- *Corresponding Author:
- Baoming He
Department of Neurology, Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital, Chengdu, Sichuan 610072, China
E-mail: hbmneuro@163.com
| This article was originally published in a special issue, “Drug Development in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(5) Spl Issue “8-13” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This paper primarily discusses about the influence of atorvastatin plus aspirin on efficacy and inflammatory factors in patients with cerebral venous thrombosis. The study participants comprised 240 cerebral venous thrombosis patients selected between January 2020 and June 2022 who were grouped as a conventional group (n=107) treated with aspirin and a research group (n=133) treated by atorvastatin+aspirin based on the therapy they received. Statistical and comparative analysis were made regarding the curative efficacy, adverse events (gastrointestinal discomfort, nausea, vomiting and dizziness), inflammatory factors (interleukin-6, tumor necrosis factor-alpha, procalcitonin, high-sensitivity C-reactive protein), platelet parameters (hematocrit, platelet adhesion rate), nerve function injury (National Institute of Health Stroke Scale) and activities of daily living. Based on the analysis results, the research group had a higher overall response rate, a lower complication rate and reduced inflammatory factors, platelet parameters and National Institute of Health Stroke Scale scores than the conventional group, as well as notably higher post-treatment activities of daily living, with statistical significance. From the above, it can be concluded that atorvastatin+aspirin is significantly effective in treating cerebral venous thrombosis, validly inhibiting inflammation, improving platelet parameters, alleviating neurological impairment and enhancing patients’ activities of daily living.
Keywords
Atorvastatin, aspirin, cerebral venous thrombosis, therapeutic efficacy, inflammatory factors
Cerebral Venous Thrombosis (CVT) is a kind of cardio-cerebrovascular condition in brain, belongs to the most common type of cerebral infarction and often occurs in the middle-aged and elderly people, with bad living habits, diet and environmental conditions as the pathogenic factors[1,2]. Its pathological mechanism is related to the stenosis or occlusion of the local cerebrovascular lumen caused by atherosclerosis and other reasons, which will cause the formation of local clots and thrombosis that can lead to ischemia, hypoxia and necrosis of brain tissue, seriously and negatively impacting the health of the patients[3,4]. It also has the characteristics of acute, rapid and serious onset of disease, with high mortality and disability rates, mainly presenting with numbness of limbs, dysarthria and month-tongue deviation clinically[5]. Current major treatments for CVT include thrombectomy, thrombolysis and drug therapy, however, the former two approaches, both of which are non-conservative treatments, have a high risk of intracranial hemorrhage and may threaten the life of the patients[6,7]. Therefore, this study focuses on the clinical treatment options for CVT patients from the perspective of pharmacotherapy to improve clinical outcomes and provide them with relatively safe and effective treatment methods.
Atorvastatin (ATO) as a statin, is a 3-Hydroxy-3-Methylglutaryl-Coenzyme A (HMG-CoA) reductase inhibitor and has been shown in animal experiments that it can inhibit inflammation and platelet activation, protect vascular endothelial cells and alleviate cerebral edema[8,9]. It has also been indicated to improve the outcomes of cerebrovascular Adverse Events (AEs) in patients with spontaneous subarachnoid hemorrhage by down-regulating Aquaporin-4 (AQP4) and Endothelin-1 (ET-1) levels and maintaining vascular autoregulation[10]. A rat experiment reported that ATO can inhibit neuronal apoptosis in hypoxic-ischemic neonatal rats by activating a molecular pathway involved in Brain-Derived Neurotrophic Factor (BDNF), thus alleviating brain damage[11]. While as a synthetic drug, Aspirin (ASP) has anti-inflammatory, anticoagulant and antithrombotic effects, as well as a certain preventive action against colorectal carcinoma, myocardial infarction and other cardiovascular events[12,13]. One study reported that using ATO in combination with other drugs may be beneficial in further reducing the risk of reinfarction, stroke and death compared with ASP alone, suggesting that ATO+ASP may contribute to a better clinical outcome[14].
Accordingly, we conducted relevant validation, mainly analyzing the effect of ATO+ASP on the efficacy and Inflammatory Factors (IFs) of CVT patients, which can fill the gap of the current lack in research on the role of ATO plus ASP in the treatment of CVT to some certain extent.
