- *Corresponding Author:
- Jianjing Zhang
Department of Gerontology, Wenzhou Seventh People’s Hospital, Wenzhou, Zhejiang 325000, China
E-mail: 15088551438@163.com
| This article was originally published in a special issue, “Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “266-273” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Bipolar disorder represents a significant global health challenge, marked by recurrent episodes of mania and depression. Traditional treatments often exhibit limitations, prompting the exploration of alternative therapeutic strategies. This study investigated the potential efficacy of Chaihu-Shugan-San, a traditional Chinese herbal formula, in managing bipolar disorder symptoms. 90 individuals diagnosed with bipolar disorder underwent 2 w intervention, randomly assigned to Chaihu-Shugan-San and placebo group. Symptom severity was rigorously assessed using established scales, such as Hamilton depression and anxiety rating scales, brief psychiatric rating scale and young mania rating scale. To further validate these findings, an animal model induced bipolar disorder-like symptoms with ouabain. The results revealed a significant improvement in symptom scores across all measures in the Chaihu-Shugan-San group compared with the placebo group, suggesting a promising role for Chaihu-Shugan-San in addressing the complexities of bipolar disorder. Chaihu-Shugan-San treatment exhibited a multifaceted impact, not only reducing manic-like behaviors and locomotor activity but also mitigating depression-like behaviors. This dual modulation of manic and depressive symptoms depicts Chaihu-Shugan-San as a potential holistic therapeutic approach for bipolar disorder. Exploration of Chaihu-Shugan-San introduces a novel avenue, emphasizing the importance of integrating traditional herbal medicine into psychiatric care. Chaihu-Shugan-San emerges as a promising, fostering choice for improved management strategy in bipolar disorder.
Keywords
Bipolar disorder, Chaihu-Shugan-San, depression, open field test, ouabain, forced swim test
Bipolar disorders, encompassing bipolar I and II are debilitating conditions marked by recurrent episodes of mania, hypomania and depression, which affect a substantial percentage of the global population[1,2]. The socio-economic impact of bipolar disorders extends beyond individual suffering, posing challenges to employment, social relationships and overall well-being[3,4]. While conventional treatments, including pharmacotherapy and psychotherapy play pivotal roles, their efficacy is often accompanied by limitations, prompting a growing interest in Complementary and Alternative Medicine (CAM) [5,6]. In this landscape, traditional herbal medicine emerges as a promising frontier for addressing the complexities of bipolar disorder. Herbal remedies, with their potential to offer therapeutic benefits with fewer side effects, have gained attention, such as Passiflora incarnate (passion flower), Matricaria recutita L. (chamomile), Scutellaria lateriflora (skullcap), Ginkgo biloba (Ginkgo) and Rhodiola rosea (golden root)[7].
Traditional Chinese medicine, deeply rooted in a holistic understanding of health and balance, provides a rich tapestry of herbal remedies which are strong and effective. Chaihu-Shugan-San (CHSGS) is a traditional Chinese herbal formula with the composition of seven different herbs[8]. CHSGS effectively treats functional dyspepsia, reduces anxiety and depression symptoms, enhances gastric emptying rates and influences the bacterial community structure in affected patients[9]. CHSGS combined with Selective Serotonin Reuptake Inhibitors (SSRIs) had a significant improvement on the depressive symptom in patients with Parkinson's Disease compared to the SSRIs alone[10]. Network pharmacology analysis indicated that complex mechanisms of energy metabolism, neurotransmitter metabolism, inflammation and hormone metabolic processes were closely associated with the anti-depressive effects of CHSGS[11].
In the light of historical applications and contemporary findings regarding CHSGS, this study aims to explore its potential efficacy in managing bipolar disorder symptoms. We hypothesize that CHSGS with its multifaceted impact on emotional well-being and previous indications of anti-depressive effects could offer a holistic therapeutic approach for individuals with bipolar disorder. The primary objective of this research is to rigorously assess the symptom severity in bipolar disorder patients undergoing 2 w intervention with CHSGS by comparing it to a placebo group. Additionally, we aim to further validate the clinical findings and unravel potential mechanistic insights into CHSGS's impact on manic and depressive behaviors, by employing an animal model induced with bipolar disorder-like symptoms.
