- *Corresponding Author:
- S. K. Dunga
Department of Pharmacy, Annamalai University, Chidambaram, Tamil Nadu 608002, India
E-mail: dungakiran@gmail.com
| Date of Received | 21 December 2023 |
| Date of Revision | 23 July 2024 |
| Date of Acceptance | 22 October 2024 |
| Indian J Pharm Sci 2024;86(5):1875-1882 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Lithiasis is the formation of stony concretions in the body, affecting people worldwide and having a high recurrence rate. Herbal medicines are effective in treating kidney stones and preventing the recurrence of the disease. The study aimed to evaluate the antilithiatic activity of the hydroalcoholic extract of Syzygium jambos fruit in rats. Lithiasis was induced in adult male Wistar albino rats by oral administration of 0.75 % ethylene glycolated water for 28 d. The hydroalcoholic extract of Syzygium jambos was administered orally every day at doses of 200 and 400 mg/kg, while Cystone (750 mg/kg, p.o.) served as the reference standard for 14 d. The antilithiatic activity was then assessed after 28 days of treatment. The effect of hydroalcoholic extract of Syzygium jambos was examined through biochemical analysis of serum (total protein, creatinine, uric acid, BUN, bilirubin, calcium, oxalate, lactate dehydrogenase), urine (creatinine, uric acid, urea, calcium, oxalate, magnesium, citrate), and pancreatic and renal tissues (superoxide dismutase, catalase, glutathione, protein carbonyl content, malondialdehyde, nitrite, tumor necrosis factor-alpha, and interleukin-6) at the end of the 28th d. The hydroalcoholic extract of Syzygium jambos exhibited potential antioxidant, crystal inhibitory and anti-inflammatory activities, which may contribute significantly to its effectiveness against pancreatic and renal calculi in ethylene glycol-induced rats. These effects are likely attributed to the polyphenols and flavonoids present in the Syzygium jambos fruit.
Keywords
Syzygium jambos, cystone, ethylene glycol, crystal inhibition, kidney stones
Nephrolithiasis is a condition in which crystal deposits typically form in the kidneys. It is a growing urinary disease that affects approximately 12 % of the global population and is associated with an increased risk of end-stage renal disease[1]. There are several causes of kidney stones. Lithiasis is a condition in which stones are formed due to the concentration of mineral salts. The kidneys, urinary tract, pancreas, and gallbladder are the organs most affected by this condition[2]. These stones are classified based on their location and cause: uroliths, pancreatoliths, and renoliths, respectively. The most common type of kidney stone is calcium oxalate, which forms in Randall's plaques on the surface of the renal papilla[3]. The process of stone formation is complex and results from several physicochemical phenomena, including supersaturation, nucleation, growth, aggregation, and the retention of urinary stone components in renal tubular cells[4].
Currently, kidney stones are managed through surgical procedures such as Extracorporeal Shock Wave Lithotripsy (ESWL), Ureteroscopy (URS), and Percutaneous Nephrolithotomy (PNL), or through the use of synthetic drugs. However, these methods have side effects and can be very expensive[5]. Medicinal herbs have traditionally been used to treat kidney stones even before the advent of modern treatments, as they have been proven to be effective, natural remedies. Therefore, treatment with medicinal plants is recommended because they contain phytochemicals that may have litholytic properties[6]. Several risk factors contribute to the development of stone disease, including supersaturation of oxalate crystals, decreased excretion of citrate, oxidative stress, and genetic predisposition to certain metabolic disorders[7]. Antioxidants with low molecular weight such as ascorbate, Alpha (α)-tocopherol, cysteine, thioredoxin, and vitamins may have anti-lithiasis effects against stone disease caused by oxidative stress[8]. Therefore, research has focused on phytochemical-based antioxidants for the treatment of undissolved stone disease. Syzygium jambos. L., (S. jambos) also known as rose apple, has a long history in traditional medicine and is widely used in various cultures[9]. Different parts of S. jambos contain phenolic and flavonoid content that help reduce oxidative stress, inflammation, and apoptosis in the pancreas. Based on the mentioned reports[10], the present study was conducted to investigate the effect of S. jambos on the antilithiatic activity in stone formation caused by Ethylene Glycol (EG) in albino Wistar rats.
Materials and Methods
Fruit materials:
Ripe fruits of S. jambos were collected from a growing area in Guntur, Andhra Pradesh, India, and authenticated by Prof. Satya Narayana Raju, Taxonomist, Department of Botany and Microbiology, Acharya Nagarjuna University. Dry the fruits in the shade and grind them into fine powder. The powder was suspended in 2 % acacia gum and used for experimental studies.