Materials and Methods
General information:
The research participants were 240 CVT patients admitted to Sichuan Provincial People's Hospital between January 2020 and June 2022, including 107 patients in the conventional group (ASP therapy) and 133 patients in the research group (ATO+ASP therapy). The study was approved by the Sichuan Provincial People's Hospital Ethics Committee and informed consent was obtained from each subject. The two patient cohorts showed no statistical significance in baseline data (p>0.05), which was clinically comparable.
Inclusion and exclusion criteria:
The patients who met the diagnostic criteria for CVT, with the onset-to-admission time within 72 h, no contraindications to the study medication (ASP and ATO) and cooperation with the study were included in the study. Patients who received prior treatment with hemostatic, coagulation and antifibrinolytic drugs; CVT patients who are suffering combination with cerebral hemorrhage, myocardial infarction and/or coagulation dysfunction; limb paralysis and hemiplegia; history of mental illness or dysarthria and malignant tumors were excluded.
Research methods:
The conventional group was treated with ASP, which was taken once daily before meals or bedtime, 100 mg a time, for 6 mo. The research group was given ATO on the basis of ASP (the same administration method and dosage as the conventional group). ATO was taken orally at bedtime with a dose of 20 mg once a day for 6 mo.
Observation indicators:
The clinical efficacy of the drugs was analyzed by the evaluation criteria and divided into certain categories. The category cured refers to the disappearance of signs and symptoms, the recovery of language ability, the improvement of muscle strength to grade IV or above and the ability of self-care after treatment; a marked response means that the signs and symptoms basically disappear after treatment, the muscle strength is improved by 2 levels vs. before treatment and the language ability is basically normal; a response corresponds to the improved signs and symptoms after treatment, enhanced muscle strength by one level vs. before treatment, and improved language ability; non-response is translated as unaltered or worsened signs and symptoms after treatment. The Overall Response Rate (ORR) is the sum of cured, marked response and response as a percentage of the total number of patients. Post-treatment AEs such as gastrointestinal discomfort, nausea, vomiting and dizziness, were observed and recorded.
Before and after treatment, 6 ml of venous blood of upper limbs was taken on an empty stomach and the serum was separated by centrifugation to quantify Interleukin-6 (IL-6), Tumor Necrosis Factor-alpha (TNF-α), Procalcitonin (PCT) and high-sensitivity C-Reactive Protein (hs-CRP) by Enzyme-Linked Immunosorbent Assays (ELISAs).
Venous blood (3 ml) was extracted from patients before and after treatment and centrifuged to collect the supernatant. The Hematocrit (Hct) and platelet adhesion rate were detected by Coulter LH 750 automatic blood routine analyzer.
Patients’ neurological impairment and Activities of Daily Living (ADL) before and after treatment were assessed using the National Institutes of Health Stroke Scale (NIHSS) and ADL scoring system whose score range was 0-42 and 0-100, respectively. A higher NIHSS score suggests more serious neurological impairment, while a higher ADL scale score indicates a stronger ADL ability.
Statistical analysis:
Continuous variables, represented by (x̄±s), were compared between groups using independent sample t tests. Categorical variables were described in the form of n (%), and inter-group differences were identified by Chi-square (χ²) tests. This study used Statistical Package for the Social Sciences (SPSS) 18.0 software for statistical analysis and p<0.05 as the significant threshold.
Results and Discussion
Baseline data of the two groups was compared and shown in Table 1. No significant inter-group differences were identified in baseline data such as sex, age, course of disease and complications between the two groups of patients (p>0.05).
| Indicators | Conventional group (n=107) | Research group (n=133) | χ²/t | p |
|---|---|---|---|---|
| Gender | ||||
| Male | 61 (57.01) | 74 (55.64) | 0.045 | 0.83 |
| Female | 46 (42.99) | 59 (44.36) | ||
| Age (years old) | 63.15±5.95 | 62.95±5.54 | 0.269 | 0.79 |
| Course of disease (h) | 6.20±1.58 | 6.07±1.64 | 0.62 | 0.54 |
| Complications | ||||
| Hypertension | 37 (34.58) | 41 (30.83) | 0.516 | 0.77 |
| Coronary heart disease | 34 (31.78) | 42 (31.58) | ||
| Hyperlipidemia | 36 (33.64) | 50 (37.59) |
Table 1: Comparison of baseline data between the two groups of patients.