Materials and Methods
General information:
90 study individuals with bipolar disorder were selected between January 2019 and January 2020 and were randomly assigned into CHSGS group and placebo group, each with 45 cases. Patients in the CHSGS group received CHSGS treatment (fig. 1) where the CHSGS formula included 6 g of Bupleuri Radix (Chaihu) and Citrus reticulata pericarp (chenpi), 4.5 g each of Paeonia Radix (shaoyao), Citrus aurantium (zhiqiao), xiangfu (Cyperus rhizome) and Ligusticum chuanxiong rhizome (chuanxiong), along with 1.5 g of Glycyrrhiza Radix (gancao). The administration of the medication involved warm ingestion of the prepared decoction with the above mentioned ingredients in 300 ml of water twice daily for a period of 2 w.
All the patients participated voluntarily and have provided informed consent. This experiment received approval from the ethics committee of our hospital and adhered to the principles of the Declaration of Helsinki.
Inclusion criteria: Patients meeting the International Classification of Diseases (ICD)-10 diagnostic criteria for bipolar disorder[12]; patients of age range of (18-60) y; patients with normal intelligence; patients with no severe physical illnesses; patients who do not consume alcohol, drugs or other psychoactive substances and patients with no language comprehension disorders were included in the study.
Exclusion criteria: Pregnant or lactating patients; patients having risk of suicide or with drug allergies; patients with mental disorders, mental disorders due to psychoactive substances and non-substance-induced mental disorders, schizophrenia, schizoaffective disorders, neuroses, and other types of mental illnesses were excluded from the study.
Treatment method:
Hamilton Depression Rating Scale (HDRS), which is a 21-item scale was used to assess the severity of depressive symptoms, with scores ranging from 0-50[13]. Similarly, Hamilton Anxiety Rating Scale (HARS) was used to measure anxiety symptoms severity through 14 items on a Likert scale (0-4), yielding a total score from 0 to 56[14]. 24-item Brief Psychiatric Rating Scale (BPRS) was also used in order to focus on the general psychopathology and psychotic symptoms where the score on a Likert scale of 1-7, with a total score range of 24-168[15] and Young Mania Rating Scale (YMRS) was used to assess the severity of manic symptoms using 11 items, with a total score range of 0-60[16].
Bipolar disorder symptoms induced by ouabain:
Male Sprague-Dawley rats approximately weighing about 200 g, (n=12) were individually housed, maintaining a 12:12 light-dark cycle. Bipolar disorder was induced using ouabain per the previous reported protocols[17,18]. Rats received anesthesia of 75 mg/kg of ketamine and 15 mg/kg of xylazine; cannulae were placed 3.5 mm deep in the dorsal skull (2.5 mm lateral and 1 mm caudal to bregma). 0.5 µl/5 min of 1 mM Intracerebroventricular (ICV) ouabain injection was administered manually for 3 d (1st, 3rd and 7th d).
Animal treatment:
Post-surgery, rats were cared for in individual polyacrylic cages with warm cloth. 35 mg/kgof gentamycin was given for 3 d while lignocaine gel relieved pain and neosporin powder prevented bacterial infection. Rats exhibited recovery within 2-3 h post-surgery, with their health which was diligently monitored for 7 d. Starting on the 8th d, rats received 4.8 g/kg CHSGS aqueous solution (CHSGS group, n=6) or an equivalent volume of normal saline (saline group, 1.0 ml/100 g, n=6) via gavage twice daily at 12 h interval for 4 w, concluding on the 28th d[19].
