- *Corresponding Author:
- Zuoyan Liu
Department of Rehabilitation Medicine, West China Hospital, Sichuan University/West China School of Nursing, Sichuan University, People's Republic of China
E-mail: lzy18782248342@163.com
| This article was originally published in a special issue, “Recent Progression in Pharmacological and Health Sciences” |
| Indian J Pharm Sci 2024:86(2) Spl Issue “243-248” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To assess the effectiveness of Xingnaojing injection in conjunction with Ginkgo biloba extract in managing post-stroke cognitive impairment. The period from June 2022 to June 2023 saw the treatment of 100 patients with post-stroke cognitive impairment at our hospital, all of whom were included in this study. The patients were then randomly assigned to an observation group (n=50) and a control group (n=50). While the control group was provided with intravenous infusion of Xingnaojing injection, the observation group, following the control group’s protocol, underwent treatment involving a combination of Xingnaojing injection and Ginkgo biloba extract injection. Both groups underwent therapy for a continuous 14 d period. During the treatment, the evaluation criteria encompassed the effectiveness of treatment, neural function (brain-derived neurotrophic factor, nerve growth factor), cognitive function (National Institute of Health Stroke Scale, activities of daily living, mini-mental state examination), neurotransmitter levels, and any unfavorable responses to the drugs. A notable distinction was observed, with the overall effective rate being significantly higher in the observation group (94.23 %) than in the control group (77.08 %). The incidence of adverse drug reactions showed no notable difference between the control and observation groups. Significant clinical effectiveness is evident in the treatment of poststroke cognitive impairment with the utilization of Xingnaojing injection in conjunction with Ginkgo biloba extract. It effectively enhances neural and cognitive function while ensuring safety for patients. The potential exists for this treatment approach to emerge as a key strategy in stroke rehabilitation and offer valuable insights for clinical treatment decisions. Nevertheless, additional extensive and prolonged follow-up studies are necessary to verify and reinforce these outcomes.
Keywords
Xingnaojing injection, Ginkgo biloba extract, post-stroke cognitive impairment, efficacy
Despite the substantial progress in modern medical technology and treatment methods, Post-stroke Cognitive Impairment (PSCI) remains a prevalent and worrisome consequence of stroke, significantly affecting the quality of life and social functioning of individuals[1,2]. Cognitive impairment following a stroke involves various cognitive limitations, affecting attention, memory, language, and executive function[3,4]. These limitations have a significant impact on patient’s everyday tasks, social skills, and work capacity[5-7], thereby presenting substantial challenges to patients and their families[8,9]. Treatment options for PSCI are currently limited, and while medications like acetylcholinesterase inhibitors are commonly utilized, their effectiveness is debated and frequently linked to adverse reactions[10].
The urgent and critical need for effective treatments for PSCI is the reason why Xingnaojing injection, a traditional Chinese medicine injection widely employed in cerebrovascular disease treatment[11,12], is being sought. Comprised of multiple herbal ingredients, it exerts effects such as promoting blood circulation, resolving stasis, dispelling wind, and clearing heat, thus improving cerebral blood flow, protecting neuronal cells, and facilitating neural functional recovery[13]. Rich in flavonoid compounds, Ginkgo biloba (G. biloba) extract originates from the leaves of G. biloba[14]. Research has suggested that G. biloba extract has the potential to prevent and treat cerebrovascular diseases, owing to its antioxidative, anti-inflammatory, and cerebral blood flow-enhancing properties[15,16].
Although there is a shortage of comprehensive clinical research evaluating its efficacy and safety, the combination of Xingnaojing injection and G. biloba extract holds promise in improving PSCI as a novel treatment approach. The objective of this research is to evaluate the therapeutic effectiveness of Xingnaojing injection in conjunction with G. biloba extract for PSCI, therefore providing more effective treatment choices and guidance for medical professionals.
