- *Corresponding Author:
- R. K. K. Arya
Herbal Medicine Division, Defence Institute of Bio-Energy Research (DRDO) Field Station, Pithoragarh, Uttarakhand 263139,263001, India
E-mail: rajeshwararya@gmail.com
| Date of Submission | 16 June 2020 |
| Date of Revision | 03 August 2022 |
| Date of Acceptance | 09 February 2022 |
| Indian J Pharm Sci 2022;84(1):182-188 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Pyracantha crenulata (Deoxynivalenol) M. Roemis an evergreen shrub of family Rosaceae extensively reported for antihypertensive, antispasmodic, diuretic, sedative and vasodilatation properties. Despite its clinical uses, analysis of its nutritional profile and elemental composition, as well as the phytochemical investigations revealed the other probable applications of the plant. Quantitative biochemical analysis showed the presence of substantial quantities of carbohydrates, protein, crude, fibre and ascorbic acid along with the physicochemical characteristic of the plant. The elemental composition analysis revealed the presence of several macro elements and microelements, such as sodium, potassium, calcium, lithium, zinc, copper, manganese, iron and cobalt. The phytochemical investigations confirm the presence of medicinally active components such as alkaloids, saponins, glycosides, tannins and phenols. The in vitro antioxidant activity assessed using 1,1-diphenyl-2-picrylhydrazyl, 2,2-azinobis(3-ethylbenzothiazoline-6- sulfonic acid), scavenging assay and quantitative phytochemical analysis showed that the leaves extract having IC50 value 10.61 μg/ml and 12.73 μg/ml, by 2,2-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) and 1,1-diphenyl-2-picrylhydrazyl assay, and the phytochemical, such as total phenolic (128.78 mg/g), tannin (385.15 mg/g) and flavonoid contents (22.81 mg/g) respectively. In essence, the lyophilized leaves extract exhibits higher total phenolic, tannin and flavonoid contents and antioxidant activity. In summary, these results provide insightful information that the leaves of the plant endowed with several active principles, elemental composition and other biochemical characteristics that can be useful in the therapeutic activities as well as for the nutritional applications.
Pyracantha crenulata (Deoxynivalenol) M. Roemis an evergreen shrub of family Rosaceae extensively reported for antihypertensive, antispasmodic, diuretic, sedative and vasodilatation properties. Despite its clinical uses, analysis of its nutritional profile and elemental composition, as well as the phytochemical investigations revealed the other probable applications of the plant. Quantitative biochemical analysis showed the presence of substantial quantities of carbohydrates, protein, crude, fibre and ascorbic acid along with the physicochemical characteristic of the plant. The elemental composition analysis revealed the presence of several macro elements and microelements, such as sodium, potassium, calcium, lithium, zinc, copper, manganese, iron and cobalt. The phytochemical investigations confirm the presence of medicinally active components such as alkaloids, saponins, glycosides, tannins and phenols. The in vitro antioxidant activity assessed using 1,1-diphenyl-2-picrylhydrazyl, 2,2-azinobis(3-ethylbenzothiazoline-6- sulfonic acid), scavenging assay and quantitative phytochemical analysis showed that the leaves extract having IC50 value 10.61 μg/ml and 12.73 μg/ml, by 2,2-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) and 1,1-diphenyl-2-picrylhydrazyl assay, and the phytochemical, such as total phenolic (128.78 mg/g), tannin (385.15 mg/g) and flavonoid contents (22.81 mg/g) respectively. In essence, the lyophilized leaves extract exhibits higher total phenolic, tannin and flavonoid contents and antioxidant activity. In summary, these results provide insightful information that the leaves of the plant endowed with several active principles, elemental composition and other biochemical characteristics that can be useful in the therapeutic activities as well as for the nutritional applications.
Keywords
Quantitative, elemental composition, scavenging assay, Rosaceae, flavonoid.