Measurement of behavioral parameters:
Behavioral parameters were evaluated through the Open Field Test (OFT), forced swim test and locomotor activity. During OFT conducted on the 1st, 7th, 14th, 21st and 28th d, rats explored an open field, with activities such as crossings, rearings and time spent in the center monitored. Locomotor activity, measured on the 1st, 9th, 18th and 27th d involved 3 min habituation period followed by 5 min recording using an actophotometer. Forced swim test was also conducted on the same days, included placing rats in water-filled tanks, recorded their movements for 5 min, and noting immobility time during a 6 min exposure, with the first 2 min as habituation.
Statistical analysis:
Statistical analysis was conducted using GraphPad Prism 8.0. Categorical data were presented as counts and analyzed using the Chi-square (χ²) test. For normally distributed continuous data (mean±Standard Deviation (SD)), statistical comparisons were performed using the t-test or two-way Analysis of Variance (ANOVA) followed by Sidak's multiple comparisons test after confirming normality with the Shapiro-Wilk test. Non-normally distributed data, presented as median and Interquartile Range (IQR) were analyzed using the Mann-Whitney test or Wilcoxon matched-pairs signed rank test. Results with a significance level of p<0.05 were considered statistically significant.
Results and Discussion
Demographic characteristics of CHSGS and placebo groups were studied. Baseline characteristics indicated no significant differences between the groups (Table 1). Median age in the CHSGS group was 44 y (IQR: 32.5-52.5) and in the placebo group was 41 y (IQR: 35-51.5), showing no statistically significant difference (p=0.732). Gender distribution demonstrated 27 females and 18 males in the CHSGS group, while 25 females and 20 males in the placebo group, with no significant disparity between the groups (p=0.831). Marital status, education level and bipolar type also exhibited comparable distributions with p-values of 0.218, 0.136 and 0.477, respectively.
| Demographic characteristics | CHSGS group (n=45) | Placebo group (n=45) | p |
|---|---|---|---|
| Age (y) | 44 (32.5-52.5) | 41 (35-51.5) | 0.732 |
| Gender | |||
| Female | 27 | 25 | |
| Male | 18 | 20 | 0.831 |
| Marital status | |||
| Single never married | 15 | 12 | |
| Married | 17 | 25 | |