Animals:
Animals were obtained from Mahavir Research Institute, Hyderabad. Male Wistar rats 180-200 g were used in this study. The animals were kept under standard environmental conditions (23±1°) with a relative humidity of 50±10 %, on a 12:12 light-dark cycle. They were provided with water and a standard laboratory diet (70 % carbohydrates, 25 % protein, 5 % lipids), Hindustan Lever, Bangalore. After pretrial randomization, animals were housed and handled according to Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines. The animal experiments were approved by the CPCSEA Institutional Animal Ethics Committee (IAEC) under approval number 011/IAEC/NCPA/PhD 21-22.
Experimental procedure:
Group 1 served as the normal control (vehicle), while Groups 2 to 5 were treated with 0.75 % EG in drinking water until 28th d to induce stone formation. Group 2 was the disease control (0.75 % EG), group 3 received 0.75 % EG+standard (750 mg/kg of cyston), and groups 4 and 5 received 0.75 % EG+S. jambos at doses of 200 mg/kg and 400 mg/kg, respectively. EG (0.75 %) in drinking water was administered to groups 2 to 5 until 28th d to induce stone formation[11]. Chronic administration of 0.75 % EG to male Wistar rats resulted in hyperoxaluria and hypercalciuria. The formation of urinary stones occurs due to excessive saturation of urine with certain solutes, such as calcium and oxalate. Elevated levels of oxalate and calcium in the urine and kidneys can lead to the formation of kidney stones.
Biochemical assays:
After 28 d of HAFSJ treatment, the mice were anesthetized. Blood was collected from the retroorbital plexus, and serum was separated. Urine was collected using metabolic cages. Both serum and urinary parameters were assessed using Coral kits. The pancreas and kidneys were dissected and washed with ice-cold saline. The pancreas and kidneys were homogenized in 2 mL of ice-cold phosphate buffer (20 mm, pH 7.4) using a glass homogenizer with Teflon pestle, and then centrifuged. The supernatant was collected for analysis. The total and soluble protein content in the lens homogenate was estimated using the Lowry method[12].
Superoxide Dismutase (SOD) activity was measured using the Nitroblue tetrazolium (NBT) reduction method. To prepare the reaction, add 0.1 ml of kidney homogenate supernatant to 1.2 ml of 0.052 M sodium pyrophosphate buffer (pH 8.3), followed by 0.1 ml of 186 μm phenazonium methosulfate, 0.3 ml of 300 μm NBT, and 0.2 ml of 780 μm Nicotinamide Adenine Dinucleotide+Hydrogen (NADH). The reaction mixture was incubated at 30° for 90 s, and the reaction was stopped by adding 1 ml of glacial acetic acid. The reaction mixture was stirred vigorously, shaken with 4 ml of n-butanol, and centrifuged at 4000 rpm for 10 min. The absorbance of the organic layer was measured at a wavelength of 560 nm[13]. Catalase (CAT) activity was assessed based on the ability of the test samples to degrade Hydrogen peroxide (H2O2). The procedure involved mixing 20 μl of kidney homogenate with an incubation mixture containing 1 M Tris-Hydrogen Chloride (HCl), 5 mm Ethylenediaminetetraacetic Acid (EDTA) (pH 8.0), and 900 μl of 10 mm H2O2 as the enzyme substrate, followed by 30 μl of distilled water. The ability to decompose H2O2was measured at 230 nm using a spectrophotometer (Agilent Technologies, Cary 100)[14]. Reduced glutathione (GSH) activity was measured using the following method: Add 0.2 ml of kidney homogenate, 0.2 ml of 0.8 mm EDTA, 0.1 ml of sodium azide, 0.1 ml of 4 mM GSH, 0.1 ml of H2O2 solution, and 0.4 ml of 0.4 M EDTA in phosphate buffer (pH 7). Incubate for 10 min at 37°, then allow the tube to cool to room temperature. Add 0.5 ml of 10 % Trichloroacetic Acid (TCA), centrifuge for 10 min at 2000 rpm, and add 0.1 ml of 0.04 % 5,5-dithio-bis-(2-nitrobenzoic acid (DTNB) solution to the supernatant. The optical density of the resulting mixture was measured at 420 nm[15]. Malondialdehyde (MDA) levels in kidney homogenates were measured using the method described by Ohkawa et al.[15]. To prepare the mixture, 0.1 ml of kidney homogenate was mixed with 2 ml of distilled water, followed by 0.1 ml of Thiobarbituric Acid (TBA) and 1 ml of TCA. The resulting mixture was heated in a water bath, and after cooling, butanol was added. The organic phase was separated by centrifugation, and the optical density was measured at a wavelength of 532 nm. At the end of the study period, urine analysis was performed 24 h after urine collection[16]. Commercial kits were used to estimate urine volume, as well as the levels of creatinine, uric acid, and urea. Creatinine Clearance (CCr) was calculated using the following formula:
Ccr (ml/min/kg)=[urinary chromium (mg/dl)×urine volume (ml)/serum chromium (mg/dl)×(1000/body weight (g)) ×(1/1440 (min)).