Medicinally important plants are an incredible[1] source of herbal remedies used from ancient times for the care, cure and prevention of various diseases [2]. In recent times, herbal medicines have received unprecedented attention across the globe because of their minimal or no side effects during the therapy. Generally, a medicinal plant displays multi-faceted pharmacological responses due to the presence of a variety of active components. These active secondary metabolites synthesized by the plants have revealed a wide array of therapeutic activities ranging from anticancer, antimicrobial, antidiuretic to skincare [3]. Plant-based drug discovery is a multidisciplinary approach which employs biological,ethno botanical and biochemical techniques in the path of drug discovery [4,5]. Standardization is an essential step to assess the quality of crude drugs based on the principal components. Therefore, the standardization required crucial knowledge about its organoleptic characters, proximate composition along with the phytoconstituents and pharmacological effects [6]. In recent years, there has been a paradigm shift in the public to follow herbal based medications rather than western medicine. Primarily, it accounts for the severe adverse effects caused by these drugs. Therefore, the researchers endeavor to tap the ancient wisdom of traditional remedies to discover active components responsible for the therapeutic effects [7].
Antioxidants are the compounds having characteristics to prevent the oxidation of biomolecules (e.g. lipids, nucleic acids, proteins, and DNA) [8] by Reactive Oxidative Species (ROS) such as hydroxyl radical, peroxides, superoxide. Thus, antioxidant agents avert the occurrence of oxidative stress-related diseases, like diabetes-mellitus, brain stroke, Parkinson’s disease, rheumatoid arthritis, Alzheimer’s disease and cancer as well [9].
Phytochemicals such as phenolic compounds, carotenoids, flavonoids, proanthocyanidins, benzoic acid derivatives, coumarins, stilbenes and lignin’s, are the naturally occurring antioxidants which prevent the occurrence of oxidative-stress related diseases [10].
Pyracantha crenulata (P. crenulata) (D. Don) M. Roem (Syn. Crataegus crenulata Roxb.) (Rosaceae) commonly cultivated as an ornamental plant, grows at the altitude of 900-2400 m in the North-Western Himalaya. Among its many vernacular names like Himalayan Firethorn, Nepalese firethorn or Hawthorn, the plant also known as Ghingaru in Uttarakhand state of India [11]. It is a bushy and dense plant found widely in barren lands. The evergreen plant attains height up to 5-12 feet, branched extensively with lateral dark green leaves and orange-red pulpy berries [12]. The different parts of the plant contain many bioactive molecules viz. vitexin4 rhamnoside, vitexin, leucocynidine, leucoanthocyanidin, flavonoids, flavonol, kaempferol, glycoside, quercetin, beta-sitosterol and oligomeric saponins etc. [13]. It holds medicinal utilization in the treatment of cardiac failure, paroxysmal tachycardia,myocardial weakness, hypertension and arteriosclerosis. Besides, the fruits manifest antispasmodic, diuretic, sedative and vasodilatation properties [14]. The analysis of the proximate, elemental composition and the phytochemical investigations will give a prelude to the therapeutic potential and other possible applications of the plant [15]. The leaves of P. crenulata has not well explored so far for their nutritional profile. Therefore, this study focused on the phytochemical screening, proximate and elemental composition along with the in vitro antioxidant potential of the leaves extract.
Materials and Methods
Plant sample:
The leaves of Ghingaru (P. crenulata) (fig. 1) collected from Pithoragarh, Uttarakhand (India) dehydrated, powdered and stored carefully in a tightly-closed container for further studies. Further, the plant authenticated by the Botanical Survey of India (BSI), Dehradun (Accession No. 502).
Chemicals:
Reagents purchased from Sigma Chemicals USA were of analytical grade. 2,6-dichlorophenolindophenol, sodium carbonate, Potassium Permanganate (KMnO4), Potassium Persulfate (K2S2O8), Potassium Ferricyanide (K3Fe(CN)6), Ferric Chloride (FeCl3), Aluminum Chloride (AlCl3), Potassium Acetate (CH3COOK), Sodium Hydroxide (NaOH), Ascorbic acid, DPPH1,1- diphenyl-2-picrylhydrazyl (DPPH), tannic acid, 2,2-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), oxalic acid, sodium bicarbonate, Folin- Ciocalteau reagent, Fehling’s solution and Ninhydrin.
Extraction:
The dried powder of leaves (100 g) was successively extracted by cold maceration three times with 400 ml of 25 % ethanol and kept for 24 h. Each time with occasionally shaking and the whole organic filtrate collected was concentrated in vacuo using a rotary vacuum evaporator. Further, the semisolid residue was lyophilized. The percentage yield of lyophilized extract was 23 %. For the determination of antioxidant activity, total phenolic, flavonoids and tannin contents, the solution prepared by dissolving lyophilized leaves extract with water (100 mg/10 ml).