The treatment efficacy was evaluated and subdivided according to the criteria of cure, marked response, response and non-response. The ORR of the research group was calculated to be 92.48 %, which was significantly higher than that of 73.83 % in the conventional group (p<0.05) (Table 2).
| Indicators | Conventional group (n=107) | Research group (n=133) | χ² | p |
|---|---|---|---|---|
| Cure | 29 (27.10) | 72 (54.14) | - | - |
| Marked response | 20 (18.69) | 43 (32.33) | - | - |
| Response | 30 (28.04) | 8 (6.02) | - | - |
| Non-response | 28 (26.17) | 10 (7.52) | - | - |
| ORR | 79 (73.83) | 123 (92.48) | 45.686 | <0.001 |
Table 2: Comparison of clinical efficacy of drugs between the two groups of patients.
We statistically calculated the incidence rate of AEs such as gastrointestinal discomfort, nausea, vomiting, and dizziness in both cohorts, and found an obviously lower incidence of AEs in the research group, compared with the conventional group (15.89 % vs. 7.52 %) (p<0.05) (Table 3).
| Indicators | Conventional group (n=107) | Research group (n=133) | χ² | p |
|---|---|---|---|---|
| Gastrointestinal discomfort | 6 (5.61) | 3 (2.26) | - | - |
| Nausea | 3 (2.80) | 2 (1.50) | - | - |
| Vomiting | 3 (2.80) | 2 (1.50) | - | - |
| Dizziness | 5 (4.67) | 3 (2.26) | - | - |
| Total | 17 (15.89) | 10 (7.52) | 4.16 | 0.041 |
Table 3: Comparison of AE’S between the two groups of patients.
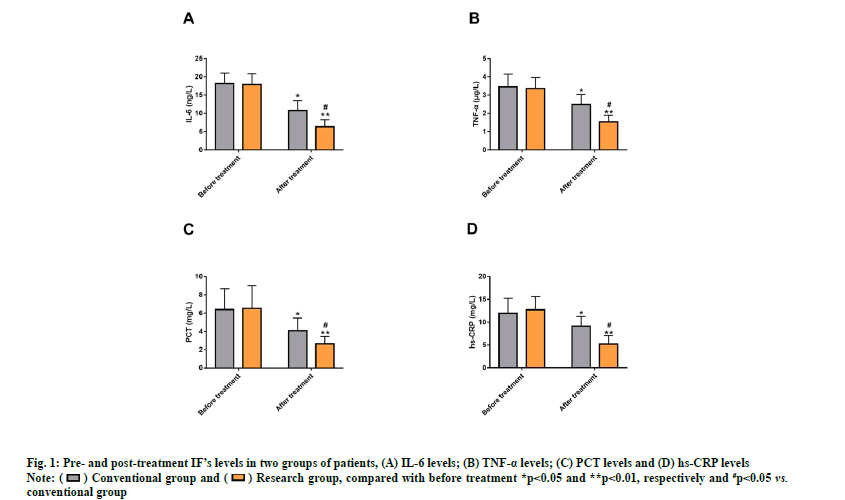
ELISA detection of IFs such as IL-6, TNF-α, PCT and hs-CRP revealed no evident inter-group differences before treatment (p>0.05) and the IFs were markedly reduced after treatment (p<0.05) with even lower levels in the research group (p<0.05) (fig. 1).
