- *Corresponding Author:
- Xin Yi
Department of Human Anatomy, Medical School, Nantong University, Nantong, Jiangsu 226001, China
E-mail: yixin@ntu.edu.cn
| Date of Received | 17 December 2021 |
| Date of Revision | 04 November 2022 |
| Date of Acceptance | 13 July 2023 |
| Indian J Pharm Sci 2023;85(4):1025-1032 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore how microRNA-29a-3p influences the differentiation of neural stem cells into neurons and to identify any potential mechanisms involved. The program TargetScan, available online was utilized to examine and make predictions about the microRNA-29a-3p located upstream that regulate doublecortin X messenger ribonucleic acid. Ultimately, microRNA-29a-3p was preferred as the main central point of the investigation. The targeting of doublecortin X gene by microRNA-29a-3p was validated by employing a dual-luciferase reporter gene system. Before subjecting to adherent differentiation, the neural stem cells were transfected with microRNA-29a-3p inhibitor, microRNA-29a-3p mimic and normal control. MicroRNA and doublecortin X messenger ribonucleic acid levels were measured through quantitative reverse transcription-polymerase chain reaction and doublecortin X protein levels were determined through Western blot assays. The differentiation of neuronal precursor cells was detected by doublecortin X immunofluorescence. Neuronal differentiation was detected by microtubule-associated protein 2 immunofluorescence. According to the dual-luciferase reporter gene system, miR-29a-3p targeted the doublecortin X gene; microRNA-29a-3p significantly decreased in differentiated cells for 3 d was observed, along with a significant reduction in levels of doublecortin X protein and messenger ribonucleic acid. On the other hand, a significant raise in microRNA-29a-3p in differentiated cells of microRNA-29a-3p mimic group for a period of 3 d was noticed with a significant drop in protein levels, doublecortin X messenger ribonucleic acid and doublecortin X immunofluorescence-positive neuronal precursor cells. From the microtubule-associated protein 2 immunofluorescence, it was confirmed that microRNA-29a-3p inhibitor group significantly elevated the total number of neurons, while microRNA-29a-3p mimic reduced the neurons on 7 d. Therefore, low expression of microRNA-29a-3p promote differentiation of neural stem cells into neurons via targeting doublecortin X.
Keywords
MicroRNA-29a-3p, doublecortin X, neural stem cells, neurons, differentiation
The Central Nervous System (CNS) is an important component of the human body, coordinating the physiological activities of various organs in response to changes in the internal and external environment. Once the CNS is damaged, it can bring tremendous pain to patients and a heavy burden to society and families[1,2]. With the aging population and the frequent occurrence of traffic accidents, there are more and more cases of degenerative diseases and injuries of the CNS[3,4]. Currently, the main method of treating such diseases is to supplement the neurotransmitter deficiency caused by the loss of neurons and achieve a balance of neurotransmitters. However, this cannot fundamentally solve the problem of neuron degeneration and loss, whereas using new neurons to replace missing ones can fundamentally solve the problem. Therefore, cell replacement therapy is universally considered an ideal method[5-9].
Neural Stem Cells (NSCs) are a type of stem cell that have been identified not only in embryonic brain tissue but also in the human adult brain, particularly in the regions of sub ventricular zone and the dentate gyrus, which are two areas where neuronal cells are generated in adult mammals[10]. Other areas such as the septum, striatum and spinal cord have also been found to contain NSCs[11,12]. Numerous factors in the body can affect the migration and differentiation of NSCs[13,14]. Researchers are currently focusing on investigating the various intrinsic and extrinsic factors that play a crucial role in influencing the differentiation of NSCs, specifically towards the development of neuronal cells. Research findings indicate that under natural differentiation conditions, both inside and outside the body, the vast majority of NSCs differentiate into glial cells, with very few becoming neuronal cells[15,16]. Identifying the factors and molecular mechanisms that affect NSCs differentiation into neuronal cells is imperative, hence urgent, in order to guide the differentiation of sufficient numbers of neuronal cells to meet therapeutic needs.