Materials and Methods
General information:
The research subjects comprised 100 individuals with PSCI who received treatment at our hospital from June 2022 to June 2023. Using a random number table, the patients were allocated into a control group (n=50) and an observation group (n=50). The control group’s treatment involved administering Xingnaojing injection intravenously to 27 male and 23 female patients, with an average age of (68.32±9.14) y and duration of illness of (18.98±2.49) h. On the other hand, the observation group's treatment entailed combining G. biloba extract injection with the control group’s treatment and was administered to 28 male and 24 female patients, with an average age of (68.76±9.01) y and duration of illness of (18.68±2.86) h. The above data comparison revealed no statistically significant variances (p>0.05), denoting comparability. All patients participating in this study have submitted informed consent forms, and the study has received approval from our hospital’s ethics committee.
Inclusion criteria: Age ≥45 y, onset to hospital admission within 24 h; noticeable decrease in cognitive function, with two or more cognitive impairments, including orientation, attention, language, visual spatial function, executive function, and motor control and informed consent from the patient’s family.
Exclusion criteria: Computed Tomography (CT)-verified intracerebral hematoma or subarachnoid hemorrhage; severe impairment of additional organs; autoimmune disorders; malignant neoplasms and hypersensitivity to the medications utilized in this study.
Methods:
Routine Western medical treatments, covering the control of blood pressure and blood sugar, regulation of blood lipids, dehydration for reducing intracranial pressure, anticoagulation, antiplatelet therapy, and the provision of neurotrophic supplementation, were administered to all patients. Xingnaojing injection intravenous infusions (Approval No: National Medical Products Administration Z53021640, Dali Pharmaceutical Co., Ltd., specifications 10 ml) were administered to the control group as a 20 ml/d dosage within a 250 ml 5 % glucose solution, once daily for 7 d, for a total of two treatment cycles. The observation group was treated with a combined administration of G. biloba extract injection (from Yuke Kang Pharmaceutical Group Co., Ltd., National Medical Products Administration H20070226, 5 ml:17.5 mg) and the regimen utilized in the control group. The injection was added to 250 ml of 5 % glucose solution and administered intravenously at a rate of 20 ml/d continuously for 14 d.
Observation indices:
Clinical efficacy: A lack of improvement or worsening of clinical symptoms after 14 d of treatment resulted in categorizing it as ineffectiveness, which contributed to a 17 % reduction in the National Institute of Health Stroke Scale (NIHSS) score[17]. Substantial relief of clinical symptoms with an NIHSS score decrease of 18 % to 45 % was deemed as improvement, whereas nearly complete disappearance of clinical symptoms with an NIHSS score decline of 46 % to 90 % was characterized as marked improvement following treatment. An NIHSS score decrease of over 90 % and the complete disappearance of clinical symptoms were both indicative of a cure.
Overall effective rate=Number of cured cases+marked improvement cases+improvement cases/total cases×100 %
Neural function: Patient’s peripheral blood (4 ml) was collected before and after treatment in the morning on an empty stomach, followed by centrifugation at a centrifugation radius of 13.5 cm and a speed of 3000 r/min for 8 min to obtain serum, which was preserved in a refrigerator at -30° until further testing. Following strict adherence to the instructions provided with the kits, reagent kits from Shanghai Hengyuan Co., Ltd. were utilized to measure the serum levels of Nerve Growth Factor (NGF) and Brain-Derived Neurotrophic Factor (BDNF) with an enzyme-linked immunosorbent assay[18].
Treatment outcome assessment standards: Assessment of the severity of brain functional impairment in the two groups was conducted using the NIHSS score, where higher scores denoted more severe brain functional impairment. Utilizing the Modified Barthel Index (MBI), the assessment of Activities of Daily Living (ADL) was carried out to determine patient’s independence in their daily affairs, where scores of 100 points signified normal function, ≥60 points indicated basic self-care, 41 to 59 points suggested moderate functional impairment and the need for assistance in daily activities, 21 to 40 points signaled severe functional impairment and substantial reliance on support for daily living, and ≤20 points indicated complete dependence on others for daily activities[19]. The evaluation of cognitive function in both groups involved utilizing the Mini-Mental State Examination (MMSE) to assess time and place orientation, language, attention and calculation, immediate memory, short-term memory, and visuospatial abilities. Correct responses were awarded 1 point, with incorrect or unknown responses receiving 0 points, contributing to a total score range of 0 to 30[20,21].