To measure the protein carbonyl content in the homogenate, 1 ml of 10 mM 2,4-Dinitrophenylhydrazine (DNPH) in 2 M HCl was added to the reaction mixture. The samples were incubated at room temperature for 30 min. Then, 1 mL of cold TCA (10 % w/v) was added to the mixture, and the samples were centrifuged at 3000×g for 10 min. The protein pellet was washed 3 times with 2 ml of ethanol/ethyl acetate (1:1, v/v) and dissolved in 1 ml of guanidine hydrochloride (6 M, pH 2.3). The absorbance of the samples was measured at a wavelength of 370 nm[17]. Tumor Necrosis Factor-α (TNF-α) and Interleukin-6 (IL-6) levels in liver homogenates were measured using commercial TNF-α and IL-6 Enzyme-Linked Immunoassay (ELISA) kits (eBioscience Inc., San Diego, California) and Roshe ELISA kits (United States of America (USA)), following the manufacturer's instructions.
Results and Discussion
Treatment of kidney stones depends on several important factors, including stone location, size,composition, and patient symptoms. While significant advances have been made in the surgical management of kidney stones over the past decade, drug treatments have not been as successful in preventing new stone formation and reducing urinary stone recurrence[18]. Currently, kidney stones are regarded as a complex syndrome influenced by multiple factors, and treatment plans should be individualized, taking into account the potential damage caused by stone-forming factors[19]. The cause of this disorder is multifactorial, involving genetics, diet, and reduced physical activity. Calcium-containing stones are the most common type of kidney stone (75 %-90 %), followed by magnesium ammonium phosphate (struvite) stones (10 %-15 %), uric acid stones (10 %-3 %), and cystine stones (1 %-0.5 %)[20]. Recent human studies suggest that a diet rich in vegetables and fruits may help prevent urinary stones. Regular consumption of a plant-based diet increases urine pH and output, and reduces the formation of stones, with compounds like phytate, citrate, potassium, and magnesium associated with decreased supersaturation of calcium oxalate and uric acid[21]. Additionally, inhibitors have been identified in S. jambos, which provides edible fruits in various forms, such as juice, jelly, and jam. This report highlights the presence of polyphenols, flavonoids, tannins, and sterols in different parts of the S. jambos plant[22].
Various methods are available to screen for potential antilithiatic drugs, but EG is considered superior because it primarily targets the kidneys. This substance is administered orally and metabolized into four organic acids: glyceraldehyde, glycolic acid, glyoxylic acid, and finally, oxalic acid[23]. EG activates glycolate oxidase, leading to the formation of free radicals and lipid peroxidation. It also increases levels of creatinine, urea, and uric acid, ultimately causing necrosis[24]. These metabolites become supersaturated and contribute to the initiation and progression of stone disease. This study aimed to investigate the role of HAFSJ in stone formation in the kidneys and pancreas. HAFSJ was induced using EG as a model, with the experiment conducted over 28 d. The in vitro antioxidant activity of S. jambos in EG-induced stone formation was evaluated, with its major phytochemical components potentially contributing to this activity.
Creatinine is a byproduct of creatine breakdown, which is an important component of muscle. It is excreted from the body through the kidneys. When the kidneys function abnormally, the creatinine level in the blood increases because less creatinine is excreted in the urine[25]. Uric acid is primarily responsible for the cancer-preventive potential of plasma. The kidneys also play a key role in removing urea and other waste substances from the blood. Approximately 90 % of excess nitrogen is excreted through the kidneys in the form of urea, which is primarily produced during protein digestion in the liver. In many cases, protein intake in humans and other organisms exceeds the amount required for anabolic processes[26], leading to the production of large amounts of urea. This increased urea production creates an osmotic load on the kidneys. Serum bilirubin is considered a reliable marker of liver function, as it reflects the liver's ability to process bilirubin into bile. Elevated bilirubin levels can lead to hyperbilirubinemia. Table 1 shows that in EG-induced rats, levels of creatinine, uric acid, urea, and bilirubin were elevated, while protein levels were reduced. However, treatment with HAFSJ significantly reverted these levels to normal. Many studies have shown that the administration of EG to experimental animals reduces urine output, induces hyperoxaluria, and subsequently leads to hypercalciuria, which increases the retention and excretion of oxalate[27]. EG also increases the activity of Lactate Dehydrogenase (LDH), an enzyme involved in oxalate synthesis in the liver and kidneys. LDH is considered a marker of cell damage, as it is released when cells or cell membranes are damaged, leading to cell death. Elevated LDH activity indicates increased oxalate synthesis and greater kidney epithelial cell damage[28]. An increase in serum LDH levels was observed in rats treated with high doses of oxalate in the EG group. However, this treatment significantly reduced serum calcium, oxalate, and LDH levels (Table 2), and inhibited both oxalate synthesis and epithelial cell damage.
