/kg-1 alteplase as an intravenous bolus for 1 min, and the remaining 90 % was administered with continuous infusion pump over 1 h period. Besides, symptomatic treatments such as inhibiting platelet aggregation, dilating blood vessels and improving blood circulation were provided, and routine drugs such as statins and butylphthalide were given.
Similarly, the research group received 15 ml of edaravone dexborneol injection which was mixed with 100 ml of 0.9 % sodium chloride injection for intravenous dripping twice a day and the treatment cycle of both the groups continued for 14 d.
Observation indicators:
Effective rate: The effective rate of treatment was evaluated on the 1st and 14th d of the treatment using the National Institutes of Health Stroke Scale (NIHSS)[15]; ≥90 % was considered as basic recovery, reduction from 46 % to ≤90 % was termed as significant improvement, reduction percentage ranging from 19 % to ≤46 % was considered as improvement and <19 % reduction in the NIHSS score was considered as ineffectiveness.
Side effects of medication: We observed and recorded the cases of side effects of medication such as nausea, vomiting, chest tightness, fever, dizziness and headache after treatment, and calculated the total incidence.
Nerve function: Patients’ nerve function was assessed before and after treatment using the NIHSS. The scale has a total score of 42, with the score in direct proportion to the neurological dysfunction. The disability degree was assessed by the modified Rankin Scale (mRS) (score range is between 0-6)[16]; the score is directly proportional to the degree of disability.
Activities of Daily Living (ADL) ability: The patients' ADL ability was evaluated with the help of Barthel Index (BI)[17]; on a 100-point scale, higher scores suggest better ADL ability.
Serum indicators: 5 ml of venous blood was collected before and 14 d after treatment and then the serum was obtained by centrifugation. NO was detected by colorimetry and serum indicators such as Endothelin-1 (ET-1) and Matrix Metalloproteinase-2 (MMP-2) were quantified by Enzyme-Linked Immunosorbent Assay (ELISA).
Statistical analysis:
This study used Statistical Package of Social Sciences (SPSS) version 22.0 software for statistical analysis. Expressed by (x±s), continuous variables were comparatively analyzed by the independent samples t-test. Categorical variables, expressed by n (%), were comparatively analyzed using the Chi square (χ2) test. Statistical significance is reported at the p<0.05 level unless and otherwise noted.
Results and Discussion
The general data such as mean age, Body Mass Index (BMI), gender, lesion area, hypertension and diabetes between the research and control groups were compared. We found no significant difference (p>0.05) (Table 1).
| Factors | n | Control group (n=56) | Research group (n=67) | χ2/t | p |
|---|---|---|---|---|---|
| Sex | 0.100 | 0.751 | |||
| Male | 75 | 35 (62.50) | 40 (59.70) | ||
| Female | 48 | 21 (37.50) | 27 (40.30) | ||
| Mean age (y) | 123 | 60.73±7.9 | 60.33±9.57 | ||
| BMI (kg/m2) | 123 | 22.04±2.75 | 22.72±2.86 | ||
| Lesion area (cm2) | 123 | 14.82±1.84 | 15.38±2.46 | ||
| Hypertension | 0.412 | 0.521 | |||
| Yes | 61 | 26 (46.43) | 35 (52.24) | ||
| No | 62 | 30 (53.57) | 32 (47.76) | ||
| Diabetes | 0.684 | 0.408 | |||
| Yes | 33 | 13 (23.21) | 20 (29.85) | ||
| No | 90 | 43 (76.79) | 47 (70.15) |
Table 1: General Information of the Patients, N (%) (X±S)
Efficacy of patients of the two groups was comparatively evaluated. The total effective rate was 78.57 % in the control group and 92.54 % in the research group, with a statistical inter-group difference (p<0.05) (Table 2).