Comparison of brain neurotransmitters: Using high-performance liquid chromatography-mass spectrometry, the levels of serotonin 5-Hydroxytryptamine (5-HT), Norepinephrine (NE), and Dopamine (DA) in peripheral blood (4 ml) were measured from patients prior to and following treatment on an empty stomach.
Occurrence of adverse reactions: Incidence of adverse drug reactions during treatment was recorded.
Statistical analysis:
Utilizing the Statistical Package for the Social Sciences (SPSS) 25.0, the analysis was performed, comparing the measurement data, presented as mean±standard deviation, between the two groups via an independent sample t-test. Utilizing the Chi-square (χ²) test, the count data were analyzed. The criteria for establishing statistical significance were defined at a level of p<0.05.
Results and Discussion
A statistically significant difference was observed, with the overall effective rate of treatment in the observation group (94.23 %) being higher than that of the control group (77.08 %) (p<0.05) as shown in Table 1.
| Group (n=50) | Cured | Marked improvement | Improvement | Ineffectiveness | Overall effective rate |
|---|---|---|---|---|---|
| Observation | 11 (22.00) | 21 (42.00) | 14 (28.00) | 4 (8.00) | 46 (92.00) |
| Control | 7 (14.00) | 14 (28.00) | 16 (32.00) | 13 (26.00) | 37 (74.00) |
| χ² | 5.741 | ||||
| p | 0.017 |
Table 1: Curative effect.
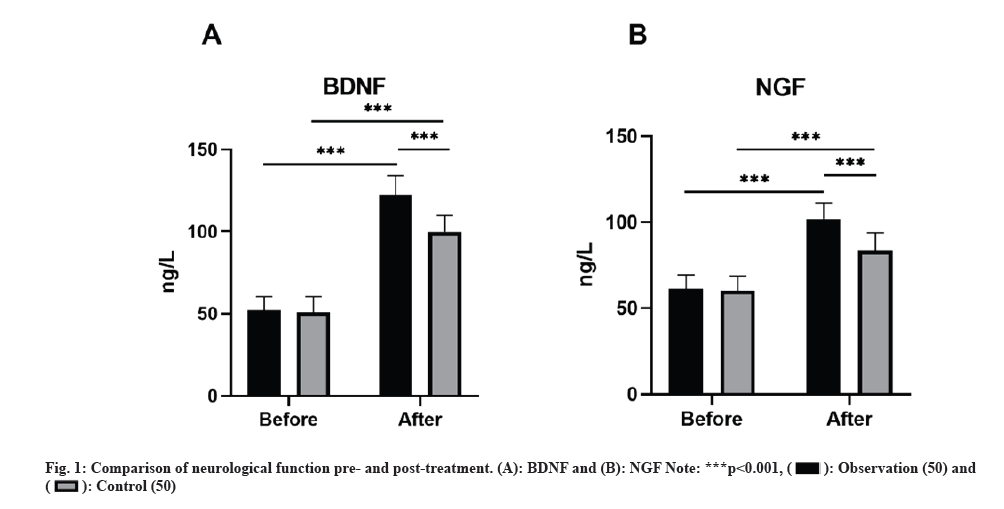
No remarkable discrepancies were observed in the baseline neurological function indicators (BDNF and NGF) between groups (p>0.05). Nevertheless, subsequent to the treatment, all neurological function indicators exhibited higher levels compared to baseline, with the observation group displaying greater levels than the control group (p<0.05) as shown in fig. 1.
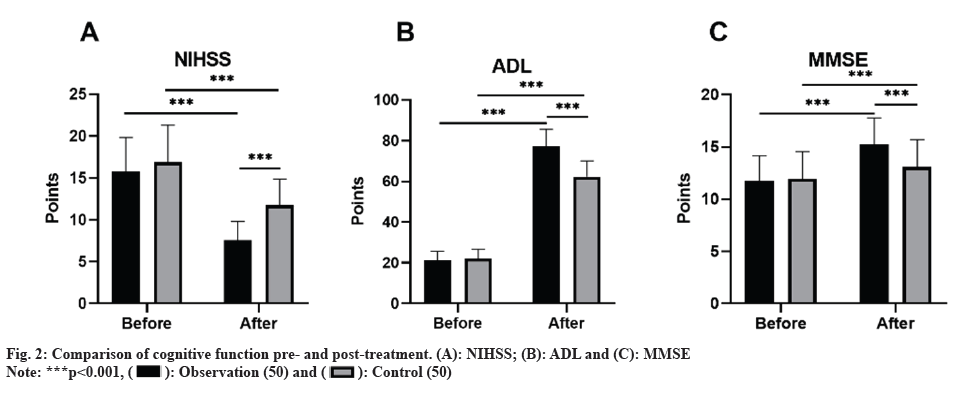
Initially, no marked discrepancies in the NIHSS, ADL, and MMSE scores were found. However, after the treatment, both groups exhibited a decrease in NIHSS scores, alongside an increase in ADL and MMSE scores compared to baseline, and the observation group indicated lower NIHSS scores and higher ADL and MMSE scores as opposed to the control group (p<0.05) as shown in fig. 2.