| Disrupted marriage | 13 | 8 | 0.218 |
| Education level | |||
| Less than high school | 7 | 2 | |
| High school | 26 | 25 | |
| College degree and higher | 12 | 18 | 0.136 |
| Bipolar type | |||
| I | 34 | 32 | |
| II | 11 | 13 | 0.477 |
Table 1: Demographic characteristics of CHSGS and Placebo groups.
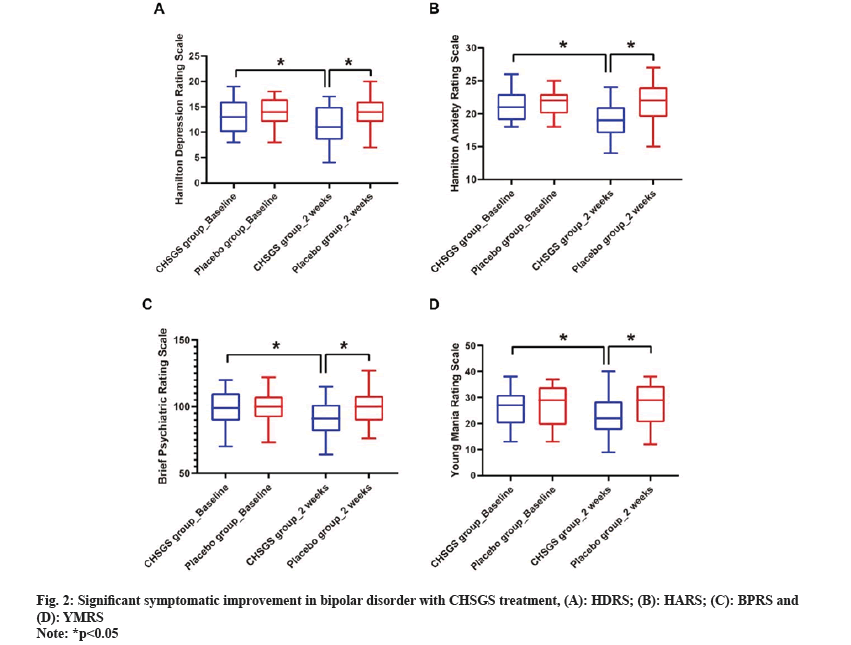
Further, CHSGS treatment demonstrated significant symptomatic improvement in bipolar disorder. CHSGS group exhibited significant improvements in various symptom scores after treatment. Prior to intervention, there were no significant differences in scores between the CHSGS and placebo groups (p>0.05). However, following 2 w CHSGS treatment, notable reductions in scores were observed across multiple assessment measures (p<0.05), including HDRS scale ranged from 13 (10-16) to 11 (8.5-15) (fig. 2A), HARS score was between 21 (19-23) to 19 (17-21) (fig. 2B) while BPRS score ranged from 99 (89.5-110) to 91 (81.5-101.5) (fig. 2C) and YMRS score was between from 27 (20-31) to 22 (17.5-28.5) (fig. 2D). Conversely, the placebo group showed no significant changes in symptom scores during the same period.
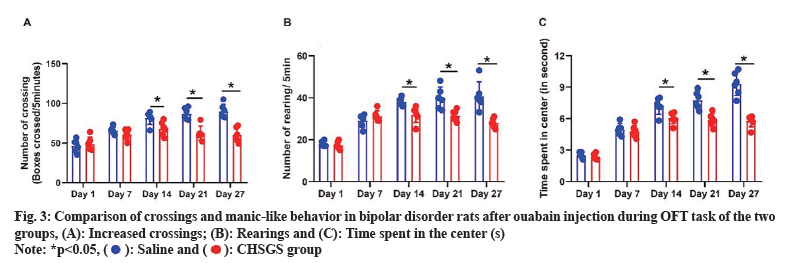
It is found that CHSGS treatment reduces manic-like behavior in ouabain induced bipolar disorder rats during the Open Field Task (OFT). After 3 d of administering ouabain injection, animals exhibited manic-like behaviors which were characterized by increased crossings (fig. 3A), rearings (fig. 3B), and time spent in the center (fig. 3C) during the OFT task. No significant difference was observed between saline and CHSGS groups on the 1st and 7th d (p>0.05). However, CHSGS group showed a progressive reduction in the number of crossings, rearings and time spent in the center compared to the saline group on the 14th, 21st and 28th d (p<0.05).
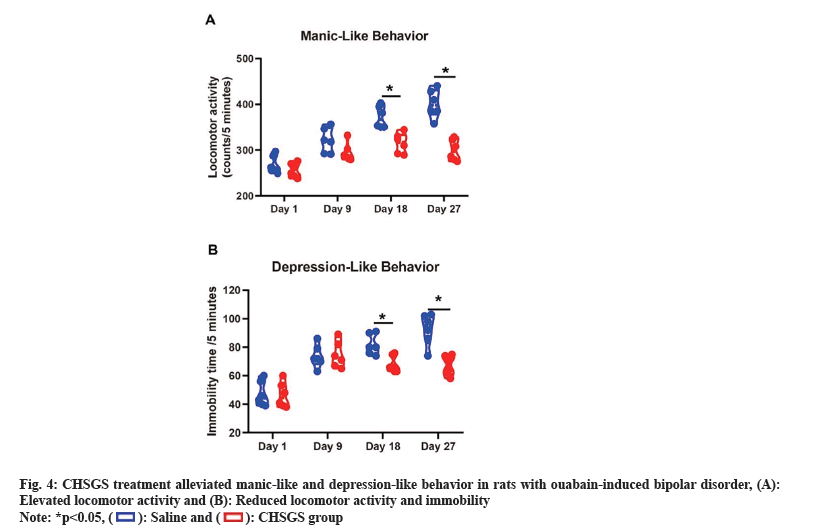
Similarly, CHSGS treatment mitigated manic-like and depression-like behavior in ouabain-induced bipolar disorder rats. Rats received ouabain on the 1st, 3rd and 7th d, exhibiting significantly elevated locomotor activity. Concurrently, ouabain had a substantial impact on immobility time in bipolar disorder-like rats. On the 18th and 27th d, the CHSGS group displayed significantly reduced locomotor activity and immobility time compared to the saline group (p<0.05) (fig. 4).