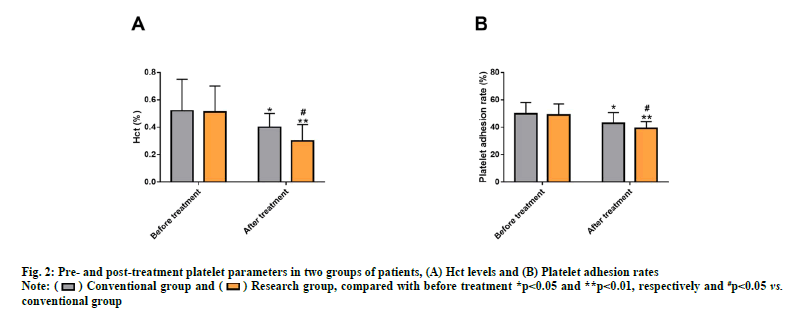
Measurements of platelet parameters (Hct and platelet adhesion rate) using an automatic blood routine analyzer also showed no notable inter-group differences before treatment (p>0.05). After treatment, a marked reduction in these two platelet parameters was observed (p<0.05), especially in the research group (p<0.05) (fig. 2).
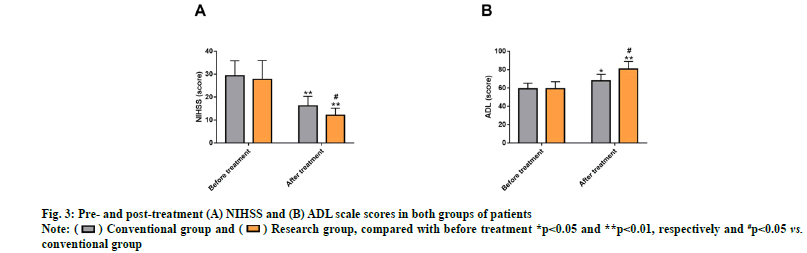
The NIHSS and ADL scores of both groups were evaluated to analyze the influences of the two drug therapies on CVT patients’ neurological impairment and ADL. The two cohorts differed insignificantly in NIHSS and ADL scores before treatment (p>0.05). After treatment, markedly reduced NIHSS score that was lower than that of the conventional group was determined in the research group (p<0.05), while the ADL score was obviously elevated and was higher compared with the conventional group (p<0.05) (fig. 3).
In this study, the efficacy was compared between the conventional group receiving ASP therapy and the research group receiving ATO+ASP. The determined ORR was found to be notably higher in the research group vs. the conventional group (92.48 % vs. 73.83 %), indicating that ATO+ASP therapy can effectively ameliorate the symptoms and signs of CVT patients and restore their language ability and muscle strength. The therapeutic mechanism of ASP in cardio-cerebrovascular diseases is associated with its inhibition of platelet aggregation, which is beneficial to prevent recurrent arterial thrombosis and thus plays a certain antithrombotic role[15,16]. ASP has been demonstrated to reduce the risk of venous thromboembolism by 42 %[17,18]. The cerebral vascular protective effect of ATO is related to its alleviation of cerebral vasospasm and its mediating effect on the structural and functional remodeling of vascular endothelial cells, so as to be protective of cerebrovascular auto-regulation[19]. Vogt et al. proposed that the reduction of cardiovascular risk by ATO is linked to the dose- and time-dependent improvement of renal function[20]. Through safety observation, the major AEs in both groups were determined to be gastrointestinal discomfort, followed by dizziness, and finally nausea and vomiting. Moreover, an evidently lower incidence of AEs were identified in the research group compared with the conventional group (7.52 % vs. 15.89 %), suggesting a higher safety profile of the combined treatment compared with ASP monotherapy.
Inflammation-induced necroptosis is shown to be not only related to the pathogenesis of neurodegeneration, cancer and autoimmune diseases, but also participates in the initiation and progression of ischemic diseases. Inhibition of inflammatory reaction is therefore conducive to preventing the development of pathological processes such as ischemic diseases[21]. Besides, research has also linked the activation of pro-IFs (IL-6 and TNF-α) to the acceleration of atherosclerosis progression, which can also lead to plaque instability under the action of various pro-IFs[22]. ELISA results regarding IFs in the two groups revealed that the post-treatment levels of indexes such as IL-6, TNF-α, PCT and hs-CRP in the research group decreased significantly and were lower than those in the conventional group, indicating more potent inhibitory effect of the combined medication on inflammation than monotherapy in CVT patients. Similarly, it was indicated in one study that ATO+ASP inhibited the release of pro-IFs like IL-6 in bone marrow stromal cells of stroke patients, demonstrating that ATO+ASP may have a synergistic inhibitory effect on inflammatory responses[23]. Further, according to the analysis of platelet parameters, Hct and platelet adhesion rate were notably reduced in the research group and lower than those in the conventional group after treatment, suggesting that the combined treatment can effectively improve the platelet parameters of CVT patients and thus improve their brain blood circulation. Finally, we used NIHSS and ADL scales to evaluate the neurological deficits and the ADL of the two groups, respectively. The research group treated with ATO+ASP also showed higher ADL and lower NIHSS after treatment, indicating that combined treatment can significantly improve patients’ ADL ability and reduce neurological deficits. In the research of Yu et al. ATO+sodium ozagrel not only significantly inhibits inflammatory reactions in patients with type 2 diabetes mellitus complicated with lacunar infarction, but also alleviates neurological deficits, similar to our findings[24]. Another study reported that ATO calcium plus ASP is not only better than ASP alone in the treatment of patients with acute ischemic stroke, but also contributes to a lower incidence of complications and shorter hospitalization time, as well as a certain improvement effect on patients’ survival outcomes[25].