Organoleptic study:
The organoleptic study encompasses the study of morphological and sensory characters of crude drugs. The organoleptic evaluation carried out by the reported method, which includes the whole drug characteristic such as color, odour, taste, texture, size and shape [16,17]. Dried leaves powder used for organoleptic evaluation.
Physicochemical screening:
According to the World Health Organization (WHO) protocols, various physicochemical parameters viz. loss on drying, pH value, ash values and extractive values evaluated to assess the identity, purity and strength of the plant samples [18,19].
Qualitative phytochemical screening:
Phytochemical profiling was done by performing standard chemical tests for the presence of alkaloids, reducing sugars, monosaccharaides, glycosides, saponins, phenolic compounds, amino acids, proteins, steroids and terpenoids [20,21].
Determination of proximal composition:
The quantitative evaluation of the nutritional value and organic content was performing to measure the actual percentage proximate composition. It includes the quantification of various parameters according to the standard methods of analysis. The parameters examined are-total protein, fat, total carbohydrate, moisture contents, crude fiber and mineral contents [22]. The estimation of total carbohydrate, protein content, crude fiber, ascorbic acid and chlorophyll contents were carried out by Enthrone method [23], Lowry’s method [24], Maynard’s method, 2, 6- dichlorophenol-indophenol method and Arnon method, respectively [21,25].
Determination of elemental composition:
Mineral content is a measure of the amount of specific inorganic components, such as Calcium (Ca), Sodium (Na), Phosphorus (P), Magnesium (Mg), Zinc (Zn), copper and cadmium present within a sample [26]. Microelements and microelements were estimated by wet digestion method by using a triple acid mixture. Estimation of microelements (Na, K, Ca and Li) were carried out by flame photometer (foto flame 620256 NP, PEI-AIMIL Instrumentation,), while microelements (Fe, Co, Mn, Cu and Zn) determined by Atomic Absorption Spectrophotometer (model no. 4129, Electronic Corporation of India Ltd, Hyderabad). The instruments calibrated by using standard solutions (0.20-10 mg/l) [27] .
Determination of total phenolic, tannin and flavonoid contents:
The phenolic compounds present in the plant material are responsible for their potential antioxidant activity. The evaluation of phytochemical constituents such as tannin, flavonoids and phenolic contents carried out according to the reported methods. The estimation of total tannin, phenolic and flavonoid contents were determine by Folin-Denis method [25], Folin-Ciocalteau method and Aluminum chloride colorimetric method, respectively [28,29,30]
Determination of in vitro antioxidant activity:
An antioxidant biomolecule can interrupt the oxidation of biomolecules by oxidising themselves. The total antioxidant potential of plant material was estimated by DPPH and ABTS scavenging assay, according to the above-reported method. For the antioxidant assays, the solution of plant extracts (100 mg/10 ml) diluted according to the required concentrations. The Ascorbic acid (0.1 mg/ml) was use as the reference standard for all methods.
ABTS scavenging assay:
The antioxidant capacity was evaluate by the reported method depends on the scavenging of ABTS (2,2-azinobis (3-ethyl benzothiazoline-6-sulfonic acid)) free radical [31]. In this method, 7 mm ABTS solution and 2.45 m potassium persulphate solution mixed in equal volume and allow to react them for 12 h-16 h in the dark to produce a blue-green chromogenic, ABTS radical action (ABTS•+). Before, performing the assay, the resultant blue-green solution diluted with the methanol for obtaining the initial absorbance of 0.702±0.001 at 734 nm. The antioxidant effects observed by mixing 1 ml of ABTS solution with 00-50 μl of test samples (makeup to 1 ml) and measured the absorbance after 5-7 min at 734 nm, the decrease in the absorbance observed due to hydrogen donating availability of ABTS radical action, inducing a change in color of ABTS•+ radical action to colorless ABTS [32]. The percentage of free radical scavenging activity of ABTS•+ radicals were determining at different concentrations by using the formula:
Percentage FRSA=Control absorbance-Test Absorbance)/Control Absorbance×100
The IC50 value calculated as:
IC50 value (μg/ml) = (Concentration of test/50 % nearest FRSA)×50
1,1-diphenyl-2-picrylhydrazyl scavenging assay:
The antioxidant capacity estimated by the reported method relies on the scavenging of DPPH free radical [33]. Herein, DPPH methanol solution (2 ml, 0.135 mm) mixed with different aliquots of extract, after 30 min, the absorbance was measured at 517 nm against methanol as blank. Ascorbic acid used as a reference standard. The percentage of free radical scavenging activity of DPPH radicals was determined at different concentrations, also IC50 value calculated by using the formula mentioned above [34].