The observed significant symptomatic improvement in bipolar disorder patients treated with CHSGS aligns with the principles of traditional Chinese medicine that support the formula's composition. Bipolar disorder (multifaceted nature), characterized by episodes of mania and depression, presents a challenge in finding treatments that effectively address both poles of the disorder[20,21]. Conventional pharmacological interventions often focus on mood stabilization but may not fully capture the complexity of bipolar disorder symptomatology. CHSGS, a widely-used herbal prescription known for its efficacy in treating neurologic impairment and depression[22,23] demonstrates broad-spectrum effect in alleviating both depressive and manic symptoms, as evidenced by the reduction in HDRS, HARS, BPRS and YMRS scores.
Functional enrichment analysis has revealed potential mechanisms underlying the therapeutic effects of CHSGS. Notably, Phosphoinositide-3-Kinase/Protein Kinase B (PI3K/Akt) signaling pathway emerges as a key player, prominently affected by CHSGS in the treatment of major depressive disorder[24,25]. Additionally, CHSGS exerts its antidepressant effects by promoting hippocampal angiogenesis and neurogenesis through the activation of the Sirtuin 1 (SIRT1)/Forkhead box protein O1 (FOXO1) axis, involving the regulation of Vascular Endothelial Growth Factor A (VEGFA) and Brain Derived Neurotrophic Factor (BDNF)[26]. It also regulates Nuclear Factor Kappa B (NF-κB)-mediated BDNF expression through the modulation of gut microbiota and inflammation[27]. CHSGS demonstrates antidepressant effects by modulating the ratio of hippocampal Estrogen Receptor Alpha (ERα) and Beta (β) messenger Ribonucleic Acid (mRNA) (ERα/ER β ratio) in rats, providing a potential therapeutic target for perimenopausal anxiety and depression[28]. Meanwhile, CHSGS exhibits antidepressant effects by downregulating MicroRNA-124 (miR-124), relieving the inhibition of the Mitogen-Activated Protein Kinase 14 (MAPK14) and Glutamate Ionotropic Receptor Alpha-amino-3-hydroxy-5-Methyl-4-Isoxazolepropionic Acid (AMPA) type subunit 3 (GRIA3) signaling pathways and promoting hippocampal synapse formation in a rodent model of depression[22]. Furthermore, CHSGS impacts brain functional network connectivity, energy metabolism, neurotransmitter levels, and inflammatory processes in the hippocampus, contributing to its anti-depressive effects in perimenopausal depression[11]. Importantly, CHSGS demonstrates therapeutic efficacy against post-stroke depression by inhibiting neuroinflammation through modulation of the Janus Kinase/Signal Transducers and Activators of Transcription 3 (JAK/STAT3)-Glycogen Synthase Kinase 3 (GSK3) β/Phosphatase and Tensin homolog (PTEN)/Akt pathway[29]. This multifaceted impact on diverse molecular pathways highlights CHSGS as a promising holistic therapeutic approach, offering potential integration into psychiatric care for improved management strategies in various depressive conditions.
The incorporation of an animal model induced by ouabain administration in rats provides valuable insights into the behavioral effects of CHSGS in a controlled experimental setting. The ouabain-induced model, mimicking both manic and depressive symptoms in rats[17,20,30] aligns with the characteristics of bipolar disorder and strengthens the translational relevance of CHSGS. The reduction in manic-like behaviors, such as increased crossings, rearings and time spent in the center during the OFT, coupled with decreased immobility time in the forced swim test, highlights the potential of CHSGS to address both poles of bipolar disorder.