| Groups | Total protein (mg/dl) | Creatinine (mg/dl) | Uric acid (mg/dl) | BUN (mg/dl) | Bilirubin (mg/dl) |
|---|---|---|---|---|---|
| Control | 7.23±0.44 | 0.72±0.03 | 3.27±0.42 | 20.56±0.77 | 0.70±0.08 |
| EG (0.75 %) | 3.28±0.31# | 1.76±0.08# | 8.00±0.29# | 26.99±0.67# | 1.21±0.09# |
| Cystone (750 mg/kg) | 6.33±0.27*** | 0.72±0.05*** | 4.12±0.27*** | 22.21±0.12*** | 0.77±0.04*** |
| HAFSJ (200 mg/kg) | 3.73±0.21* | 1.03±0.05** | 7.04±0.30** | 26.02±0.77** | 0.94±0.06*** |
| HAFSJ (400 mg/kg) | 5.77±0.16*** | 0.93±0.05*** | 5.81±0.29*** | 23.48±0.61*** | 0.85±0.02*** |
Note: All values are expressed as Mean±Standard Error of the Mean (SEM) statistical comparisons were made by using One-way Analysis of Variance (ANOVA) followed by Dunnett’s multiple comparison tests using GraphPad Prism version 8 and found significantly different at p>0.05, *p<0.05, **p<0.01, ***p<0.001 when compared to disease control and #p<0.001 when disease control is compared with the control group
Table 1: Effect of HAFSJ on total protein Levels in EG-induced lithiasis in rats (N=6)
| Groups | Calcium (mg/dl) | Oxalate (mg/dl) | LDH (U/l) |
|---|---|---|---|
| Control | 0.44±0.07 | 31.45±1.10 | 220.42±2.11 |
| EG (0.75 %) | 1.29±0.03# | 43.33±0.44# | 409.00±4.22# |
| Cystone (750 mg/kg) | 0.82±0.04*** | 34.19±1.31*** | 250.22±3.21*** |
| HAFSJ (200 mg/kg) | 1.10±0.05* | 39.44±1.17** | 383.09±5.24* |
| HAFSJ (400 mg/kg) | 0.98±0.06*** | 38.29±0.82*** | 290.66±3.90*** |
Note: All values are expressed as Mean±SEM. Statistical comparisons were made by using One way ANOVA followed by Dunnett’s multiple comparison tests using GraphPad Prism version 8 and found significantly different at nsp>0.05, *p<0.05, **p<0.01, ***p<0.001 when compared to disease Control and #p<0.001 when disease control is compared with control group
Table 2: Effect of HAFSJ on calcium levels in EG-induced lithiasis in rats (N=6)
Urine pH is a critical factor in the formation of kidney stones. A low urine pH reduces the solubility of calcium oxalate stones, promoting stone formation[29]. In the present study, both urine volume and urine pH significantly decreased in the disease group by 28th d. This suggests that the solubility of calcium oxalate is reduced, leading to urine supersaturation with oxalate and calcium ions, which initiates stone formation. In contrast, the urine pH of treated mice increased significantly, indicating that HAFSJ may enhance the solubility of calcium oxalate stones and reduce the supersaturation of ions in the urine. In patients with kidney stones, a reduction in glomerular filtration leads to decreased urine output, resulting in the accumulation of waste products, such as nitrogenous compounds like urea and creatinine, and a decrease in creatinine clearance[30]. In this study, urinary uric acid concentration increased in the EG model group. As shown in Table 3, the increase in creatinine, urea, and uric acid excretion was significantly suppressed in the cystone and HAFSJ treatment groups.