| Factors | Control group (n=56) | Research group (n=67) | χ2 | p |
|---|---|---|---|---|
| Basic recovery | 18 (32.14) | 26 (38.81) | ||
| Marked improvement | 11 (19.64) | 21 (31.34) | ||
| Improvement | 15 (26.79) | 15 (22.39) | ||
| Ineffectiveness | 12 (21.43) | 5 (7.46) | ||
| Effective rate | 44 (78.57) | 62 (92.54) | 4.995 | 0.025 |
Table 2: Efficacy of Two Groups of Patients, N (%)
Side effects of medication in two groups were evaluated. Both the groups showed no notable difference in side effects of medication such as nausea and vomiting, chest tightness, fever, dizziness and headache (p>0.05) (Table 3).
| Factors | Control group (n=56) | Research group (n=67) | χ2 | P |
|---|---|---|---|---|
| Nausea and vomiting | 0 (0.00) | 3 (4.48) | ||
| Chest tightness | 2 (3.57) | 0 (0.00) | ||
| Fever | 2 (3.57) | 2 (2.99) | ||
| Dizziness and headache | 1 (1.79) | 2 (2.99) | ||
| Total | 5 (8.93) | 7 (10.45) | 0.080 | 0.777 |
Table 3: Side Effects of Medication in Two Groups, N (%)
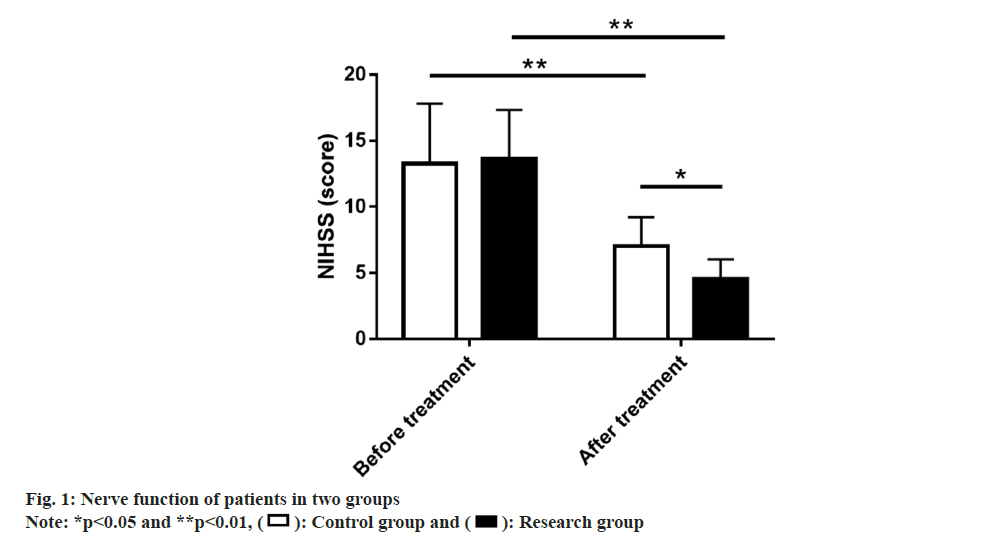
Similarly nerve function in both groups was also compared. The NIHSS assessment of nerve function revealed no marked inter-group difference in NIHSS scores before treatment (p>0.05); the score decreased evidently after treatment (p<0.01), with even lower NIHSS score in the research group (p<0.05) (fig. 1).
