- *Corresponding Author:
- J. N. Zhang
Department of Anesthesiology, raditional Chinese Medicine Hospital of Wuxi, Wuxi, Jiangsu 214071, P. R. China
E-mail: zjn_wxzy@126.com
| This article was originally published in a special issue,
“Advanced Targeted Therapies in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(1) Spl Issue “36-42” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the effect of baicalin on cognitive function and nerves after splenectomy in aged rats is the main objective of the study. Aged rats were randomly divided into a sham operation group, a model group (to construct a splenectomy surgical model) and a baicalin treatment group (baicalin was given after the model was constructed). The pathological conditions like interleukin-6, tumor necrosis factor alpha, interleukin-1 beta content; brain detection by terminal deoxynucleotidyl transferase dUTP nick end labeling method apoptosis of tissue cell; western blot method was used to detect the expression of related proteins and real-time fluorescence quantitative polymerase chain reaction method was used to detect the messenger ribonucleic acid levels of related genes. Compared with the control group, the brain tissue cell apoptosis increased and the expression of inflammatory factors increased in the model group; compared with the model group, the brain tissue apoptosis of the baicalin treatment group decreased, the expression of Ki67 positive cells increased and the inflammation-related factor interleukin-6, tumor necrosis factor alpha, interleukin-1 beta levels were significantly decreased, N-methyl D-aspartate receptor subtype 2B, phosphorylated-extracellular signal-regulated kinase, phosphorylated-cAMP response element-binding protein expressions were significantly increased. Baicalin can improve postoperative cognitive dysfunction and nerve injury in aged rats after splenectomy by improving the pathological state of brain tissue, attenuating the inflammatory response and promoting apoptosis of brain tissue.
Keywords
Baicalin, postoperative cognitive dysfunction, inflammatory factor, neuroprotection
Postoperative Cognitive Dysfunction (POCD) is a central nervous system complication after surgical anesthesia, mainly manifested by disorders of consciousness, cognition, learning, memory, orientation and psychomotor behavior[1]. It can delay the recovery time of patients, increase the risk of other complications and have a serious impact on prognosis[2,3]. The exact mechanism of POCD is still unclear.
The traditional Chinese medicine, baicalin is known to have neuroprotective effects. Baicalin is a flavonoid extracted from the dried rhizomes of Astragalus and is the main pharmacological component of Astragalus. Baicalin has antiviral, anti-inflammatory, anti-apoptotic and antioxidant functions[4-7]. It can penetrate the blood-brain barrier and exert neuroprotective effects through antiinflammatory and antiapoptotic pathways, thereby providing effective protection against cerebral ischemia and other injuries[8,9]. In an in vitro model, baicalin also showed protective effects against neuronal damage induced by amyloid beta peptide (Aβ). Baicalin has an obvious protective effect on cerebral ischemia-reperfusion injury, which may be related to its antioxidant effect in the early stage of cerebral ischemia-reperfusion[10].
It is known that baicalin has protective and repairing effects on learning and memory impairment in rats, but its protective effect and molecular mechanism on POCD caused by splenectomy in aged rats are still unclear. Therefore, this study conducted splenectomy in aged rats and intraperitoneal injection of baicalin to explore the effect of baicalin on post-splenectomy in aged rats from the aspects of brain tissue pathological state, inflammatory factor expression and N-methyl D-aspartate Receptor Subtype 2B (NR2B)/Extracellular Signal-Regulated Kinase (ERK)/cAMP Response Element-Binding Protein (CREB) signaling pathway. Cognitive dysfunction and neuroprotective mechanisms provide a relevant theoretical basis for the clinical treatment and drug development of POCD and neuroinflammation.
Materials and Methods
Animals used in the study:
36 male Sprague-Dawley rats of 18 mo old, weighing 500-700 g, were provided by Shanghai Song Chi Animal Center. Animal room feeding environment was maintained at a room temperature of 22°±2°, relative humidity 50 %-60 % and 12 h alternating light and dark. Rats were raised of Specific Pathogen Free (SPF) and all experiments were conducted in accordance with the outlined ethical principles for medical research. The work was approved by the ethics Committee of Wuxi Hospital of Traditional Chinese Medicine affiliated to Nanjing University of Chinese Medicine.