Taken together, ATO+ASP can effectively ameliorate the signs and symptoms of CVT patients and restore their language and muscle strength, with significant curative effects and a high safety profile. Moreover, it can inhibit inflammation, improve platelet parameters, alleviate neurological deficits, and improve ADL, with clinical application value.
Conflict of interests:
The authors declared no conflict of interests.
References
- Ding J, Song B, Xie X, Li X, Chen Z, Wang Z, et al. Inflammation in cerebral venous thrombosis. Front Immunol 2022;13:1-12.
[Crossref] [Google scholar] [PubMed]
- Safina DR, Esin RG, Khakimova AA, Alimbekova LR. Cerebral venous thrombosis. Zh Nevrol Psikhiatr Im S S Korsakova 2022;122(3-2):11-6.
- Mehta A, Danesh J, Kuruvilla D. Cerebral venous thrombosis headache. Curr Pain Headache Rep 2019;23(7):1-6.
[Crossref] [Google scholar] [PubMed]
- Saposnik G, Barinagarrementeria F, Brown Jr RD, Bushnell CD, Cucchiara B, Cushman M, et al. Diagnosis and management of cerebral venous thrombosis: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42(4):1158-92.
[Crossref] [Google scholar] [PubMed]
- Aghamiri SH, Mansouri B, Mehrpour M, Karani SM, Ghaffari M, Lima BS, et al. Efficacy of mechanical thrombectomy in stroke patients with large vessel involvement. Eur J Transl Myol 2022;32(2):1-7.
[Crossref] [Google scholar] [PubMed]
- Lansberg MG, O'Donnell MJ, Khatri P, Lang ES, Nguyen-Huynh MN, Schwartz NE, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2):e601S-36S.
[Crossref] [Google scholar] [PubMed]
- Guyatt GH, Norris SL, Schulman S, Hirsh J, Eckman MH, Akl EA, et al. Methodology for the development of antithrombotic therapy and prevention of thrombosis guidelines: Antithrombotic Therapy and Prevention of Thrombosis: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2):53S-70S.
[Crossref] [Google scholar] [PubMed]
- Velarde GP, Choudhary N, Bravo-Jaimes K, Smotherman C, Sherazi S, Kraemer DF. Effect of atorvastatin on lipogenic, inflammatory and thrombogenic markers in women with the metabolic syndrome. Nutr Metab Cardiovasc Dis 2021;31(2):634-40.
[Crossref] [Google scholar] [PubMed]
- Cheng ZJ, Dai TM, Shen YY, He JL, Li J, Tu JL. Atorvastatin pretreatment attenuates ischemic brain edema by suppressing aquaporin 4. J Stroke Cerebrovasc Dis 2018;27(11):3247-55.
[Crossref] [Google scholar] [PubMed]
- Chen J, Li M, Zhu X, Chen L, Yang S, Zhang C, et al. Atorvastatin reduces cerebral vasospasm and infarction after aneurysmal subarachnoid hemorrhage in elderly Chinese adults. Aging 2020;12(3):2939-51.
[Crossref] [Google scholar] [PubMed]
- Yu L, Liu S, Zhou R, Sun H, Su X, Liu Q, et al. Atorvastatin inhibits neuronal apoptosis via activating cAMP/PKA/p-CREB/BDNF pathway in hypoxic-ischemic neonatal rats. FASEB J 2022;36(4):1-14.