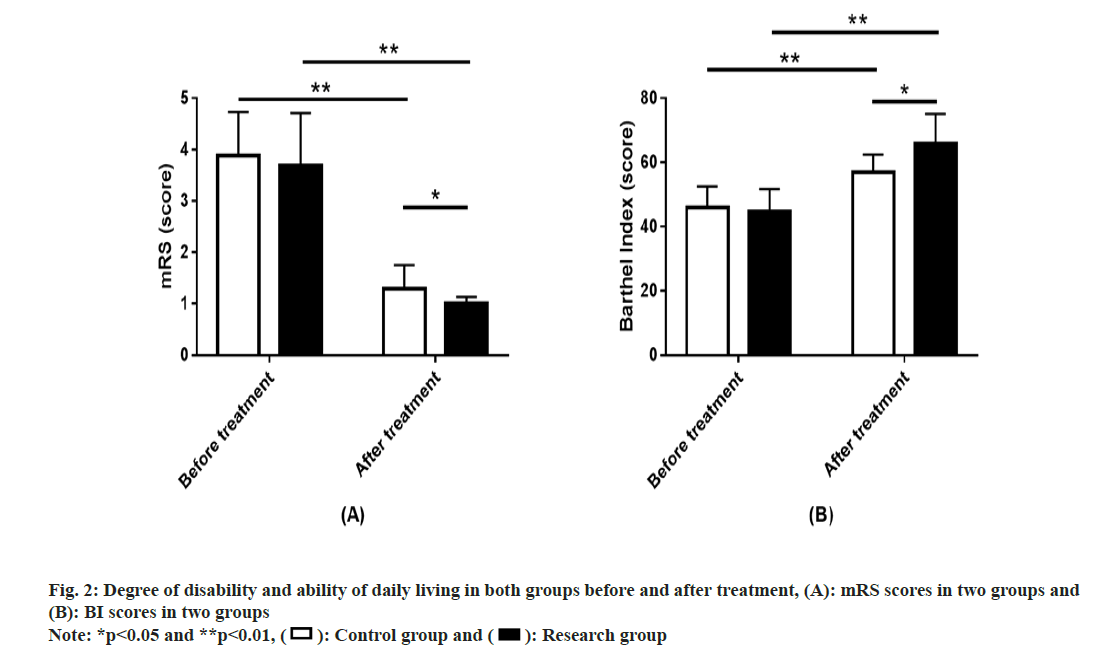
Disability degree and ADL ability in both the groups was evaluated. We employed the mRS and BI to assess patients’ disability degree and ADL ability, respectively. The two groups showed similar scores of the two scales before treatment (p>0.05). After treatment, mRS score showed a significant downward trend in both groups, while the BI score showed a significant increase (p<0.01), with lower mRS score and higher BI score in the research compared with the control group (p<0.05) (fig. 2).
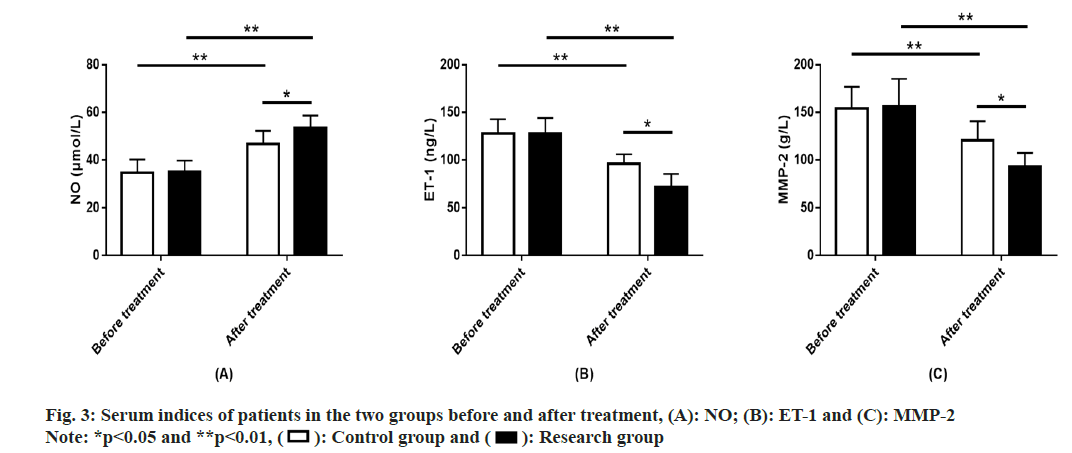
Serum indices of two groups of patients were studied. Measurements of NO, ET-1 and MMP- 2 showed that these indices of the two groups were comparable before treatment (p>0.05). After treatment, NO in the two groups increased significantly, while ET-1 and MMP-2 decreased significantly (p<0.01). NO in the research group was significantly higher compared with the control group after treatment, while ET-1 and MMP-2 were significantly lower (p<0.05) (fig. 3).