Animal model:
The rats were anesthetized by intraperitoneal injection of pentobarbital sodium 50 mg/kg. After anesthesia, the rats were placed on the operating table and the spleen was dissociated through a central incision in the abdomen. The spleen was resected after double ligation and the abdominal cavity was closed after hemostasis was confirmed.
36 rats were fed for 1 w and randomly divided into sham operation group (n=8), splenectomy group (n=8) and baicalin group (n=20). The aged rats in the baicalin group were intraperitoneally injected with 100 mg/kg and 20 mg/kg baicalin on the day before operation. The others were injected with the same amount of normal saline. Except for the sham group, splenectomy was performed on the 2nd d. In the sham group, only the abdominal cavity was opened after anesthesia without partial splenectomy and the other steps were the same, with continuous injection for 7 d.
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) analysis:
3 μm paraffin sections were dewaxed in xylene for 10 min, replaced with fresh xylene and dewaxed for 10 min and then with absolute ethanol, 95 % ethanol, 85 % ethanol and 75 % ethanol for 10 min each. After that wash with distilled water, then wash 3 times with 1×Tris-Buffered Saline (TBS) for 5 min each, then dropwise add proteinase K, incubate at room temperature for 20 min, wash with 1×TBS buffer, incubate with 3 % Hydrogen peroxide (H2O2) for 5 min at room temperature and wash 2-3 times with TBS buffer, 5 min each time and then add 1×Terminal deoxynucleotidyl Transferase (TdT) equilibration buffer dropwise, at room temperature for 10-30 min, add label, incubate at 37° for 1.5 h, wash with TBS, add stop solution, incubate at 37° for 5 min, block with blocking buffer for 10 min at room temperature and add 1× conjugate. After incubation for 30 min, 3,3’-Diaminobenzidine (DAB) was added dropwise for color development. After the color development was terminated, methyl green or hematoxylin was added dropwise for counterstaining. The slides were mounted with neutral gum and observed under a light microscope.
Hematoxylin and Eosin (H and E) staining:
5 μm sections of fixed tissues were stained with H and E, according to standard procedures, neutral gum seal. The changes in the structure and morphology of the brain tissue of the rats in each group were observed under a light microscope. Photographs were taken with the Olympus image analysis system and analyzed with ImageJ software.
Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) analysis:
The hippocampal tissue was preserved at -80°, total Ribonucleic Acid (RNA) was extracted by TRIzol. Qiagen miRNeasy mini kit is used to extract exosomal microRNA (miRNA). SuperScriptTM first-strand synthesis system (Invitrogen) was used to prepare complementary Deoxyribonucleic Acid (cDNA) for qRT-PCR analysis by SYBRTM Green Master Mix (Qiagen). The 2-ΔΔCt method was used to calculate the normalized relative expression of beta-actin. Gene primer sequences were shown in Table 1.
| Gene | Primer sequence (5’ to 3’) | Size |
|---|---|---|
| TNF-α | F: GATCGGTCCCAACAAGGAGG | 20 |
| R: TCCCTCAGGGGTGTCCTTAG | 20 | |
| IL-1β | F: TTGCTTCCAAGCCCTTGACT | 20 |
| R: GGTCGTCATCATCCCACGAG | 21 | |
| IL-6 | F: AGAGACTTCCAGCCAGTTGC | 20 |
| R: AGTCTCCTCTCCGGACTTGT | 20 | |
| NR2B | F: GGGTCACGCAAAACCCTTTC | 19 |
| R: CCTTGTTTTTGACGCCCCTG | 20 | |
| ERK | F: CAACCAGAACAACTGGCTGC | 20 |
| R: GCCCAAAGCTCCTGACTTCT | 20 | |
| CREB | F: ACTCAGCCGGGTACTACCAT | 20 |
| R: GCACTGCCACTCTGTTCTCT | 20 | |
| GAPDH | F: GCGAGATCCCGCTAACATCA | 20 |
| R: CTCGTGGTTCACACCCATCA | 20 |
Note: F: Forward Primer; R: Reverse Primer; Size: Primer size; TNF-α: Tumor Necrosis Factor alpha; IL-1β: Interleukin 1 beta; IL-6: Interleukin 6; NR2B: N-methyl D-aspartate Receptor Subtype 2B; ERK: Extracellular Signal-Regulated Kinase; CREB: cAMP Response Element-Binding Protein; GAPDH: Glyceraldehyde-3-Phosphate Dehydrogenase
Table 1: Nucleotide Sequences of Primers used for Quantitative RT-PCR detection for mRNA
Western blot:
The hippocampal tissue was preserved at -80° and protein was quantified by Bicinchoninic Acid (BCA) method. The total cell lysate was separated by Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and blotted on polyvinylidene fluoride membrane (Millipore). Then, the membrane was blocked with 5 % skimmed milk at room temperature for 1 h and with specific antibodies against NR2B (Abcam, ab183942), ERK (Abcam, ab184699), phosphorylated-ERK (p-ERK) (Abcam, ab76299), CREB (CST, #9197), p-CREB (Abcam, ab32096) and beta-actin (Abcam, ab6267). The blot was visualized using SuperSignalTM West Pico PLUS chemiluminescent substrate (SD251210, Thermo Fisher Scientific, Inc).