MicroRNAs (miRNAs) are tiny Ribonucleic Acid (RNA) molecules that are non-coding, and are of almost 21-22 nucleotides in terms of their length. They regulate the gene expression by binding to partly matching sequences present in the 3' Untranslated Region (UTR), which could be of one or more target messenger RNA (mRNAs). Numerous reports suggest that the miRNAs significantly contribute to various biological properties, such as cell proliferation, cell differentiation, and cell apoptosis. Moreover, having a crucial function in the neural development and physiology, miRNAs are found in large numbers in the CNS[17,18]. Recent studies have also shown that miRNAs participate in proliferation and differentiation of stem cells via regulating expression of many stem cell regulatory factors[19-22].
Doublecortin X (DCX) is broadly expressed in neural precursor cells and is a protein associated with microtubules after migration during mitosis and is a key protein involved in neuronal differentiation and a major marker of neural precursor cells[23]. TargetScan (http://www.targetscan.org) was utilized in this study for the purpose of analyzing and predicting miRNAs that govern DCX mRNA, and analysis results showed miR-29a-3p was one of them. Through relevant literature, it was report miR-29a-3p is involved in various cellular processes[24-29], but its role in NSCs to neuronal differentiation process is unclear. We speculate miR-29a-3p may participate in process of NSCs to neuronal differentiation by targeting DCX.
Materials and Methods
Primary culture of NSCs:
After 15 d of pregnancy, SD rats were put to death using Carbon dioxide (CO2) anesthesia, which was supplied by the Animal Experiment Center of Nantong University, sterile fetuses were extracted and bilateral cerebral cortex was isolated and placed in Dulbecco's Modified Eagle Medium (DMEM) culture fluid. After carefully removing the meninges and blood vessels, the tissue was thoroughly washed three times with D-Hank's solution to eliminate any traces of blood and clots. Subsequently, the contents were finely minced into a paste using ophthalmic scissors. The resulting mixture was then filtered through a 200-mesh sieve and collected in a 10 ml centrifuge tube. The combination was centrifuged at a rate of 1000 r/min for three cycles of 3 min each and the liquid above the remaining solid materials was removed. The growth medium containing 1 % N2 (Gibco), 10 ng/ml basic Fibroblast Growth Factor (bFGF), and 20 ng/ml human Epidermal Growth Factor (EGF) was added. These bottles were then placed in a cell culture incubator, maintaining a temperature of 37° and an atmosphere with 5 % CO2. To support cell growth, the culture medium was replenished every 3-4 d and set final cell density to 1×106 cells/ml. When density of neural cell spheres was high, 0.25 % trypsin was used for digestion to obtain neural sphere clones, which were then passaged.
Cell transfection:
At a concentration of 5×103/ml, fourth generation NSCs were cultured in a 6-well culture plate, and were subsequently transfected with miR-29a-3p mimic, inhibitor or corresponding Negative Controls (NC) from Themo Fisher, United States of America (USA) using lipofectamine 2000, following the instructions on transfection. A blank control group was also established and the cells were incubated at 5 % CO2, 37° incubator. After being transfected 5 h, the cells were cultured for an additional 8 h using DMEM as the medium before they were collected for the subsequent experiment.
Differentiation culture of NSCs:
Placed the above transfected or transfected NSCs (density of 5×104/ml) in 24-well culture plate, and divided into control, mimic NC, inhibitor NC, mimic, and inhibitor groups. The culture medium used consisted of a mixture of DMEM/F12 at a 1:1 ratio, supplemented with 2 % B27 and 2 % Fatal Bovine Serum (FBS). The cells were then incubated in a cell culture incubator with a 5 % CO2 concentration set at 37°. The medium was refreshed every 3 d to ensure optimal cell nourishment and growth.