Statistical analysis:
The data were characterize by Mean±Standard Deviation (SD) (n=3). The obtained data were interpreted using statistical package for the social sciences 16.0 Software.
Results and Discussion
.The organoleptic study of morphological characters is essential to differentiate similar species by visual appearance. The findings presented as in Table 1.
| S.No. | Characters | Pyracantha crenulata dried leaves powder |
|---|---|---|
| 1. | Color | Dark green |
| 2. | Odour | Characteristic |
| 3. | Taste | Astringent |
| 4. | Texture | Fibrous, rough |
Table 1: Macroscopic Features of Dried Leaves Powder of Pyracantha Crenulata
The identity, purity and strength of P. crenulata leaves were estimate by various parameters on a dry weight basis. The common physicochemical characteristics of dried leaves powder presented in Table 2. The finding from the analysis revealed that leaves have 6.67 ±0.321 % loss on drying, 23.84±0.116 % ash value, 2.85±0.169 % acid insoluble ash, 4.77±0.174 % water soluble ash, 26.82±0.098 % water soluble extractive value, 17.68±0.126 % alcohol soluble extractive value, pH 6.69±0.045 (1 %) and pH 6.75±0.055 (10 %). The physicochemical screening can provides valuable information about the purity and quality, which is essential for the standardization of the herbal drug.
| S.No. | Parameters (percentage D.W.) | Results (Mean±SD) |
|---|---|---|
| 1. | Loss on drying | 6.67±0.321 |
| 2. | Ash value | 23.84±0.116 |
| 3. | Acid insoluble ash | 2.85±0.169 |
| 4. | Water-soluble ash | 4.77±0.174 |
| 5. | Alcohol soluble extractive value | 17.68±0.126 |
| 6. | Water-soluble extractive value | 26.82±0.098 |
| 7. | pH 1 % | 6.69±0.045 |
| 8. | pH 10 % | 6.75±0.055 |
Table 2:physicochemical characteristic of pyracantha crenulata
The qualitative phytochemical screening carried out on the lyophilized extract of P. crenulata leaves. It indicates the presence of various bioactive constituents which are essential for the biological activities. The analysis revealed the presence of alkaloids, glycosides, flavonoids, tannins, monosaccharaides, reducing sugars, saponin, protein, steroids and phenols (Table 3).
| S.No. | Phytochemical constituents | Test performed | Results |
|---|---|---|---|
| 1. | Reducing sugars | Fehling’s test Benedict test |
++ |
| 2. | Monosaccharides | Barfoed’s test | ++ |
| 3. | Alkaloids | Dragendorff’s test Mayer’s Test Hager’s test |
++ ++ |
| 4. | Steroids | Salkowski reaction | - |
| 5. | Protein | Biuret test | ++ |
| 6. | Glycosides | Keller-Killani Test | ++ |
| 7. | Saponin | Foam Test | ++ |
| 8. | Flavonoids | Lead acetate test | ++ |
| 9. | Tannins and Phenols | Ferric chloride test Dilute iodine solution Dilute HNO3 solution |
++ - |
| 10. | Amino acid | Ninhydrin test | ++ |
Note: Key: ++=Presence, - =Absence
Table 3: Qualitative Phytochemical Characteristic of Pyracantha Crenulata
Phytochemicals present in crude drugs significantly contribute to the nutritional composition of the human diet. The balanced diet comprises of appropriate quantity of nutrients like carbohydrate, proteins, fibers and fats. Therefore, it is necessary to assess the nutritional profile of the plant. The quantitative evaluation of P. crenulata leaves represented in Table 4, revealed the approximate nutritional composition. The leaves contain carbohydrates (42.86±0.262), protein (6.139±0.0175), total crude fiber (12.494±0.427) and fat (2.10±0.100 %) mg/100 g on a dry basis. The leaves also showed the high concentration of ascorbic acid (81.727±0.195 mg/100 g) and chlorophyll concentration (0.968±0.00252 mg/100 g chlorophyll a, 0.4313±0.00153 mg/100 g chlorophyll b) showing the photosynthetic abilities of the plant.