The insufficiency of cerebral blood supply in AIS patients is related to the blockage of cerebral blood supply caused by embolisms formed by various factors in the body's blood system that enter the cerebral vessels with blood circulation[18]. AIS is the most common serious manifestation of cerebrovascular disease and patients may represent the clinical symptoms such as dizziness, hemiplegia, aphasia and cerebral edema, accompanied by varying degrees of neurological impairment[19]. There is an urgent need for an effective treatment that can effectively relieve the symptoms of AIS and inhibit the disease, which is of great significance for improving the nerve function of patients.
Primarily, this study determined an evidently higher total effective rate of treatment in the research group (92.54 %) compared with the control group (78.57 %), suggesting that edaravone dexborneol injection has higher curative effect in AIS than the conventional alteplase therapy. In the study of Zhang et al.[20], edaravone dexborneol injection used in AIS model rats was confirmed to be effective in treating cerebral ischemic injury by activating Mitogen-Activated Protein Kinase Phosphatase 1 (MKP-1) related molecular pathways, alleviating neurodeficit symptoms in rat hippocampal CA1 region, inhibiting both apoptosis and neuronal damage. Side effects of medication such as nausea, vomiting, chest tightness, fever, dizziness and headache were not significantly different between the two groups, indicating that edaravone dexborneol injection would not increase the side effects of medication compared with conventional treatment with certain tolerance and safety. According to the evaluation of nerve function by NIHSS, the NIHSS score of the research group reduced markedly after treatment and lower compared with the control group, demonstrating the ability of edaravone dexborneol injection to validly inhibit neurological impairment compared with conventional treatment, which is more beneficial in protecting brain function. In addition, the degree of disability and the ADL ability of patients were evaluated by the mRS and BI, respectively. It was found that the mRS score of the research group after treatment was markedly lowered than the control group, while the BI score was markedly elevated and higher compared with the control group. This shows that edaravone dexborneol injection has a prominent positive effect on reducing the degree of disability and improving the ADL ability compared with conventional treatment. In the study of Li et al.[21], the nerve function and quality of life of AIS patients were also improved by edaravone dexborneol injection, consistent with our research results. A randomized, double-blind trial pointed out that edaravone dexborneol injection more effectively improved the nerve function of AIS patients on 90th d than edaravone, similar to our findings[22].
On the other hand, when the blood vessels are stimulated during AIS progression, the inflammatory microenvironment will be unbalanced, i.e., there will be excessive release of abnormal inflammatory factors and abnormal increase of oxygen free radicals, which will cause the adsorption of white blood cells by cerebral microvascular endothelial cells, resulting in the blockage of intracranial capillaries and related brain tissue damage[23,24]. NO which is a vasodilator modulator, is beneficial in protecting blood vessels from the negative effects of various factors[25]. ET-1 is an active polypeptide that favors vasoconstriction, its levels rise abnormally and are often associated with endothelial damage[26]. MMP-2 is closely linked to AIS progression, can enhance inflammatory microenvironment imbalances and promote arterial plaque formation, thereby accelerating the deterioration of AIS[27,28]. Serum indices such as NO, ET-1 and MMP- 2 were measured in this study. NO was found to be evidently higher in the research group vs. control group after treatment, while ET-1 and MMP-2 were significantly lower, which indicates that edaravone dexborneol injection can promote vasodilation, inhibit endothelial injury and prevent AIS progression by adjusting the aforementioned indicators.
To sum up, edaravone dexborneol injection has high curative effect in the treatment of AIS patients with a safety profile equivalent to that of conventional treatment. It can significantly alleviate neurological deficits and disability in patients, while exerting beneficial effects on the improvement of ADL ability and serum indices such as NO, ET-1 and MMP-2, which has clinical promotion value.