Real-time Polymerase Chine Reaction (PCR):
The cells that were cultivated were directly lysed using Trizol, and total RNA was extracted. As per the standard guidelines available in the first-strand complementary Deoxyribonucleic Acid (cDNA) synthesis kit of the HiScript Q RT SuperMix for qPCR (+gDNA wiper) from Vazyme Biotech, 1 μg of complete RNA was transformed to cDNA. The Fast Start Universal SYBR Green Master (Roche) was used for real-time PCR in accordance with the StepOnePlus™ Real-Time PCR system (Applied Biosystems). The primers used in the experiment were designed by Sangon Biotech and RiboBio companies. U6: forward 5′-CTCGCTTCGGCAGCACATA -3; Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH): forward 5′-ATTCCATGGCACCGTCAAGGCTGA-3′, reverse 5′-TTCTCCATGGTGGTGAAGACGCCA-3′; DCX: forward 5'-AGCCTGAGCAGCACCTGTGA-3', reverse 5'-CGTCCTCGCCAATGGAAGAA-3'; miR-29a-3p: forward 5′-CTCGCTTCGGCAGCACATA-3'. To compute the relative expression level of genes, the 2-△△CT technique was employed.
Western blot:
Extract total cellular protein using the pierce kit, determine the concentration and conduct gel electrophoresis. Transferred to a membrane using the Bio-Rad semi-dry transfer system, blocked with 5 % skimmed milk under room temperature for 120 min. The primary and secondary antibodies were incubated at 4° (for overnight) and at room temperature (for 2 h), respectively. Finally, used the Bio-Rad Chemiluminescence kit to visualize. Scanned and quantified each band density using the Shine-tech Image System. The primary antibodies used were; mouse anti-DCX antibody (1:1000, Abcam), and mouse anti-β-actin antibody (1:1200, Abcam). The secondary antibody used in the current study was a goat anti-mouse Immunoglobulin G (IgG) conjugated with horseradish peroxidase (1:5000, Pierce).
Immunofluorescence:
Initially, cells were fixed by employing paraformaldehyde solution (4 %) at room temperature for 30 min. Later, cells were blocked for 2 h with Phosphate Buffer Solution (PBS) (0.01 mol/l, pH 7.4) containing Bovine Serum Albumin (BSA) (0.5 %) and Triton X-100 (0.3 %). Finally, cells were exposed to incubation with the primary and secondary antibodies at 4° (for overnight) and at room temperature (for 2 h), respectively. The cell nuclei were counterstained with Hoechst 33342 (1:1000, Pierce) and mounted with glycerol after PBS washing. The primary antibodies used were; mouse anti-DCX antibody (Abcam) at a dilution of 1:500, and a dilution of 1:500 for mouse anti-beta (β) Microtubule-Associated Protein 2 (MAP-2) antibody (Abcam). The secondary antibody used in the current study was a goat anti-mouse IgG secondary antibody that was conjugated with Alexa Fluor® 488 at a dilution of 1:1000.
Luciferase experiment:
The PCR method amplified wild-type or mutant 3'UTR sequences of the DCX gene and cloned them into the pGL3 luciferase reporter plasmid (Promega). By employing Lipo2000 reagent (ThemoFisher), NSCs were then transfected with luciferase reporter plasmid, miR-29a-3p mimic or NC (ThemoFisher) and Renilla luciferase. A dual-luciferase reporter assay kit (Promega) was employed to determine the Luciferase activity.
Statistical analysis:
The data is presented in the format of mean value accompanied by standard deviation. GraphPad Prism 6 software was employed to examine the data, employing one-way analysis of variance to compare multiple groups, and independent t-test to compare two groups. p<0.05 is indicative of statistical significance.