| Groups | Urine VOLUME | Urine pH | Urine creatinine (mg/dl) | Urine uric acid (mg/dl) | Urine urea (mg/dl) |
|---|---|---|---|---|---|
| Control | 15.00±0.16 | 7.23±0.22 | 0.64±0.02 | 4.16±0.11 | 26.33±0.90 |
| EG (0.75 %) | 8.88±0.34# | 3.49±0.50# | 1.31±0.06# | 7.43±0.20# | 36.30±0.72# |
| Cystone (750 mg/kg) | 13.44±0.11*** | 7.13±0.15*** | 0.76±0.03*** | 4.31±0.04*** | 26.12±0.80*** |
| HAFSJ (200 mg/kg) | 10.56±1.02** | 5.23±0.47** | 0.98±00.04** | 5.76±0.23** | 32.79±1.15* |
| HAFSJ (400 mg/kg) | 15.22±0.61*** | 7.13±0.71*** | 0.92±00.08*** | 4.99±0.10*** | 29.86±0.86*** |
Note: All values are expressed as Mean±SEM. Statistical comparisons were made by using one-way ANOVA followed by Dunnett’s multiple comparison tests using GraphPad Prism version 8 and found significantly different at nsp>0.05, *p<0.05, **p<0.01, ***p<0.001 when compared to disease control and #p<0.001 when disease control is compared with the control group
Table 3: Effect of HAFSJ on urine volume and Urine PH EG-induced lithiasis in rats
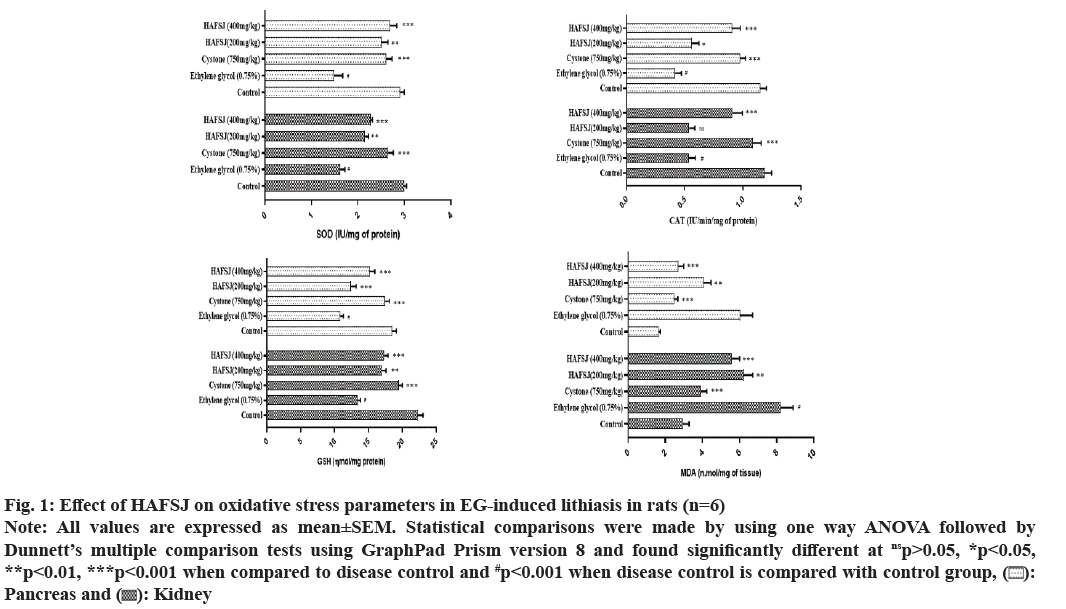
Calcium-containing stones are the most common type, accounting for 70 %-80 % of all kidney stones, with calcium phosphate and calcium oxalate being the predominant forms. The formation of calcium oxalate stones occurs due to the supersaturation of urine with calcium and oxalate[31]. Factors contributing to the formation of calcium oxalate stones include acidic urine, low urine output, hypercalciuria (increased calcium concentration in urine, which leads to the precipitation of calcium salts), hyperoxaluria (high oxalate concentration in urine), and calcium salt deposits due to elevated calcium levels in urine[32]. A low citrate concentration in urine leads to high pH and hyperuricuria (acidic urine dissolves uric acid, contributing to the formation of uric acid stones). Magnesium forms a complex with oxalate, reducing the supersaturation of calcium oxalate, thereby decreasing the rate of calcium oxalate crystal growth and nucleation. Similarly, citrate forms complexes with calcium ions, resulting in a soluble complex that lowers supersaturation levels[33]. Treatment with HAFSJ significantly inhibited crystal promoters (calcium and oxalate) and promoted crystal inhibitors (magnesium and citrate) in EG-induced lithiatic rats, as shown in Table 4. Tubular ischemia caused by hyperoxaluria may play a significant role in initiating a series of programmed events leading to cell death. Renal tubular apoptosis is associated with increased Reactive Oxygen Species (ROS) levels in mice[34]. Additionally, increased renal ROS levels, along with the impairment of key antioxidant enzymes such as SOD, CAT, and Glutathione Peroxidase (GPx), have been reported in obstructed kidneys in vivo. CAT is a widely distributed enzyme in living organisms that catalyzes the decomposition of hydrogen peroxide into water and oxygen. The GSH system includes GSH, GSH reductase, GSH peroxidase, and GSH S-transferase[35]. Lipid peroxidation has been positively correlated with cellular oxalate, oxalate binding, Gamma (γ)-glutamyl carboxylase, and calcium levels, and negatively correlated with GSH and vitamin E levels in rat tissues. It was observed that the levels of SOD, CAT, and GSH were significantly enhanced, while MDA levels were depleted in the pancreatic and renal tissues of rats treated with cystone and HAFSJ, compared to lithiasis control rats (fig. 1).