Quantification and statistical analysis:
All data are expressed as mean±Standard Error of the Mean (SEM). We used a two-tailed Student’s t-test to compare the two groups. Use analysis of variance to compare multiple groups and then perform Bonferroni post testing. All experiments were repeated at least 3 times, p<0.05 was considered statistically significant.
Study approval:
The Animal Care and Use Committee of the Experimental Animal Research Center, Wuxi Hospital of Traditional Chinese Medicine, Nanjing University of Chinese Medicine reviewed and approved all animal care programs and experiments. All rats were kept in the SPF special facility of the Experimental Animal Research Center of Nanjing University.
Results and Discussion
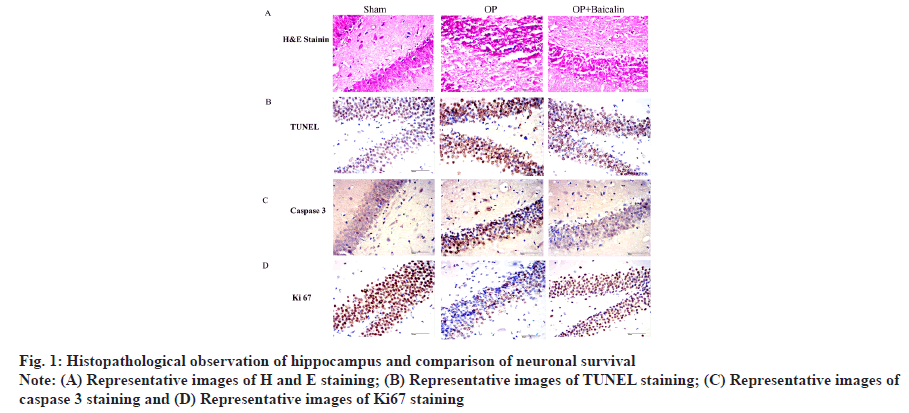
Histopathological observation of hippocampus and comparison of neuronal survival was explained in detail. To explore the effect of baicalin on aged rats after splenectomy, we set up three groups of experimental rats and randomly divided them into sham group, splenectomy group and baicalin treatment group after splenectomy. Brain tissue was collected 3 d after the experiment. Perform immunohistochemical experiments. H and E staining showed that the vertebral neurons in the hippocampal Cornu Ammonis (CA1) area of the rats in the sham-operated group had complete morphology, neat arrangement and clear and complete nuclei. Splenectomy of rat hippocampal CA1 area showed vertebral neuron damage, sparse cell arrangement, reduced cytoplasm, nuclear pyknosis and blurred nucleoli. After splenectomy, the damage of vertebral body neurons in the hippocampal CA1 region of the rats in the baicalin treatment group was lighter than that in the splenectomy group (fig. 1A). The results of TUNEL staining showed that, compared with the sham group, the apoptotic cells in the operation group increased and the apoptotic cells in the hippocampus area were significantly reduced after baicalin treatment (fig. 1B). The results of immunohistochemical staining showed that, compared with the sham-operated group, the expression of caspase 3 in the operation group was significantly increased and its expression was significantly decreased after treatment with baicalin. At the same time, the expression of Ki67 in the treatment group with baicalin after splenectomy was also significantly increased (fig. 1C and fig. 1D).