Results and Discussion
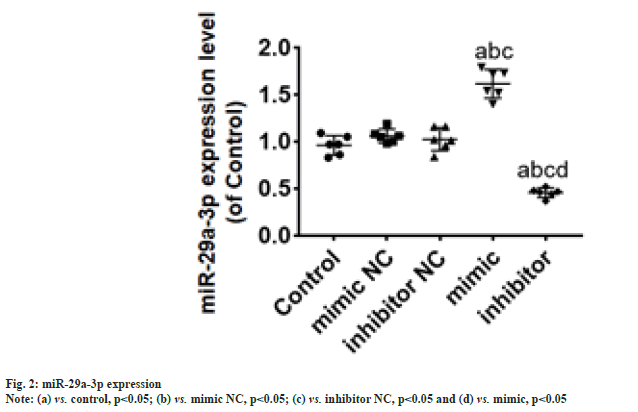
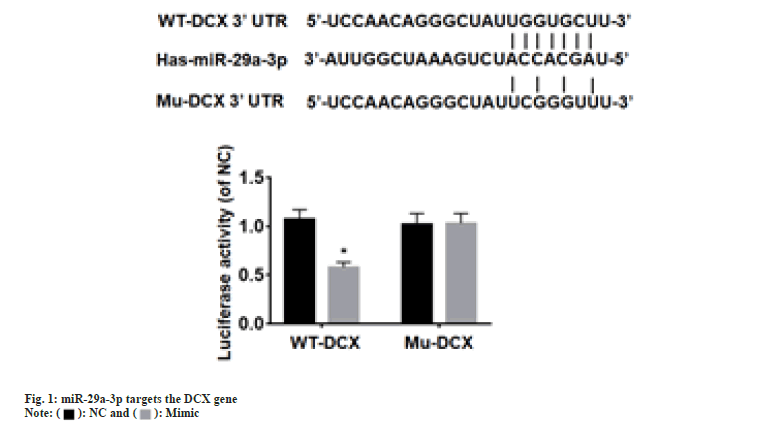
According to the luciferase experiment assay, the activity of the luciferase in the 3' untranslated region of the wild-type DCX vector was suppressed (p<0.05) by miR-29a-3p. Whereas, miR-29a-3p did not showed any effect on the mutant DCX gene vector (fig. 1). Hence, through this observation it can be concluded that miR-29a-3p chiefly targets DCX. The miR-29a-3p expression in NSCs was significantly (p<0.05) elevated by transfection of mimic group, whereas significantly reduced (p<0.05) in the transfection of inhibitor group, compared with NC group as shown in fig. 2.
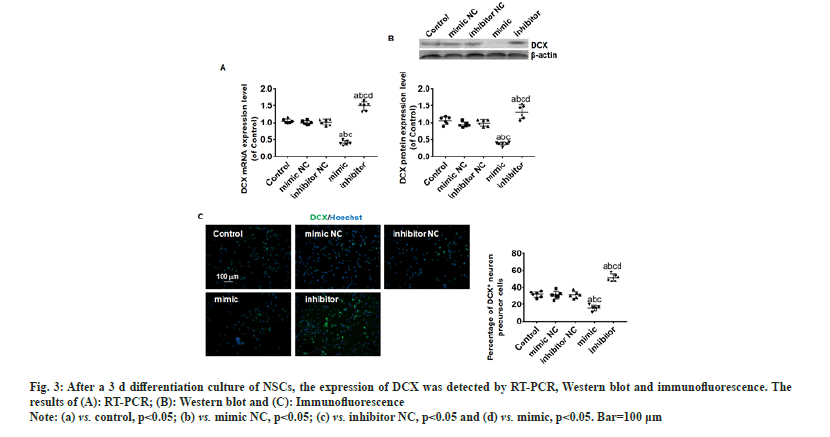
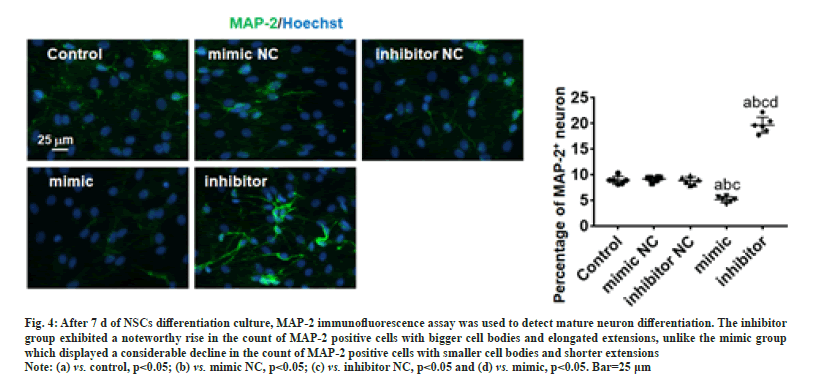
The expression of DCX was analyzed using RT-PCR, Western blot and immunofluorescence after a 3 d differentiation culture of NSCs. The results of Western blot and RT-PCR showed no significant changes in the levels of protein and DCX mRNA of NC group. Besides, a significant decrease (p<0.05) in protein and DCX mRNA levels in the mimic group, whereas a significant rise (p<0.05) in the inhibitor group (fig. 3A and fig. 3B). On the other hand, the outcomes of immunofluorescence exhibited a remarkable rise (p<0.05) in the DCX-positive cells count in the inhibitor group, while significant cells count drop (p<0.05) were noticed in mimic group (fig. 3C). After 7 d of differentiation culture, mature neuron differentiation was assessed using the MAP-2 immunofluorescence assay. Fig. 4 depicts that the inhibitor group exhibited a noteworthy rise in the count of MAP-2 positive cells with bigger cell bodies and elongated extensions (p<0.05), unlike the mimic group which displayed a considerable decline in the count of MAP-2 positive cells with smaller cell bodies and shorter extensions (p<0.05).