Funding:
This study was supported by the Sichuan Construction Project, China.
Conflict of interests:
The authors declared no conflict of interests.
References
- Hu X, de Silva TM, Chen J, Faraci FM. Cerebral vascular disease and neurovascular injury in ischemic stroke. Circ Res 2017;120(3):449-71.
[Crossref] [Google Scholar] [PubMed]
- Poredos P, Gregoric ID, Jezovnik MK. Inflammation of carotid plaques and risk of cerebrovascular events. Ann Transl Med 2020;8(19):1281.
[Crossref] [Google Scholar] [PubMed]
- Saini V, Guada L, Yavagal DR. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology 2021;97:6-16.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Li Y, Zang J, Zhang T, Li Y, Tan Z, et al. CircOGDH Is a penumbra biomarker and therapeutic target in acute ischemic stroke. Circ Res 2022;130(6):907-24.
[Crossref] [Google Scholar] [PubMed]
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke 2019;50(12):e344-418.
[Crossref] [Google Scholar] [PubMed]
- Tang L, Tang X, Yang Q. The application of tirofiban in the endovascular treatment of acute ischemic stroke: A meta-analysis. Cerebrovasc Dis 2021;50(2):121-31.
[Crossref] [Google Scholar] [PubMed]
- Wu HY, Tang Y, Gao LY, Sun WX, Hua Y, Yang SB, et al. The synergetic effect of edaravone and borneol in the rat model of ischemic stroke. Eur J Pharmacol 2014;740:522-31.
[Crossref] [Google Scholar] [PubMed]
- Chen Q, Cai Y, Zhu X, Wang J, Gao F, Yang M, et al. Edaravone dexborneol treatment attenuates neuronal apoptosis and improves neurological function by suppressing 4-HNE-associated oxidative stress after subarachnoid hemorrhage. Front Pharmacol 2022;13:1-12.
[Crossref] [Google Scholar] [PubMed]
- Kikuchi K, Tancharoen S, Takeshige N, Yoshitomi M, Morioka M, Murai Y, et al. The efficacy of edaravone (radicut), a free radical scavenger, for cardiovascular disease. Int J Mol Sci 2013;14(7):13909-30.
[Crossref] [Google Scholar] [PubMed]
- Tokumaru O, Shuto Y, Ogata K, Kamibayashi M, Bacal K, Takei H, et al. Dose-dependency of multiple free radical-scavenging activity of edaravone. J Surg Res 2018;228:147-53.
[Crossref] [Google Scholar] [PubMed]
- Jiao L, Zhang J, Li Z, Liu H, Chen Y, Xu S. Edaravone alleviates delayed neuronal death and long-dated cognitive dysfunction of hippocampus after transient focal ischemia in Wistar rat brains. Neuroscience 2011;182:177-83.
[Crossref] [Google Scholar] [PubMed]
- Wang B, Cao H, Shen T, Li M, Li YL, Cui CL, et al. Mechanism of musk and borneol on inflammatory of cerebral ischemia and reperfusion injury at different time points of acute phase in rats. Zhong Yao Cai 2015;38(10):2139-43.
[Google Scholar] [PubMed]
- Xu J, Wang Y, Wang A, Gao Z, Gao X, Chen H, et al. Safety and efficacy of edaravone dexborneol vs. edaravone for patients with acute ischaemic stroke: A phase II, multicentre, randomised, double-blind, multiple-dose, active-controlled clinical trial. Stroke Vasc Neurol 2019;4(3):109-14.
[Crossref] [Google Scholar] [PubMed]
- Mendelson SJ, Prabhakaran S. Diagnosis and management of transient ischemic attack and acute ischemic stroke: A review. JAMA 2021;325(11):1088-98.
[Crossref] [Google Scholar] [PubMed]
- Yoshimura S, Sakai N, Yamagami H, Uchida K, Beppu M, Toyoda K, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med 2022;386(14):1303-13.