Fig. 1: Effect of HAFSJ on oxidative stress parameters in EG-induced lithiasis in rats (n=6)
Note: All values are expressed as mean±SEM. Statistical comparisons were made by using one way ANOVA followed by Dunnett’s multiple comparison tests using GraphPad Prism version 8 and found significantly different at nsp>0.05, *p<0.05, **p<0.01, ***p<0.001 when compared to disease control and #p<0.001 when disease control is compared with control group, ( ): Pancreas and (
): Pancreas and ( ): Kidney
): Kidney
| Groups | Calcium (mg/dl) | Oxalate (mg/dl) | Magnesium (mg/dl) | Citrate (mg/dl) |
|---|---|---|---|---|
| Control | 3.33±0.21 | 0.52±0.05 | 0.59±0.04 | 30.23±1.77 |
| EG (0.75 %) | 8.11±0.16# | 1.53±0.03# | 0.33±0.01# | 19.56±1.19# |
| Cystone (750 mg/kg) | 3.68±0.22*** | 0.82±0.19*** | 0.56±0.06*** | 27.23±1.41*** |
| HAFSJ (200 mg/kg) | 7.11±0.09** | 1.28±0.05** | 0.50±0.05** | 22.34±0.95** |
| HAFSJ (400 mg/kg) | 4.35±0.19*** | 0.92±0.08*** | 0.55±0.03*** | 26.94±1.17*** |
Note: All values are expressed as Mean±SEM. Statistical comparisons were made by using One-way ANOVA followed by Dunnett’s multiple comparison tests using GraphPad Prism version 8 and found significantly different at nsp>0.05, *p<0.05, **p<0.01, ***p<0.001 when compared to disease control and #p<0.001 when disease control is compared with the control group
Table 4: Effect of HAFSJ on Calcium Levels in EG-induced lithiasis in rats
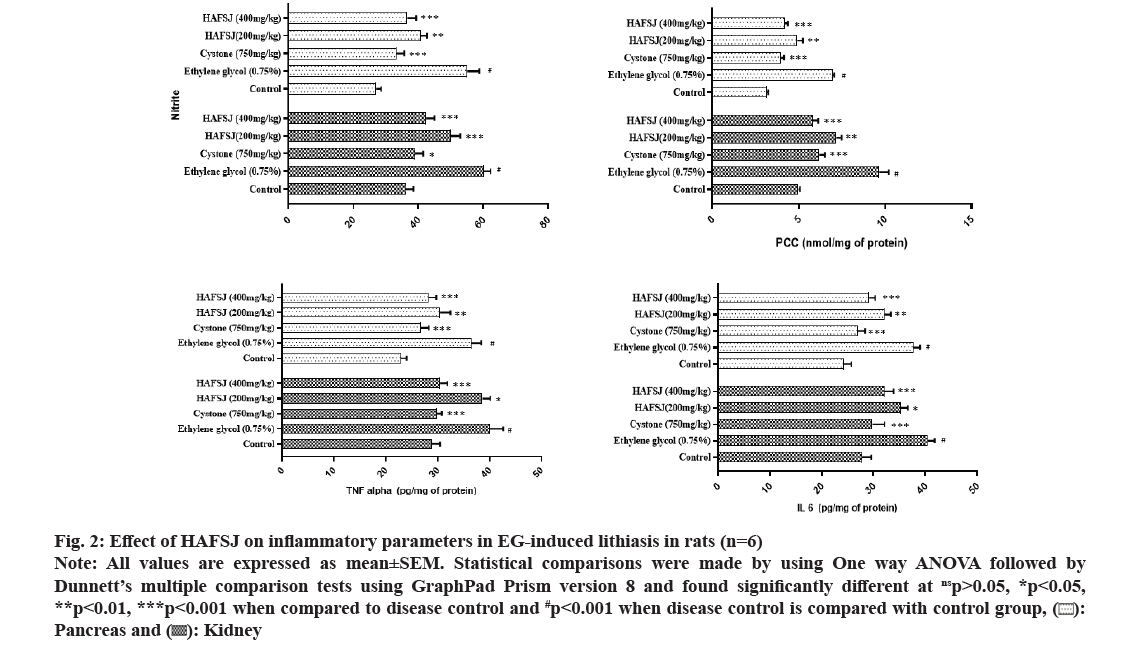
These crystals cause further damage to kidney tissue through growth or endocytosis, creating a vicious cycle of crystal damage, retention, and nucleation. As a result, the increase in plasma carbonyl content may be a coordinated action of stone forming components such as oxalate, which generates free radicals that damage cells and crystals. Nitrite plays a compensatory role in the dysregulation of the arginine-Nitric Oxide Synthase (NOS)-Nitric Oxide (NO) axis during vascular injury and inflammation. After angioplasty injury, blood vessels increase the levels of arginase-1, which is associated with heightened activity and decreased activity of the NOS enzyme. Inflammation is another key mechanism in the pathogenesis of kidney stones. It has been reported that a large accumulation of macrophages occurs in a rat kidney stone model, suggesting that inflammatory factors released by these macrophages may contribute to the formation of Calcium Oxalate (CaOx) crystals. This inflammation is typically localized around the crystal deposits. Intrarenal crystals provoke inflammation and kidney cell necrosis, a process that involves TNF receptor signaling and immune-mediated kidney disease. High levels of IL-6 have also been reported in the urine of patients with kidney stones. The present study identifies the protective role of HAFSJ in mitigating pancreatic and renal damage caused by crystal deposition, by regulating the levels of nitrite, carbonyls, and inflammatory markers TNF-α and IL-6 in ethylene glycol-induced lithiasis in rats (fig. 2).