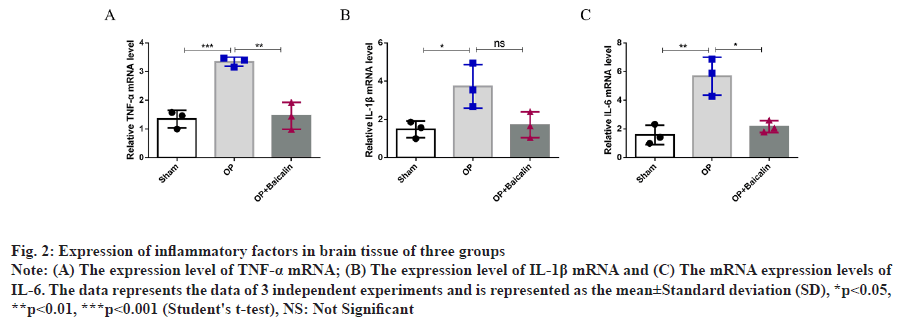
The above results show that baicalin can improve the pathological conditions of brain tissue and the morphology of neurons in the hippocampus after splenectomy in aged rats. Comparison of the relative expression levels of Tumor Necrosis Factor alpha (TNF-α), Interleukin 1 beta (IL-1β) and Interleukin 6 (IL-6) messenger RNA (mRNA) in the hippocampus was explained here. To detect the expression changes of proinflammatory factors in the hippocampus of elderly rats after splenectomy, real-time quantitative PCR experiment was conducted. Compared with the sham operation group, the mRNA and protein expressions of TNF-α, IL-1β and IL-6 in the hippocampus of operation group were significantly increased. Compared with operation group, baicalin treatment group was found in the hippocampus of rats and there was no significant difference in IL-1β mRNA level between the two groups, but there was a downward trend (fig. 2A-fig. 2C).
Fig. 2: Expression of inflammatory factors in brain tissue of three groups
Note: (A) The expression level of TNF-α mRNA; (B) The expression level of IL-1β mRNA and (C) The mRNA expression levels of
IL-6. The data represents the data of 3 independent experiments and is represented as the mean±Standard deviation (SD), *p<0.05,
**p<0.01, ***p<0.001 (Student's t-test), NS: Not Significant
The above results indicated that baicalin could reduce the expression of proinflammatory factors after splenectomy in aged rats.
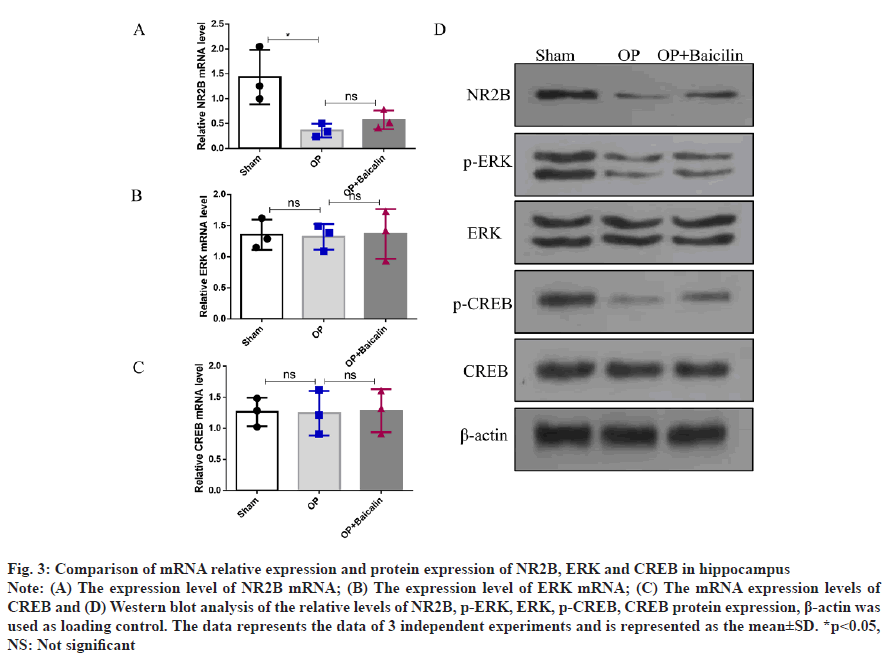
Comparison of mRNA relative expression and protein expression of NR2B, ERK and CREB in the hippocampus was shown in fig. 3. To investigate the mechanism of baicalin on nerves in aged rats after splenectomy, we detected the mRNA level and protein expression of NR2B/ERK/CREB signaling pathway. The results showed that, compared with the sham group, the relative expression of NR2B mRNA and protein in the hippocampus of the operation group was significantly decreased; compared with the operation group, the relative expression of NR2B protein in the baicalin treatment group after splenectomy was significantly increased, there was no significant difference in NR2B mRNA levels between the two groups (fig. 3A and fig. 3D). The mRNA levels of ERK and CREB did not change significantly in the three groups (fig. 3B and fig. 3C), but compared with the sham group, the relative protein expressions of p-ERK and p-CREB in the operation group were decreased. The relative expressions of p-ERK and p-CREB proteins increased in the baicalin treatment group (fig. 3D).