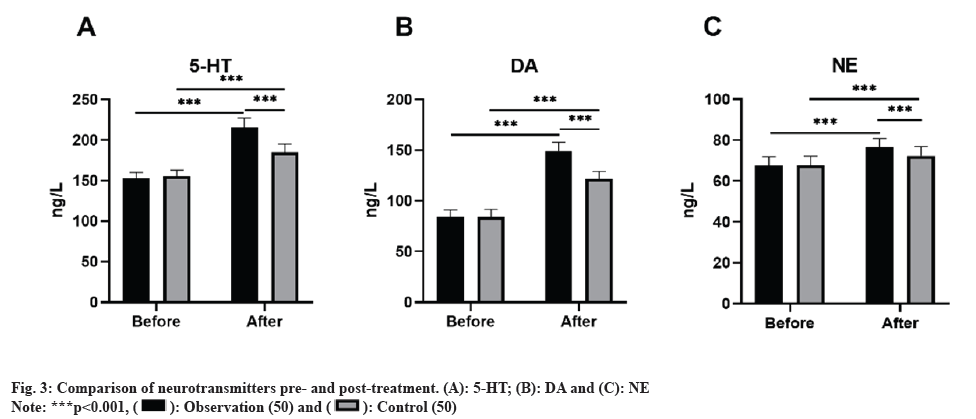
Initially, there were no notable discrepancies in the levels of neurotransmitters (5-HT, DA, and NE) between the two groups prior to treatment (p>0.05). Nonetheless, post-treatment, all neurotransmitter levels surpassed those observed at baseline, with the observation group displaying higher levels than the control group (p<0.05) as shown in fig. 3.
Adverse drug reactions were reported in 12.00 % of the control group and 8.00 % of the observation group, with no remarkable difference between groups (p>0.05) as shown in Table 2.
| Group (n=50) | Gastrointestinal discomfort | Rash | Chest distress | Palpitation | Overall incidence |
|---|---|---|---|---|---|
| Observation | 1 (2.00) | 2 (4.00) | 1 (2.00) | 0 (0.00) | 4 (8.00) |
| Control | 2 (4.00) | 1 (2.00) | 1 (2.00) | 2 (4.00) | 6 (12.00) |
| χ² | 0.444 | ||||
| p | 0.505 |
Table 2: Adverse drug reactions n (%).
Following a stroke, PSCI encompasses a range of syndromes that emerge within 6 mo, presenting as symptoms of cognitive impairment. Research indicates that about 20 % to 50 % of stroke survivors encounter alterations in cognitive function and mood, which may include depression. Stroke not only causes cognitive impairment but also speeds up the progression of cognitive dysfunction in patients, ultimately heightening the risk of dementia. Research data indicates that stroke raises the risk of dementia development in patients by 4 to 12 times. The repercussions of PSCI go beyond raising the risk of mortality to significantly affecting patients' daily life capabilities and social functions. Given the severity of cognitive impairment following stroke, assessing the treatment efficacy of PSCI is essential.
Xingnaojing injection and G. biloba extract are both frequently employed in the treatment of stroke. Based on the ancient traditional Chinese medicine An Gong Niu Huang Wan, Xingnaojing injection is a widely used intravenous infusion medication for a variety of cerebrovascular emergencies, such as acute cerebral hemorrhage, cerebral ischemia, and ischemic stroke, and has been proven effective in addressing impairment in consciousness caused by diverse etiologies. G. biloba leaves are a traditional Chinese medicinal herb recognized for their effective role in enhancing blood circulation, alleviating blood stasis, and opening up the collaterals.