[Crossref] [Google scholar] [PubMed]
- Hybiak J, Broniarek I, Kiryczyński G, Los LD, Rosik J, Machaj F, et al. Aspirin and its pleiotropic application. Eur J Pharmacol 2020;866:1-35.
[Crossref] [Google scholar] [PubMed]
- Singal AK, Karthikeyan G. Aspirin for primary prevention: Is this the end of the road? Indian Heart J 2019;71(2):113-7.
[Crossref] [Google scholar] [PubMed]
- Soodi D, vanWormer JJ, Rezkalla SH. Aspirin in primary prevention of cardiovascular events. Clin Med Res 2020;18(2-3):89-94.
[Crossref] [Google scholar] [PubMed]
- Murphy E, Curneen JM, McEvoy JW. Aspirin in the modern era of cardiovascular disease prevention. Methodist Debakey Cardiovasc J 2021;17(4):36-47.
[Crossref] [Google scholar] [PubMed]
- Davidson KW, Barry MJ, Mangione CM, Cabana M, Chelmow D, Coker TR, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force recommendation statement. JAMA 2022;327(16):1577-84.
[Crossref] [Google scholar] [PubMed]
- Hiltunen S, Putaala J, Haapaniemi E, Tatlisumak T. Long-term outcome after cerebral venous thrombosis: Analysis of functional and vocational outcome, residual symptoms, and adverse events in 161 patients. J Neurol 2016;263(3):477-84.
[Crossref] [Google scholar] [PubMed]
- Simes J, Becattini C, Agnelli G, Eikelboom JW, Kirby AC, Mister R, et al. Aspirin for the prevention of recurrent venous thromboembolism: The INSPIRE collaboration. Circulation 2014;130(13):1062-71.
[Crossref] [Google scholar] [PubMed]
- Chen JH, Wu T, Yang LK, Chen L, Zhu J, Li PP, et al. Protective effects of atorvastatin on cerebral vessel autoregulation in an experimental rabbit model of subarachnoid hemorrhage. Mol Med Rep 2018;17(1):1651-9.
[Crossref] [Google scholar] [PubMed]
- Vogt L, Bangalore S, Fayyad R, Melamed S, Hovingh GK, de Micco DA, et al. Atorvastatin has a dose‐dependent beneficial effect on kidney function and associated cardiovascular outcomes: Post hoc analysis of 6 double‐blind randomized controlled trials. J Am Heart Assoc 2019;8(9):1-9.
[Crossref] [Google scholar] [PubMed]
- Chen AQ, Fang Z, Chen XL, Yang S, Zhou YF, Mao L, et al. Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis 2019;10(7):1-18.
[Crossref] [Google scholar] [PubMed]
- Ji E, Lee S. Antibody-based therapeutics for atherosclerosis and cardiovascular diseases. Int J Mol Sci 2021;22(11):1-16.
[Crossref] [Google scholar] [PubMed]
- Satani N, Zhang X, Giridhar K, Wewior N, Cai C, Aronowski J, et al. A combination of atorvastatin and aspirin enhances the pro-regenerative interactions of marrow stromal cells and stroke-derived monocytes in vitro. Front Pharmacol 2021;12:1-11.
[Crossref] [Google scholar] [PubMed]
- Yu Y, Wang L, Zhu X, Liu YF, Ma HY. Sodium ozagrel and atorvastatin for type 2 diabetes patients with lacunar cerebral infarction. World J Diabetes 2021;12(12):2096-106.
[Crossref] [Google scholar] [PubMed]
- Li W, Ren X, Zhang L. Clinical efficacy of atorvastatin calcium combined with aspirin in patients with acute ischemic stroke and effect on neutrophils, lymphocytes and IL-33. Exp Ther Med 2020;20(2):1277-84.
[Crossref] [Google scholar] [PubMed]
 compared with before treatment *p<0.05 and **p<0.01, respectively and #p<0.05 vs. conventional group.
compared with before treatment *p<0.05 and **p<0.01, respectively and #p<0.05 vs. conventional group.