Fig. 3:Comparison of mRNA relative expression and protein expression of NR2B, ERK and CREB in hippocampus
Note: (A) The expression level of NR2B mRNA; (B) The expression level of ERK mRNA; (C) The mRNA expression levels of
CREB and (D) Western blot analysis of the relative levels of NR2B, p-ERK, ERK, p-CREB, CREB protein expression, β-actin was
used as loading control. The data represents the data of 3 independent experiments and is represented as the mean±SD. *p<0.05,
NS: Not significant
We found that baicalin can improve POCD in aged rats after splenectomy and its mechanism is related to the upregulation of the protein expressions of NR2B, p-ERK and p-CREB, indicating that baicalin exerts neuronal effects through the NR2B/ERK/ CREB signaling pathway.
POCD mostly occurs in elderly patients and its mechanism involves both the nervous system and the non-nervous system[11]. The mechanism of the nervous system is the degeneration of the central nervous system caused by age and the mechanism of the non-nervous system is the activation of the immune system caused by surgery and the occurrence of a peripheral inflammatory response, which in turn damages the central nervous system, damages the plasticity of nerves and causes damage to brain function, eventually leading to the occurrence of POCD[2,12]. Therefore, in the intervention research of POCD, inhibiting the inflammation of the central nervous system and protecting nerve cells has become the key. We used splenectomy to build a POCD model, which can successfully induce POCD and inflammatory responses in aged rats. Therefore, in this study, splenectomy was used to establish a POCD model in aged rats and to study the effect of baicalin on inhibiting the release of inflammatory factors and regulating the role of NR2B/ERK/ CREB signaling pathway on cognitive function and neuroprotection.
The Y-maze test was used to assess behavior in the previous study[13]. The results showed that baicalin could improve the exploratory ability and memory in the acute phase after splenectomy in aged rats. In this study, the mRNA levels of TNF-α, IL-6 and IL- 1β were significantly increased after splenectomy in aged rats. After treatment with baicalin, the levels of the inflammatory factors TNF-α and IL-6 decreased significantly and IL-1β levels also showed a downward trend, although the difference was not statistically significant. These results suggest that baicalin can reduce the release of inflammatory factors, which is related to the anti-inflammatory mechanism of baicalin.
TUNEL staining showed that the pyramidal neurons in the hippocampal CA1 area were counted and pyknosis, etc., indicating that the model group showed POCD, inflammation and neuronal damage in the hippocampus of the brain tissue. The number of brain tissue cells in the baicalin treatment group was higher than that in the model group, suggesting that baicalin can improve POCD in aged rats after splenectomy, inhibit brain tissue inflammation and neuronal damage. The results of this study also showed that the relative expression of NR2B mRNA and protein was lower than that of the sham-operated group, but higher than that of the model group. Glycoside can improve cognitive function after splenectomy in aged rats and its mechanism may be related to the activation of NR2B/ERK/CREB signaling pathway.
NR2B is a part of the “N-Methyl-D-Aspartate (NMDA) nerve endings” in the brain, which is related to the functional regulation of learning, memory, processing, pain perception and eating behavior[14]. Overexpression of this subunit in the forebrain can also improve the learning and memory function of rats[15]. The study showed that mice lacking NR2B in the forebrain were impaired across a range of memory tasks, exhibiting both spatial and nonspatial phenotypes[16].