Appraising the therapeutic efficacy of Xingnaojing injection in conjunction with G. biloba extract for PSCI was the main aim of this study, which yielded notable results following the randomization and 14 d treatment of 100 patients affected by PSCI. The observation group, which received treatment involving the combination of Xingnaojing injection and G. biloba extract, demonstrated a notably higher total effective rate as opposed to the control group, highlighting the beneficial therapeutic impact of this combined treatment in enhancing PSCI.
NGF and BDNF, as members of the neurotrophic factor family, are involved in regulating neuronal survival. NGF, generated in the body as a precursor, plays a pivotal part in controlling the development and sustainability of the central and peripheral nervous system by supporting neuronal survival, differentiation, and growth. Performing a crucial function, BDNF is widely distributed in the central nervous system and peripheral tissues, where it contributes to the development, differentiation, regeneration, and remodeling of neurons. This study’s results indicate that treatment resulted in a significant elevation in BDNF and NGF levels in the observation group as opposed to the control group, implying that Xingnaojing injection combined with G. biloba extract may be involved in protecting and restoring neurological function.
Induced by ischemic stroke, neurogenesis is an endogenous neural repair mechanism, wherein newly generated neurons migrate to damaged areas, replacing impaired neurons, establishing synaptic connections, and integrating into neural circuits. Following treatment, assessment of neurotransmitter levels in this study revealed a greater increase in neurotransmitter levels in the observation group as opposed to the control group. This implies that the combined use of Xingnaojing injection and G. biloba extract could have a positive impact on neurological function recovery after stroke by modulating neurotransmitter levels.
The occurrence of adverse drug reactions was relatively low in both the observation and control groups in terms of safety, showcasing the safety and practicability of the combined treatment regimen in clinical practice, with no marked distinction between groups.
The conclusions of this research strongly support the utilization of Xingnaojing injection combined with G. biloba extract in addressing PSCI. Through its enhancement of neurological function and cognitive performance, this treatment approach may positively impact the rehabilitation and life quality of stroke patients. Nevertheless, the research is restricted by factors such as small sample size and short treatment duration, potentially affecting the reliability and generalizability of the research. Thus, it is recommended to pursue further long-term, large-scale follow-up studies to confirm and validate the conclusions of this research and explore more accurate and feasible treatment approaches in the future.
Conflict of interests:
The authors declared no conflict of interests.
References
- Wu S, Wu BO, Liu M, Chen Z, Wang W, Anderson CS, et al. Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol 2019;18(4):394-405.
[Crossref] [Google Scholar] [PubMed]
- Shehjar F, Maktabi B, Rahman ZA, Bahader GA, James AW, Naqvi A, et al. Stroke: Molecular mechanisms and therapies: Update on recent developments. Neurochem Int 2023;162:105458.
[Crossref] [Google Scholar] [PubMed]
- Huang YY, Chen SD, Leng XY, Kuo K, Wang ZT, Cui M, et al. Post-stroke cognitive impairment: Epidemiology, risk factors, and management. J Alzheimer's Dis 2022;86(3):983-99.
[Crossref] [Google Scholar] [PubMed]
- Rost NS, Brodtmann A, Pase MP, van Veluw SJ, Biffi A, Duering M, et al. Post-stroke cognitive impairment and dementia. Circ Res 2022;130(8):1252-71.
[Crossref] [Google Scholar] [PubMed]
- Verdelho A, Wardlaw J, Pavlovic A, Pantoni L, Godefroy O, Duering M, et al. Cognitive impairment in patients with cerebrovascular disease: A white paper from the links between stroke ESO dementia committee. Eur Stroke J 2021;6(1):5-17.
[Crossref] [Google Scholar] [PubMed]
- Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, et al. Trajectory of cognitive decline after incident stroke. JAMA 2015;314(1):41-51.
[Crossref] [Google Scholar] [PubMed]
- Fride Y, Adamit T, Maeir A, Ben Assayag E, Bornstein NM, Korczyn AD, et al. What are the correlates of cognition and participation to return to work after first ever mild stroke? Top Stroke Rehabil 2015;22(5):317-25.