Fig. 2: Effect of HAFSJ on inflammatory parameters in EG-induced lithiasis in rats (n=6)
Note: All values are expressed as mean±SEM. Statistical comparisons were made by using One way ANOVA followed by Dunnett’s multiple comparison tests using GraphPad Prism version 8 and found significantly different at nsp>0.05, *p<0.05, **p<0.01, ***p<0.001 when compared to disease control and #p<0.001 when disease control is compared with control group, ( ): Pancreas and (
): Pancreas and (  ): Kidney
): Kidney
S. jambos contains bioactive compounds, such as quercetin, gallic acid, and cinnamic acid, which have shown potential in inhibiting crystal formation. These compounds are believed to contribute to this effect through mechanisms such as antioxidant activity, modulation of enzymatic pathways, and anti-inflammatory effects. These actions may help reduce the factors that promote crystal aggregation. By preventing the formation and accumulation of crystals, these phytochemicals could play a role in managing or preventing conditions associated with crystal deposits, such as kidney stones. However, further research is needed to confirm their efficacy and determine optimal dosages for crystal inhibition.
Conflict of interests:
The authors declared no conflict of interests.
References
- Alelign T, Petros B. Kidney stone disease: An update on current concepts. Adv Urol 2018;2018(1):3068365.
[Crossref] [Google Scholar] [PubMed]
- Dawson CH, Tomson CR. Kidney stone disease: Pathophysiology, investigation and medical treatment. Clin Med 2012;12(5):467.
[Crossref] [Google Scholar] [PubMed]
- Wang Z, Zhang Y, Zhang J, Deng Q, Liang H. Recent advances on the mechanisms of kidney stone formation. Int J Mol Med 2021;48(2):1-0.
[Google Scholar] [PubMed]
- Ratkalkar VN, Kleinman JG. Mechanisms of stone formation. Clin Rev Bone Miner Metab 2011;9(3-4):187-97.
- Cao L, Wang YQ, Yu T, Sun Y, He J, Zhong Y, et al. The effectiveness and safety of extracorporeal shock wave lithotripsy for the management of kidney stones: A protocol of systematic review and meta-analysis. Medicine 2020;99(38):e21910.
[Crossref] [Google Scholar] [PubMed]
- Nirumand MC, Hajialyani M, Rahimi R, Farzaei MH, Zingue S, Nabavi SM, et al. Dietary plants for the prevention and management of kidney stones: Preclinical and clinical evidence and molecular mechanisms. Int J Mol Sci 2018;19(3):765.
[Crossref] [Google Scholar] [PubMed]
- Janciauskiene S. The beneficial effects of antioxidants in health and diseases. Chron Obstr Pulm Dis 2020;7(3):182.
[Crossref] [Google Scholar] [PubMed]
- Ochieng MA, Ben Bakrim W, Bitchagno GT, Mahmoud MF, Sobeh M. Syzygium jambos L. Alston: An insight into its phytochemistry, traditional uses, and pharmacological properties. Front Pharmacol 2022;13:786712.
[Crossref] [Google Scholar] [PubMed]
- Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019;8(4):96.
[Crossref] [Google Scholar] [PubMed]
- Macarini AF, Mariano LN, Zanovello M, da Silva RD, Corrêa R, de Souza P. Protective role of Rosmarinic acid in experimental urolithiasis: Understanding its impact on renal parameters. Pharmaceuticals 2024;17(6):702.
[Crossref] [Google Scholar] [PubMed]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193(1):265-75.
[Google Scholar] [PubMed]
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972;247(10):3170-5.