There are also studies showing that phosphorylation of NR2B plays an important role in the behavior of water maze mice[17]. CREB is a molecule that gathers in brain cells and when they are active in the brain’s nerve cells, they can help to activate the genes involved in the formation of long-term memories[18]. Studies have found that phosphorylation of CREB plays an important role in memory acquisition and consolidation[19]. ERK is a key gene that transmits signals from the indicated receptors to the nucleus. After phosphorylation and activation of ERK, it can mediate the transcriptional activation of a series of genes and participate in a variety of biological reactions such as cell proliferation and differentiation, cell morphological maintenance and cell apoptosis. Studies have shown that ERK/Mitogen-Activated Protein Kinase (MAPK) signaling is essential for the normal development of the nervous system from neural progenitor cells in the embryonic mesoderm[20]. At the same time, inhibition of ERK activation can significantly inhibit the phosphorylation of CREB[21- 23]. Yan et al.[24] believe that the expression of NR2B is related to the ERK/ERK Kinase (MEK) signaling pathway and improves behavioral dysfunction by inhibiting neuroinflammatory responses. Zhang et al.[25] believed that by inhibiting the inflammation of the nervous system, the ability of spatial learning and memory in rats could be improved. Therefore, baicalin can inhibit neuronal inflammatory damage and improve POCD in rats by increasing the expression of NR2B/ERK/CREB protein.
In conclusion, baicalin can improve POCD in aged rats after splenectomy and its mechanism is related to the upregulation of NR2B/ERK/CREB protein expression. This study provides an experimental basis for baicalin as a preventive drug for POCD.
Acknowledgements:
We acknowledge Liang Cai, Hongwei Zhou, Songhua Li, Chennai Jiang, Mangling Xu, Jiangnan Zhang for participating in this work’s literature search, experimental validation, data analysis and manuscript writing. Liang Cai provided innovative analysis and administrative support. Jiangnan Zhang supervised the study design and critically read and edited the manuscript. All authors approved the final version for publication.
Conflict of interests:
The authors declared no conflict of interest.
References
- Lazarov O, Hollands C. Hippocampal neurogenesis: Learning to remember. Prog Neurobiol 2016;138:1-8.
[Crossref] [Google scholar] [PubMed]
- Evered LA, Silbert BS. Postoperative cognitive dysfunction and non-cardiac surgery. Anesth Analg 2018;127(2):496-505.
[Crossref] [Google scholar] [PubMed]
- Daiello LA, Racine AM, Yun Gou R, Marcantonio ER, Xie Z, Kunze LJ, et al. Postoperative delirium and postoperative cognitive dysfunction: Overlap and divergence. Anesthesiology 2019;131(3):477-91.
[Crossref] [Google scholar] [PubMed]
- Yang F, Feng C, Yao Y, Qin A, Shao H, Qian K. Antiviral effect of baicalin on Marek’s disease virus in CEF cells. BMC Vet Res 2020;16(1):1-9.
[Crossref] [Google scholar] [PubMed]
- Zhang J, Deng Y, Cheng B, Huang Y, Meng Y, Zhong K, et al. Protective effects and molecular mechanisms of baicalein on thioacetamide-induced toxicity in zebrafish larvae. Chemosphere 2020;256:127038.
[Crossref] [Google scholar] [PubMed]
- Wang Z, Ma L, Su M, Zhou Y, Mao K, Li C, et al. Baicalin induces cellular senescence in human colon cancer cells via upregulation of DEPP and the activation of Ras/Raf/MEK/ERK signaling. Cell Death Dis 2018;9(2):1-7.
[Crossref] [Google scholar] [PubMed]
- Meng X, Hu L, Li W. Baicalin ameliorates lipopolysaccharide-induced acute lung injury in mice by suppressing oxidative stress and inflammation via the activation of the Nrf2-mediated HO-1 signaling pathway. Naunyn Schmiedebergs Arch Pharmacol 2019;392(11):1421-33.
[Crossref] [Google scholar] [PubMed]
- Hu Q, Zhang W, Wu Z, Tian X, Xiang J, Li L, et al. Baicalin and the liver-gut system: Pharmacological bases explaining its therapeutic effects. Pharmacol Res 2021;165:105444.
[Crossref] [Google scholar] [PubMed]
- Wang J, Ishfaq M, Li J. Baicalin ameliorates Mycoplasma gallisepticum-induced inflammatory injury in the chicken lung through regulating the intestinal microbiota and phenylalanine metabolism. Food Funct 2021;12(9):4092-104.
- Liu J, Zhang T, Wang Y, Si C, Wang X, Wang RT, et al. Baicalin ameliorates neuropathology in repeated cerebral ischemia-reperfusion injury model mice by remodeling the gut microbiota. Aging 2020;12(4):3791-806.