Despite these promising findings, several considerations merit attention for future research endeavors. First, elucidating the precise molecular mechanisms underlying CHSGS effects, including its impact on neurotransmitter systems, inflammatory markers, and neuroplasticity, would enhance our understanding of its pharmacological actions. Long-term studies are warranted to assess the sustainability of CHSGS effects and potential side effects, considering its prolonged use. Investigating the safety profile of CHSGS is crucial for its consideration as a viable treatment option for bipolar disorder. Additionally, exploring the synergistic effects of CHSGS with conventional pharmacotherapies, including common mood stabilizers or antipsychotics, could offer enhanced efficacy and improved symptom management for bipolar disorder patients, providing valuable clinical insights.
The findings from this study underscore the potential therapeutic benefits of CHSGS in alleviating both manic and depressive symptoms in bipolar disorder. The translational evidence from the ouabain-induced rat model provides further support for the clinical observations. However, further research is warranted to elucidate the mechanisms of action, long-term effects, and potential synergies with existing treatments. The integration of traditional Chinese herbal medicine into psychiatric care presents a promising avenue for addressing the complex symptomatology of bipolar disorder. This study contributes to the growing body of literature exploring holistic approaches to mental health and opens avenues for future research in the field.
Funding:
This work was supported by the Wenzhou Scientific Research project, “The Impact of Group Work Based on the PM model on Treatment Adherence in Patients with Bipolar Disorder in remission” (Grant No: Y2020446) and the Zhejiang Provincial Basic Public Welfare Research Program, “Study on the Molecular Mechanism of Saponins in Suanzaoren Hehuan Formula for the Treatment of Depression with Insomnia through Immune-Inflammatory Activation Mediated by TLR4/NF-κB/NLRP3 Inflammasome Pathway” (Grant No: LGF21H090002).
Conflict of interests:
The authors declared no conflict of interests.
References
- Cosgrove VE, Kelsoe JR, Suppes T. Toward a valid animal model of bipolar disorder: How the research domain criteria help bridge the clinical-basic science divide. Biol Psychiatry 2016;79(1):62-70.
[Crossref] [Google Scholar] [PubMed]
- de Filippis S, Cuomo I, Kotzalidis GD, Pucci D, Zingaretti P, Porrari R, et al. Does the efficacy of asenapine in bipolar disorder increase in the presence of comorbidity with a substance use disorder? A naturalistic study. Ther Adv Psychopharmacol 2017;7(2):67-77.
[Crossref] [Google Scholar] [PubMed]
- Rapoport SI, Basselin M, Kim HW, Rao JS. Bipolar disorder and mechanisms of action of mood stabilizers. Brain Res Rev 2009;61(2):185-209.
[Crossref] [Google Scholar] [PubMed]
- Aguiar-Geraldo JM, Possamai-Della T, Menegas S, Peper-Nascimento J, Quevedo J, Valvassori SS. Folic acid does not have an anti-manic effect but protect the brain against oxidative stress in an animal model of mania induced by ouabain. J Affect Disord 2023;334:307-16.
[Crossref] [Google Scholar] [PubMed]
- Brietzke E, Mansur RB, Zugman A, Carvalho AF, Macêdo DS, Cha DS, et al. Is there a role for curcumin in the treatment of bipolar disorder? Med Hypotheses 2013;80(5):606-12.
[Crossref] [Google Scholar] [PubMed]
- Jarman CN, Perron BE, Kilbourne AM, Teh CF. Perceived treatment effectiveness, medication compliance, and complementary and alternative medicine use among veterans with bipolar disorder. J Altern Complement Med 2010;16(3):251-5.