Fig. 3: After a 3 d differentiation culture of NSCs, the expression of DCX was detected by RT-PCR, Western blot and immunofluorescence. The
results of (A): RT-PCR; (B): Western blot and (C): Immunofluorescence.
Note: (a) vs. control, p<0.05; (b) vs. mimic NC, p<0.05; (c) vs. inhibitor NC, p<0.05 and (d) vs. mimic, p<0.05. Bar=100 μm.
Fig. 4: After 7 d of NSCs differentiation culture, MAP-2 immunofluorescence assay was used to detect mature neuron differentiation. The inhibitor
group exhibited a noteworthy rise in the count of MAP-2 positive cells with bigger cell bodies and elongated extensions, unlike the mimic group
which displayed a considerable decline in the count of MAP-2 positive cells with smaller cell bodies and shorter extensions.
Note: (a) vs. control, p<0.05; (b) vs. mimic NC, p<0.05; (c) vs. inhibitor NC, p<0.05 and (d) vs. mimic, p<0.05. Bar=25 μm.
Transplantation of NSCs for CNS injury treatment has become a trending research topic over the years. Researchers hope to promote neuronal regeneration, form new synapses, shrink necrotic cavities and restore neural pathways to improve CNS function through stem cell transplantation. In basic research, it has been reported that transplantation of NSCs into animal models of CNS injury can differentiate into neurons, promote the restoration of neural conduction pathways within the CNS and thus contribute to the recovery of CNS function[30-34]. However, the transplantation of NSCs still faces many problems, such as low differentiation rates and transplant safety (such as tumor formation), which significantly affect the effectiveness and application of NSCs. miRNAs, which are 21-22 nucleotides in length, are a variety of naturally occurring, single-stranded, non-coding small RNA molecules, which are highly abundant in the CNS[17,18]. The regulation of NSC proliferation and differentiation in the CNS involves the participation of numerous factors and miRNAs are one of these regulatory factors. miRNAs are one of these regulatory factors. Currently, there are increasing reports on the study of miRNAs regulating the biological characteristics of NSCs[35-38].