[Crossref] [Google scholar] [PubMed]
- Needham MJ, Webb CE, Bryden DC. Postoperative cognitive dysfunction and dementia: What we need to know and do. Br J Anaesth 2017;119(1):115-25.
[Crossref] [Google scholar] [PubMed]
- Luo A, Yan J, Tang X, Zhao Y, Zhou B, Li S. Postoperative cognitive dysfunction in the aged: The collision of neuroinflammaging with perioperative neuroinflammation. Inflammopharmacology 2019;27(1):27-37.
[Crossref] [Google scholar] [PubMed]
- Zhang JN, Zhou HM, Jiang CH, Liu J, Cai LY. Protective effect of baicalin against cognitive memory dysfunction after splenectomy in aged rats and its underlying mechanism. J Integr Neurosci 2020;19(4):679-85.
[Crossref] [Google scholar] [PubMed]
- Loftis JM, Janowsky A. The N-methyl-D-aspartate receptor subunit NR2B: Localization, functional properties, regulation and clinical implications. Pharmacol Ther 2003 1;97(1):55-85.
[Crossref] [Google scholar] [PubMed]
- Monaco SA, Gulchina Y, Gao WJ. NR2B subunit in the prefrontal cortex: A double-edged sword for working memory function and psychiatric disorders. Neurosci Biobehav Rev 2015;56:127-38.
[Crossref] [Google scholar] [PubMed]
- Zhang YY, Liu F, Fang ZH, Li YL, Liao HL, Song QX, et al. Differential roles of NMDAR subunits 2A and 2B in mediating peripheral and central sensitization contributing to orofacial neuropathic pain. Brain Behav Immun 2022;106:129-46.
[Crossref] [Google scholar] [PubMed]
- Zhang X, Xie Y, Xu W, Liu X, Jiang S, Bao M, et al. Effects of 5-Aza on p-Y1472 NR2B related to learning and memory in the mouse hippocampus. Biomed Pharmacother 2019;109:701-7.
[Crossref] [Google scholar] [PubMed]
- Bartolotti N, Lazarov O. CREB signals as PBMC-based biomarkers of cognitive dysfunction: A novel perspective of the brain-immune axis. Brain Behav Immun 2019;78:9-20.
[Crossref] [Google scholar] [PubMed]
- Amidfar M, de Oliveira J, Kucharska E, Budni J, Kim YK. The role of CREB and BDNF in neurobiology and treatment of Alzheimer's disease. Life Sci 2020;257:118020.
[Crossref] [Google scholar] [PubMed]
- Iroegbu JD, Ijomone OK, Femi-Akinlosotu OM, Ijomone OM. ERK/MAPK signalling in the developing brain: Perturbations and consequences. Neurosci Biobehav Rev 2021;131:792-805.
[Crossref] [Google scholar] [PubMed]
- Nalinratana N, Meksuriyen D, Ongpipattanakul B. Differences in neuritogenic activity and signaling activation of madecassoside, asiaticoside and their aglycones in neuro-2a cells. Planta Med 2018;84(16):1165-73.
[Crossref] [Google scholar] [PubMed]
- Yao W, Cao Q, Luo S, He L, Yang C, Chen J, et al. Microglial ERK-NRBP1-CREB-BDNF signaling in sustained antidepressant actions of (R)-ketamine. Mol Psychiatry 2022;27(3):1618-29.
- Gündüz D, Troidl C, Tanislav C, Rohrbach S, Hamm C, Aslam M. Role of PI3K/Akt and MEK/ERK signalling in cAMP/Epac-mediated endothelial barrier stabilisation. Front Physiol 2019;10:1387.
[Crossref] [Google scholar] [PubMed]
- Yan A, Song L, Zhang Y, Wang X, Liu Z. Systemic inflammation increases the susceptibility to levodopa-induced dyskinesia in 6-OHDA lesioned rats by targeting the NR2B-medicated PKC/MEK/ERK pathway. Front Aging Neurosci 2021;12:625166.
[Crossref] [Google scholar] [PubMed]
- Zhang F, Zhang JG, Yang W, Xu P, Xiao YL, Zhang HT. 6-Gingerol attenuates LPS-induced neuroinflammation and cognitive impairment partially via suppressing astrocyte overactivation. Biomed Pharmacother 2018;107:1523-9.
[Crossref] [Google scholar] [PubMed]