[Crossref] [Google Scholar] [PubMed]
- Baek JH, Nierenberg AA, Kinrys G. Clinical applications of herbal medicines for anxiety and insomnia; targeting patients with bipolar disorder. Aust N Z J Psychiatry 2014;48(8):705-15.
[Crossref] [Google Scholar] [PubMed]
- Jianfang X, Ling Z, Yanan J, Yanliang G. The effect of Chaihu-shugan-san on cytotoxicity induction and PDGF gene expression in cervical cancer cell line HeLa in the presence of paclitaxel+cisplatin. Cell Mol Biol 2021;67(3):143-7.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Jia Y, Liu X, Yang K, Lin Y, Shao Q, et al. Effect of Chaihu-Shugan-San on functional dyspepsia and gut microbiota: A randomized, double-blind, placebo-controlled trial. J Ethnopharmacol 2024;322:1-10.
[Crossref] [Google Scholar] [PubMed]
- Feng ST, Wang XL, Wang YT, Yuan YH, Li ZP, Chen NH, et al. Efficacy of traditional Chinese medicine combined with selective serotonin reuptake inhibitors on the treatment for parkinson's disease with depression: A systematic review and meta-analysis. Am J Chin Med 2021;49(3):627-43.
[Crossref] [Google Scholar] [PubMed]
- Huang R, Gong M, Tan X, Shen J, Wu Y, Cai X, et al. Effects of Chaihu Shugan San on brain functional network connectivity in the hippocampus of a perimenopausal depression rat model. Mol Neurobiol 2023;16(3):1655-72.
[Crossref] [Google Scholar] [PubMed]
- Kaltenboeck A, Winkler D, Kasper S. Bipolar and related disorders in DSM-5 and ICD-10. CNS Spectr 2016;21(4):318-23.
[Crossref] [Google Scholar] [PubMed]
- Santi NS, Biswal SB, Naik BN, Sahoo JP, Rath B. Comparison of hamilton depression rating scale and montgomery-asberg depression rating scale: Baked straight from a randomized study. Cureus 2023;15(9):1-7.
[Crossref] [Google Scholar] [PubMed]
- Wiglusz MS, Landowski J, Cubala WJ. Psychometric properties of the polish version of the hamilton anxiety rating scale in patients with epilepsy with and without comorbid anxiety disorder. Epilepsy Behav 2019;94(5):9-13.
[Crossref] [Google Scholar] [PubMed]
- Hofmann AB, Schmid HM, Jabat M, Brackmann N, Noboa V, Bobes J, et al. Utility and validity of the Brief Psychiatric Rating Scale (BPRS) as a transdiagnostic scale. Psychiatry Res 2022;314:1-15.
[Crossref] [Google Scholar] [PubMed]
- Samara MT, Levine SZ, Leucht S. Linkage of young mania rating scale to clinical global impression scale to enhance utility in clinical practice and research trials. Pharmacopsychiatry 2022;56(1):18-24.
[Crossref] [Google Scholar] [PubMed]
- Valvassori SS, Aguiar-Geraldo JM, Possamai-Della T, da-Rosa DD, Peper-Nascimento J, Cararo JH, et al. Depressive-like behavior accompanies neuroinflammation in an animal model of bipolar disorder symptoms induced by ouabain. Pharmacol Biochem Behav 2022;219:1-13.
[Crossref] [Google Scholar] [PubMed]
- Rajkhowa B, Mehan S, Sethi P, Prajapati A, Suri M, Kumar S, et al. Activating SIRT-1 signalling with the mitochondrial-CoQ10 activator solanesol improves neurobehavioral and neurochemical defects in ouabain-induced experimental model of bipolar disorder. Pharmaceuticals 2022;15(8):1-11.
[Crossref] [Google Scholar] [PubMed]
- Li L, Jia Q, Wang X, Wang Y, Wu C, Cong J, et al. Chaihu Shugan San promotes gastric motility in rats with functional dyspepsia by regulating Drp-1-mediated ICC mitophagy. Pharm Biol 2023;61(1):249-58.