| S.No. | Nutritional Composition (mg/100 g) D.W | Results (Mean±SD) |
|---|---|---|
| 1. | Total Carbohydrate | 42.86±0.262 |
| 2. | Total Protein | 6.139±0.0175 |
| 3. | Total crude Fibre | 12.494±0.427 |
| 4. | Fat | 2.10±0.100 |
| 5. | Ascorbic acid (mg/100 g F.W.) | 81.727±0.195 |
| 6. | Chlorophyll a (mg/100 g F.W.) | 0.968±0.00252 |
| 7. | Chlorophyll b (mg/100 g F.W.) | 0.4313±0.00153 |
Table 4:Qualitative Phytochemical Characteristic Of Pyracantha Crenulata
The analysis of elemental composition reveals the presence of macro and microelements, sodium (7.3392), potassium (581.77±5.404), calcium (518.5±0.00), lithium (7.6432±0.00), zinc (35.554±0.323), copper (1.787±0.0577), manganese (9.987±0.288), iron (54.307±1.154) and cobalt (1.08±0.2078). The data shows a high concentration of calcium and potassium than the other minerals. These minerals are essential for various biological processes. The mineral analysis of P. crenulata leaves revealed the presence of a substantial amount of minerals. Elemental composition of the plant is mention in Table 5.
| S.No. | Elemental composition (mg/100 g) D.W. | Results (Mean±SD) |
|---|---|---|
| Microelements | ||
| 1. | Sodium (Na) | 7.3392±0.00 |
| 2. | Potassium (K) | 581.77±5.404 |
| 3. | Calcium (Ca) | 518.5±0.00 |
| 4. | Lithium (Li) | 7.6432±0.00 |
| Microelements | ||
| 5. | Zinc (Zn) | 35.554±0.323 |
| 6. | Copper (Cu) | 1.787±0.0577 |
| 7. | Manganese (Mn) | 9.987±0.288 |
| 8. | Iron (Fe) | 54.307±1.154 |
| 9. | Cobalt (Co) | 1.08±0.2078 |
Table 5: Elemental Composition Of Pyracantha Crenulata Leaves
Polyphenolic compounds are secondary metabolites well-known to have antioxidant activity. The quantitative evaluation of antioxidant constituents represented in Table 6 revealed the presence of a substantial phytochemical content in the lyophilized extract of P. crenulate leaves. The total phenolic,tannin and flavonoid contents were measure by standard calibration curve and expressed as Catechol Equivalent (CE) (standard curve equation y=0.026x; R2=0.998), Tannic Acid Equivalent (TAE) (y=0.012x; R2=0.993), and Quercetin Equivalent (QE) (y=0.001x; R2=0.993) in mg/g on a Dry Weight basis (D.W.). The Total Phenolic Content (TPC) calculated to be 128.783 ±0.21361 mg CE/g, Tannin Content (TTC) was 385.156±2.038 mg TAE/g and The Flavonoid Content (TFC) was 22.814±0.2292 mg QE/g.