[Google Scholar] [PubMed]
- Aebi H. Catalase In: Bergmeyer. HU methods of enzymatic analysis. Academic Press. New York; 1974. p. 673-84
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 1968;25:192-205.
[Crossref] [Google Scholar] [PubMed]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95(2):351-8.
[Crossref] [Google Scholar] [PubMed]
- Kalousova M, Skrha J, Zima T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol Res 2002;51(6):597-604.
[Google Scholar] [PubMed]
- Akram M, Jahrreiss V, Skolarikos A, Geraghty R, Tzelves L, Emilliani E, et al. Urological guidelines for kidney stones: Overview and comprehensive update. J Clin Med 2024;13(4):1114.
[Crossref] [Google Scholar] [PubMed]
- Ferraro PM, Bargagli M, Trinchieri A, Gambaro G. Risk of kidney stones: Influence of dietary factors, dietary patterns, and vegetarian–vegan diets. Nutrients 2020;12(3):779.
[Crossref] [Google Scholar] [PubMed]
- Shukla AB, Mandavia DR, Barvaliya MJ, Baxi SN, Tripathi CR. Evaluation of anti-urolithiatic effect of aqueous extract of Bryophyllum pinnatum (Lam.) leaves using ethylene glycol-induced renal calculi. Avicenna J Phytomed 2014;4(3):151.
[Google Scholar] [PubMed]
- Chauhan NL, Juvekar VA, Sarkar A. Oxidation of ethylene glycol: Unity of chemical and electrochemical catalysis. Electrochem Sci Adv 2022;2(6):e2100092.
- Salazar JH. Overview of urea and creatinine. Lab Med 2014;45(1):e19-20.
- Weiner ID, Mitch WE, Sands JM. Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin J Am Soc Nephrol 2015;10(8):1444-58.
[Crossref] [Google Scholar] [PubMed]
- Holmes RP, Knight J, Assimos DG. Lowering urinary oxalate excretion to decrease calcium oxalate stone disease. Urolithiasis 2016;44(1):27-32.
[Crossref] [Google Scholar] [PubMed]
- Makasana A, Ranpariya V, Desai D, Mendpara J, Parekh V. Evaluation for the anti-urolithiatic activity of Launaea procumbens against ethylene glycol-induced renal calculi in rats. Toxicol Rep 2014;1:46-52.
[Crossref] [Google Scholar] [PubMed]
- Carvalho M. Urinary pH in calcium oxalate stone formers: Does it matter? Brazilian J Nephrol 2018;40(1):6-7.
- Wood K, Keys T, Mufarrij P, Assimos DG. Impact of stone removal on renal function: A review. Rev Urol 2011;13(2):73.
- O’Kell AL, Grant DC, Khan SR. Pathogenesis of calcium oxalate urinary stone disease: Species comparison of humans, dogs, and cats. Urolithiasis 2017;45(4):329-36.
[Crossref] [Google Scholar] [PubMed]
- Riley JM, Kim H, Averch TD, Kim HJ. Effect of magnesium on calcium and oxalate ion binding. J Endourol 2013;27(12):1487-92.
[Crossref] [Google Scholar] [PubMed]
- Han SJ, Lee HT. Mechanisms and therapeutic targets of ischemic acute kidney injury. Kidney Res Clin Pract 2019;38(4):427.
[Crossref] [Google Scholar] [PubMed]
- Nandi A, Yan LJ, Jana CK, Das N. Role of catalase in oxidative stress?and age?associated degenerative diseases. Oxid Med Cell Longev 2019;2019(1):9613090.
[Crossref] [Google Scholar] [PubMed]
- Selvam R. Calcium oxalate stone disease: Role of lipid peroxidation and antioxidants. Urol Res 2002;30:35-47.
[Crossref] [Google Scholar] [PubMed]
- Perazella MA, Herlitz LC. The crystalline nephropathies. Kidney Int Rep 2021;6(12):2942-57.
[Crossref] [Google Scholar] [PubMed]
- Carlström M. Nitric oxide signalling in kidney regulation and cardiometabolic health. Nat Rev Nephrol 2021;17(9):575-90.
[Crossref] [Google Scholar] [PubMed]
- Watanabe S, Alexander M, Misharin AV, Budinger GS. The role of macrophages in the resolution of inflammation. J Clin Invest 2019;129(7):2619-28.
[Crossref] [Google Scholar] [PubMed]
- Mulay SR, Eberhard JN, Desai J, Marschner JA, Kumar SV, Weidenbusch M, et al. Hyperoxaluria requires TNF receptors to initiate crystal adhesion and kidney stone disease. J Am Soc Nephrol 2017;28(3):761-8.
[Crossref] [Google Scholar] [PubMed]