[Crossref] [Google Scholar] [PubMed]
- Valvassori SS, Dal-Pont GC, Varela RB, Resende WR, Gava FF, Mina FG, et al. Ouabain induces memory impairment and alter the BDNF signaling pathway in an animal model of bipolar disorder: Cognitive and neurochemical alterations in BD model. J Affect Disord 2021;282:1195-202.
- Harrison PJ, Cipriani A, Harmer CJ, Nobre AC, Saunders K, Goodwin GM, et al. Innovative approaches to bipolar disorder and its treatment. Ann N Y Acad Sci 2016;1366(1):76-89.
[Crossref] [Google Scholar] [PubMed]
- Liu Q, Sun NN, Wu ZZ, Fan DH, Cao MQ. Chaihu-Shugan-San exerts an antidepressive effect by downregulating miR-124 and releasing inhibition of the MAPK14 and Gria3 signaling pathways. Neural Regen Res 2018;13(3):837-45.
[Crossref] [Google Scholar] [PubMed]
- Chen XQ, Li CF, Chen SJ, Liang WN, Wang M, Li C, et al. The antidepressant-like effects of Chaihu Shugan San: Dependent on the hippocampal BDNF-TrkB-ERK/Akt signaling activation in perimenopausal depression-like rats. Biomed Pharmacother 2018;105:45-52.
[Crossref] [Google Scholar] [PubMed]
- Zhang S, Lu Y, Chen W, Shi W, Zhao Q, Li L, et al. Network pharmacology and experimental evidence: PI3K/AKT signaling pathway is involved in the antidepressive roles of Chaihu Shugan San. Drug Des Devel Ther 2021;15:3425-41.
[Crossref] [Google Scholar] [PubMed]
- Chen Q, Li C, Tao E, Asakawa T, Zhang Y. Exploration of a brain-liver-communication-related mechanism involved in the experimental perimenopausal depression rat model using Chaihu-Shugan-San. Neurochem Res 2022;47(5):1354-68.
[Crossref] [Google Scholar] [PubMed]
- Zhang S, Lu Y, Shi W, Ren Y, Xiao K, Chen W, et al. SIRT1/FOXO1 axis-mediated hippocampal angiogenesis is involved in the antidepressant effect of Chaihu Shugan San. Drug Des Devel Ther 2022;16:2783-801.
[Crossref] [Google Scholar] [PubMed]
- Han SK, Kim JK, Park HS, Shin YJ, Kim DH. Chaihu-Shugan-San (Shihosogansan) alleviates restraint stress-generated anxiety and depression in mice by regulating NF-kappaB-mediated BDNF expression through the modulation of gut microbiota. Chin Med 2021;16(1):1-13.
[Crossref] [Google Scholar] [PubMed]
- Chen S, Asakawa T, Ding S, Liao L, Zhang L, Shen J, et al. Chaihu-Shugan-San administration ameliorates perimenopausal anxiety and depression in rats. PLoS One 2013;8(8):1-12.
[Crossref] [Google Scholar] [PubMed]
- Fan Q, Liu Y, Sheng L, Lv S, Yang L, Zhang Z, et al. Chaihu-Shugan-San inhibits neuroinflammation in the treatment of post-stroke depression through the JAK/STAT3-GSK3beta/PTEN/Akt pathway. Biomed Pharmacother 2023;160:1-10.
[Crossref] [Google Scholar] [PubMed]
- Possamai-Della T, Dal-Pont GC, Resende WR, Aguiar-Geraldo JM, Peper-Nascimento J, Quevedo J, et al. Imipramine can be effective on depressive-like behaviors, but not on neurotrophic factor levels in an animal model for bipolar disorder induced by ouabain. Mol Neurobiol 2022;59(12):7170-81.
[Crossref] [Google Scholar] [PubMed]

 .
.
 .
.