| S.No. | Phytochemical contents (mg/g) D.W | Results (Mean±SD) |
|---|---|---|
| 1. | Total Phenolic contents | 128.783±0.2136 |
| 2. | Total Flavonoid contents | 22.814±0.2292 |
| 3. | Total Tannin contents | 385.156±2.038 |
Table 6: Phytochemical Contents Inthe Lyophilised Leaves Extract Of Pyracantha Crenulata
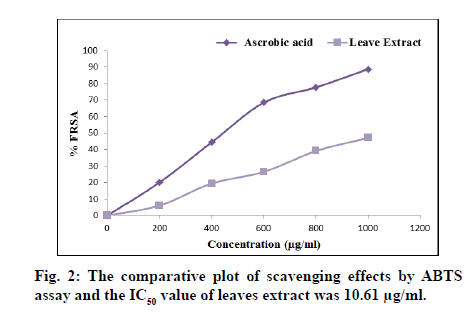
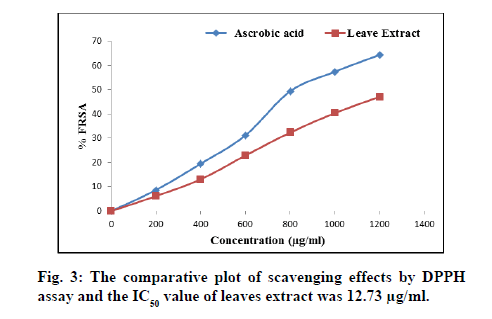
Different aliquots (200-1000 μg/ml) of ascorbic acid (standard) and P. crenulata leaves extracts were prepare for in vitro antioxidant activity by ABTS and DPPH scavenging assays. Minimum Inhibition Concentration (MIC) estimated by calculating IC50 value. MIC is a measure of the maximum antioxidant activity. In the ABTS scavenging assay, the IC50 value of the ascorbic acid and leaves extract found to be 4.49 μg/ml and 10.61 μg/ml respectively. IC50 value found to be 8.10 μg/ml and 12.73 μg/ml in ascorbic acid and extract of leaves, respectively using DPPH scavenging assay. The findings revealed that the P. crenulata leaves extract has moderate antioxidant potential in comparison to the ascorbic acid. The comparative plot of scavenging effects, i.e. percentage FRSA employing ABTS assay and DPPH assay of ascorbic acid and leaves extract shown in fig. 2 and fig. 3 respectively.
The present research strives to establish the therapeutic and dietary potential of the Himalayan plant P. crenulata leaves. The data revealed the presence of several vital phytoconstituents in the leaves extract, including alkaloids, tannins, phenolic, flavonoids, among others. Further, the findings from the quantitative biochemical and elemental composition evaluation revealed the presence of a substantial amount of carbohydrates, protein, fat, crude fibers, ascorbic acid and several microelements and microelements, respectively. Thereby, P. crenulata leaves show acceptable nutritional value. Notably, the leaves extract showed significant antioxidant activity. The results are in a satisfactory agreement with the previous work [35]. In conclusion, the leaves of P. crenulata demonstrate a valuable dietary profile and potential antioxidant activity, as well. This study warrants further research to investigate the active component(s) responsible for the antioxidant effects of the plant leaves. The study also indicates the prospective application of the leaves extracts in the herbal-based products.
Acknowledgements
The technical assistance and other facilities provided by DRDO are duly acknowledged. The logistic support provided by Director DIBER is also thankfully acknowledged.
Conflict of interests:
The authors declared no conflict of interest.
References
- Guerra RN, Pereira HA, Silveira LM, Olea RS. Immunomodulatory properties of Alternanthera tenella colla aqueous extracts in mice. Braz J Med Biol Res 2003;36:1215-9.
[Crossref] [Google Scholar] [PubMed]
- Kalia AN. Text Book of Industrial Pharmacognosym. Oscar Publication; 2005.
- Timothy O, Idu M, Falodun A, Oronsaye FE. Preliminary phytochemistry and antimicrobial screening of methanol extract of Baissea axillaris hau. Leaf J Biol Sci 2008;8:239-41.
- Jachak SM, Saklani A. Challenges and opportunities in drug discovery from plants. Curr Sci 2007;92:1251-7.
- Jatawa S, Paul R, Tiwari A. Indian medicinal plants: A rich source of natural immuno-modulator. Int J Pharmacol 2011;7(2):198-205.
- Garg P, Sardana S. Herbal drug standardization: An overview.Eur J Biomed Pharm Sci2016; 3(8):531-33.
- Franzen F, Bolini HMA. The medicinal and nutritional importance of plant extracts and the consumption of healthy foods-a review. Act Sci Nutr Health 2019;3(7):131-6.
- Liao KL, Yin MC. Individual and combined antioxidant effects of seven phenolic agents in human erythrocyte membrane ghosts and phosphatidylcholine liposome systems: importance of the partition coefficient. J Agric Food Chem 2000;48(6):2266-70.
[Crossref] [Google Scholar] [PubMed]
- Alam MN, Bristi NJ, Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J 2013;21(2):143-52.
[Crossref] [Google Scholar] [PubMed]
- Shah R, Kathad H, Sheth R, Sheth N. In vitro antioxidant activity of roots of Tephrosia purpurea Linn. Int J Pharm Sci 2010;2(3):30-3.