In neural precursor cells, DCX, a protein that is associated with microtubules, is expressed extensively after migration during mitosis and is a key protein involved in neuronal differentiation and a major marker of neural precursor cells. To analyze and predict the miRNAs regulating DCX mRNA, we utilized the online software targets can located at http://www.targetscan.org in our study, and one of the results from the analysis was that miR-29a-3p was identified. According to the findings of Pan et al.,[24] their data demonstrates that Eicosapentaenoic Acid (EPA) therapy has the potential to alleviate Lipopolysaccharide (LPS) induced NLRP8 inflammasomes activation in microglial cells. This effect is achieved by activating autophagy through the regulation of the miR-29a-3p/MAPK3 signaling pathway. These results shed light on the underlying mechanism by which miR-29a-3p contributes to the antidepressant effects of EPA. Wang et al.[25] reported lack of oxygen increased the level of circOXNAD1 originating from Human Umbilical Cord Mesenchymal Stem Cell (HuMSCs) in exosomes. Exosomes circOXNAD1 alleviates SCI through sponge miR-29a-3p, thereby improving Forkhead Box Protein O3a (FOXO3a) expression. By binding to its promoter region, SOX10 was found by Chen and co-workers to enhance the miR-29a-3p expression, which targeted Acyl-CoA Ligase 4 (ACSL4) expression and suppressed iron-triggered cell death in hippocampal neurons of Intracerebral Hemorrhage (ICH) mice[27]. The above study indicates miR-29a-3p is related in multiple biological functions of the CNS, but its role in NSC differentiation is still unclear. This research utilized the dual-luciferase reporter system to confirm whether DCX is the intended gene of miR-29a-3p. The findings displayed that miR-29a-3p has no impact on the luciferase activity of the 3'UTR of the mutant DCX gene vector, while restrains the wild-type DCX vector. With these results, it could be concluded that miR-29a-3p specifically targets DCX. Therefore, we speculate miR-29a-3p is involved in neural differentiation of NSCs by regulating the expression of DCX. As per the RT-PCR and Western blot results, a significant reduction in levels of protein and DCX mRNA was noticed in mimic group, while inhibitor group displayed a significant rise in both, compared with NC group. These results suggest that miR-29a-3p suppresses DCX expression. According to the DCX immunofluorescence results, there was a notable upsurge in the DCX-positive cells count in the inhibitor group, while a significant reduction of cells in the mimic group. The results indicate that inhibition of miR-29a-3p expression enhance NSCs to differentiate into neuronal precursor cells. After 7 d of NSCs differentiation culture, MAP-2 immunofluorescence assay was used to detect mature neuron differentiation. In the group that was given the inhibitor, there was a noteworthy increase in the number of cells exhibiting higher levels of MAP-2 expression, accompanied by larger cell bodies and longer extensions. Instead, a notable decrease in the cells of mimic group, accompanied by shorter extensions and smaller cell bodies. The outcomes specify that suppressing expression of miR-29a-3p leads to the mature neurons’ development from NSCs. To conclude, the low miR-29a-3p expression promote differentiation of NSCs into neurons via targeting DCX.
Funding:
The support for this study was provided by grants from the National College Student Innovation and Entrepreneurship Training Program.
Ethical approval:
The Institutional Animal Care and Use Committee of Nantong University approved the animal experiments.
Authors' contribution:
Xin Yi designed the study and proofread this paper. Xinyu Shi wrote this draft paper. Xinyu Shi, Haonan Zhou and Chaolun Dai did experiment. Experimental data was collected and analyzed by Xinyu Shi and Shanhong Li. The final manuscript was read and approved by all the authors.
Conflict of interests:
The authors declared no conflict of interests.
References
- Deng S, Gan L, Liu C, Xu T, Zhou S, Guo Y, et al. Roles of ependymal cells in the physiology and pathology of the central nervous system. Aging Dis 2023;14(2):468-83.
- Rajendran R, Kunnil A, Radhakrishnan A, Thomas S, Nair SC. Current trends and future perspectives for enhanced drug delivery to central nervous system in treatment of stroke. Ther Deliv 2023;14(1):61-85.
[Crossref] [Google Scholar] [PubMed]
- Schumacher M, Guennoun R, Mattern C, Oudinet JP, Labombarda F, de Nicola AF, et al. Analytical challenges for measuring steroid responses to stress, neurodegeneration and injury in the central nervous system. Steroids 2015;103:42-57.
[Crossref] [Google Scholar] [PubMed]
- Ten-Blanco M, Flores A, Cristino L, Pereda-Perez I, Berrendero F. Targeting the orexin/hypocretin system for the treatment of neuropsychiatric and neurodegenerative diseases: From animal to clinical studies. Front Neuroendocrinol 2023;69:101066.
[Crossref] [Google Scholar] [PubMed]
- Tso D, McKinnon RD. Cell replacement therapy for central nervous system diseases. Neural Regen Res 2015;10(9):1356-8.
[Crossref] [Google Scholar] [PubMed]
- Matsui T, Akamatsu W, Nakamura M, Okano H. Regeneration of the damaged central nervous system through reprogramming technology: Basic concepts and potential application for cell replacement therapy. Exp Neurol 2014;260:12-8.
[Crossref] [Google Scholar] [PubMed]
- Bofanova NS, Eliseeva AR, Onchina VS. Modern principles of therapy for patients with spinal muscular atrophy. Zh Nevrol Psikhiatr Im SS Korsakova 2023;123(3):34-40.