[Crossref] [Google Scholar] [PubMed]
- Lo Coco D, Lopez G, Corrao S. Cognitive impairment and stroke in elderly patients. Vasc Health Risk Manag 2016;12:105-16.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Bi X. Post-stroke cognitive impairment: A review focusing on molecular biomarkers. J Mol Neurosci 2020;70(8):1244-54.
[Crossref] [Google Scholar] [PubMed]
- Whyte EM, Lenze EJ, Butters M, Skidmore E, Koenig K, Dew MA, et al. An open-label pilot study of acetylcholinesterase inhibitors to promote functional recovery in elderly cognitively impaired stroke patients. Cerebrovasc Dis 2008;26(3):317-21.
[Crossref] [Google Scholar] [PubMed]
- Tian ZY, Feng LD, Xie Y, Xu DH, Zhang CY, Kong LB, et al. Chinese herbal medicine Xingnaojing injection for acute ischemic stroke: An overview of systematic reviews and meta-analysis. Front Pharmacol 2021;12:659408.
[Crossref] [Google Scholar] [PubMed]
- Zhang YM, Qu XY, Tao LN, Zhai JH, Gao H, Song YQ, et al. Xingnaojing injection ameliorates cerebral ischaemia/reperfusion injury via SIRT1-mediated inflammatory response inhibition. Pharm Biol 2020;58(1):16-24.
[Crossref] [Google Scholar] [PubMed]
- Wang L, Fan X, Chen Y, Liang X, Shen W, Zhang Y. Efficacy and safety of Xingnaojing injection for emergency treatment of acute ischemic stroke: A systematic review and meta-analysis. Front Pharmacol 2022;13:839305.
[Crossref] [Google Scholar] [PubMed]
- Nguyen T, Alzahrani T. Ginkgo biloba, StatPearls, Treasure Island (FL) ineligible companies. Disclosure: Talal Alzahrani declares no relevant financial relationships with ineligible companies: StatPearls Publishing Copyright© 2023, StatPearls Publishing LLC 2023.
- Kandiah N, Ong PA, Yuda T, Ng LL, Mamun K, Merchant RA, et al. Treatment of dementia and mild cognitive impairment with or without cerebrovascular disease: Expert consensus on the use of Ginkgo biloba extract, EGb 761®. CNS Neurosci Ther 2019;25(2):288-98.
[Crossref] [Google Scholar] [PubMed]
- Kandiah N, Chan YF, Chen C, Dasig D, Dominguez J, Han SH, et al. Strategies for the use of Ginkgo biloba extract, EGb 761®, in the treatment and management of mild cognitive impairment in Asia: Expert consensus. CNS Neurosci Ther 2021;27(2):149-62.
[Crossref] [Google Scholar] [PubMed]
- Runde D. Calculated decisions: NIH stroke scale/score (NIHSS). Emerg Med Pract 2020;22(7):CD6-7.
[Google Scholar] [PubMed]
- Nicoletti VG, Pajer K, Calcagno D, Pajenda G, Nógrádi A. The role of metals in the neuroregenerative action of BDNF, GDNF, NGF and other neurotrophic factors. Biomolecules 2022;12(8):1015.
- Pashmdarfard M, Azad A. Assessment tools to evaluate Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) in older adults: A systematic review. Med J Islamic Repub Iran 2020;34:33.
[Google Scholar] [PubMed]
- Khaw J, Subramaniam P, Abd Aziz NA, Ali Raymond A, Wan Zaidi WA, Ghazali SE. Current update on the clinical utility of MMSE and MoCA for stroke patients in Asia: A systematic review. Int J Environ Res Public Health 2021;18(17):8962.
[Crossref] [Google Scholar] [PubMed]
- Lo JW, Crawford JD, Desmond DW, Godefroy O, Jokinen H, Mahinrad S, et al. Profile of and risk factors for poststroke cognitive impairment in diverse ethnoregional groups. Neurology 2019;93(24):e2257-71.
[Crossref] [Google Scholar] [PubMed]

 .
.
 .
.
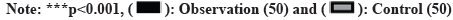 .
.