[Crossref] [Google Scholar] [PubMed]
- Ebinger M, Siegerink B, Kunz A, Wendt M, Weber JE, Schwabauer E, et al. Association between dispatch of mobile stroke units and functional outcomes among patients with acute ischemic stroke in Berlin. JAMA 2021;325(5):454-66.
[Crossref] [Google Scholar] [PubMed]
- Rahayu UB, Wibowo S, Setyopranoto I, Hibatullah RM. Effectiveness of physiotherapy interventions in brain plasticity, balance and functional ability in stroke survivors: A randomized controlled trial. NeuroRehabilitation 2020;47(4):463-70.
[Crossref] [Google Scholar] [PubMed]
- Berkman SA, Song SS. Ischemic stroke in the young. Clin Appl Thromb Hemost 2021;27:1-14.
[Crossref] [Google Scholar] [PubMed]
- Feske SK. Ischemic stroke. Am J Med 2021;134(12):1457-64.
[Crossref] [Google Scholar] [PubMed]
- Zhang W, Yang H, Gao M, Zhang H, Shi L, Yu X, et al. Edaravone dexborneol alleviates cerebral ischemic injury via MKP-1-mediated inhibition of MAPKs and activation of Nrf2. Biomed Res Int 2022:1-12.
[Crossref] [Google Scholar] [PubMed]
- Li K, Zhang Q, Lu X, Yao S. Effects of butylphthalide sodium chloride injection combined with edaravone dexborneol on neurological function and serum inflammatory factor levels in sufferers having acute ischemic stroke. J Healthc Eng 2022:1-6.
[Crossref] [Google Scholar] [PubMed]
- Xu J, Wang A, Meng X, Yalkun G, Xu A, Gao Z, et al. Edaravone dexborneol vs. edaravone alone for the treatment of acute ischemic stroke: A phase III, randomized, double-blind, comparative trial. Stroke 2021;52(3):772-80.
[Crossref] [Google Scholar] [PubMed]
- Li C, Zhao Z, Luo Y, Ning T, Liu P, Chen Q, et al. Macrophage-disguised manganese dioxide nanoparticles for neuroprotection by reducing oxidative stress and modulating inflammatory microenvironment in acute ischemic stroke. Adv Sci 2021;8(20):1-14.
[Crossref] [Google Scholar] [PubMed]
- Huang L, Chen Y, Liu R, Li B, Fei X, Li X, et al. P-Glycoprotein aggravates blood brain barrier dysfunction in experimental ischemic stroke by inhibiting endothelial autophagy. Aging Dis 2022;13(5):1546-61.
[Crossref] [Google Scholar] [PubMed]
- Xuan C, Chang FJ, Liu XC, Bai XY, Liao XL, He GW, et al. Endothelial nitric oxide synthase enhancer for protection of endothelial function from asymmetric dimethylarginine-induced injury in human internal thoracic artery. J Thorac Cardiovasc Surg 2012;144(3):697-703.
[Crossref] [Google Scholar] [PubMed]
- Arfian N, Emoto N, Vignon-Zellweger N, Nakayama K, Yagi K, Hirata K. ET-1 deletion from endothelial cells protects the kidney during the extension phase of ischemia/reperfusion injury. Biochem Biophys Res Commun 2012;425(2):443-9.
[Crossref] [Google Scholar] [PubMed]
- Kurzepa J, Kurzepa J, Golab P, Czerska S, Bielewicz J. The significance of Matrix Metalloproteinase (MMP)-2 and MMP-9 in the ischemic stroke. Int J Neurosci 2014;124(10):707-16.
[Crossref] [Google Scholar] [PubMed]
- Nie SW, Wang XF, Tang ZC. Correlations between MMP-2/MMP-9 promoter polymorphisms and ischemic stroke. Int J Clin Exp Med 2014;7(2):400-4.
[Google Scholar] [PubMed]
 ): Control group and (
): Control group and ( ): Research group
): Research group