- Singh R, Negi PS, Arya MC, Ahmed Z. Propagation techniques of Crataegus crenulata: a multipurpose plant of mid Himalayan hills. Indian Forester 2012;138(2):169-72.
- Peschel W, Bohr C, Plescher A. Variability of total flavonoids in Crataegus-Factor evaluation for the monitored production of industrial starting material. Fitoterapia 2008;79(1):6-20.
[Crossref] [Google Scholar] [PubMed]
- Sati DC. Pharmacognostical and phytochemical screening of leaf and fruit extract of Pyracantha crenulata. J Pharmacogn Phytochem 2017;6(5):2563-8.
- Negi PS, Singh R, Dwivedi SK. Evaluation of antihypertensive effect of fruit beverage of Crataegus crenulata Roxb: A wild shrub of Himalayan hills. Def Life Sci J 2018;3:146-50.
- Karthika C, Manivannan S. Pharmacognostic, physicochemical analysis and phytochemical screening of the leaves of W. trilobata L. Int J Chem Tech Res 2018(02):124-31.
- Unit P, World Health Organization. Quality control methods for medicinal plant materials. World Health Organization; 1992.
- Evans WC. Trease and evans' pharmacognosy. Elsevier Sci 2009;7:614.
- Wong KH, Cheung PC. Nutritional evaluation of some subtropical red and green seaweeds: Part I-proximate composition, amino acid profiles and some physico-chemical properties. Food chem 2000;71(4):475-82.
- Makwana SJ, Jadeja BA. Comparative analytical study of physicochemical parameters of different plant parts of Operculina turpethum . J Pharmacogn Phytochem 2016;5(5):257.
- Khandelwal K. Practical pharmacognosy. Pragati Books Pvt. Ltd 2008:220.
- Dash SP, Dixit S, Sahoo S. Phytochemical and biochemical characterizations from leaf extracts from Azadirachta indica: An important medicinal plant. Biochem Anal Biochem 2017;6(323):2161-1009.
- Cunniff P, Washington D. Official methods of analysis of aoac international. J AOAC Int 1997;80(6):127A.
- Hedge JE, Hofreiter BT, Whistler RL. Carbohydrate chemistry. Academic Press, New York 1962;17:371-80.
- Classics Lowry O, Rosebrough N, Farr A, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193(1):265-75.
- Sadasivam S, Manickam A. Biochemical methods, new age international limited. New Delhi 2008;2:4-10.
- Palma MN, Rocha GC, Valadares Filho SC, Detmann E. Evaluation of acid digestion procedures to estimate mineral contents in materials from animal trials. Asian-Australas J Anim Sci 2015;28(11):1624.
- Tewari D, Pandey HK, Sah AN, Meena HS, Manchanda A, Patni P. Pharmacognostical, Biochemical and Elemental investigation of Ocimum basilicum plants available in western Himalayas. Int J Pharm biomed Res 2012;3(2):840-5.
- Baba SA, Malik SA. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J Taibah Univ Sci 2015;9(4):449-54.
- Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 2002;10(3):178-82.
- Guglani A, Pandey HK, Arya RK, Bala M. In vitro antioxidant activity, total phenolic, flavonoid and tannin contents in the Ajuga bracteosa wall. Ex benth, grown at middle hill climatic condition of Western Himalayas. Def Life Sci J 2020;5:198-203.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999;26(9-10):1231-7.
- Meena H, Pandey HK, Pandey P, Arya MC, Ahmed Z. Evaluation of antioxidant activity of two important memory enhancing medicinal plants Baccopa monnieri and Centella asiatica. Indian J Pharmacol 2012;44(1):114.
- Kedare SB, Singh RP. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol 2011;48(4):412-22.
- Singh N, Verma P, Pandey BR. Therapeutic potential of organic Triticum aestivum Linn.(Wheat Grass) in prevention and treatment of chronic diseases: An overview. Int J Pharm Sci Drug Res 2012;4(1):10-4.
- Saklani S, Chandra S, Mishra AP. Evaluation of Nutritional profile, medicinal value and quantitative estimation in different parts of Pyrus pashia, Ficus palmata and Pyracantha crenulata. J Glob Trends Pharm Sci 2011;2(3):350-4.