[Crossref] [Google Scholar] [PubMed]
- Hart DA. Use of brain-derived stem/progenitor cells and derived extracellular vesicles to repair damaged neural tissues: Lessons learned from connective tissue repair regarding variables limiting progress and approaches to overcome limitations. Int J Mol Sci 2023;24(4):3370.
[Crossref] [Google Scholar] [PubMed]
- Mendonça L, Webster C, Boltze J, Nóbrega C. Advanced (gene and cell) therapies for central nervous system applications. Front Mol Neurosci 2023;16:1140949.
[Crossref] [Google Scholar] [PubMed]
- Tosoni G, Ayyildiz D, Bryois J, Macnair W, Fitzsimons CP, Lucassen PJ, et al. Mapping human adult hippocampal neurogenesis with single-cell transcriptomics: Reconciling controversy or fueling the debate? Neuron 2023;111(11):1714-31.
[Crossref] [Google Scholar] [PubMed]
- Yu H, Yang S, Li H, Wu R, Lai B, Zheng Q. Activating endogenous neurogenesis for spinal cord injury repair: Recent advances and future prospects. Neurospine 2023;20(1):164.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Tan J, Miao Y, Zhang Q. Neurogenesis, a potential target for intermittent hypoxia leading to cognitive decline. Curr Stem Cell Res Ther 2023.
[Crossref] [Google Scholar] [PubMed]
- Tominaga K, Sakashita E, Kasashima K, Kuroiwa K, Nagao Y, Iwamori N, et al. Tip60/KAT5 histone acetyltransferase is required for maintenance and neurogenesis of embryonic neural stem cells. Int J Mol Sci 2023;24(3):2113.
[Crossref] [Google Scholar] [PubMed]
- Huang J, Yi L, Yang X, Zheng Q, Zhong J, Ye S, et al. Neural stem cells transplantation combined with ethyl stearate improve PD rats motor behavior by promoting NSCs migration and differentiation. CNS Neurosci Ther 2023;29(6):1571-84.
[Crossref] [Google Scholar] [PubMed]
- Song S, Li Y, Huang J, Cheng S, Zhang Z. Inhibited astrocytic differentiation in neural stem cell-laden 3D bioprinted conductive composite hydrogel scaffolds for repair of spinal cord injury. Biomater Adv 2023;148:213385.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Xu L, Peng D, Zhu Y, Gu Z, Yao Y, et al. IFN-γ-STAT1-mediated CD8+ T-cell-neural stem cell cross talk controls astrogliogenesis after spinal cord injury. Inflamm Regen 2023;43(1):1-5.
[Crossref] [Google Scholar] [PubMed]
- Mohamadzadeh O, Hajinouri M, Moammer F, Tamehri Zadeh SS, Omid Shafiei G, Jafari A, et al. Non-coding RNAs and exosomal non-coding RNAs in traumatic brain injury: The small player with big actions. Mol Neurobiol 2023;60(7):4064-83.
[Crossref] [Google Scholar] [PubMed]
- Soltani S, Shahbahrami R, Jahanabadi S, Siri G, Emadi MS, Zandi M. Possible role of CNS microRNAs in Human Mpox virus encephalitis-A mini-review. J Neuro Virol 2023;29(2):135-40.
[Crossref] [Google Scholar] [PubMed]
- Zhang Q, Zhang K, Zhang C, Ge H, Yin Y, Feng H, et al. MicroRNAs as big regulators of neural stem/progenitor cell proliferation, differentiation and migration: A potential treatment for stroke. Curr Pharm Des 2017;23(15):2252-7.
[Crossref] [Google Scholar] [PubMed]
- Zhang WH, Jiang L, Li M, Liu J. MicroRNA-124: An emerging therapeutic target in central nervous system disorders. Exp Brain Res 2023;241(5):1215-26.
[Crossref] [Google Scholar] [PubMed]
- Kalhori MR, Soleimani M, Alibakhshi R, Kalhori AA, Mohamadi P, Azreh R, et al. The potential of miR-21 in stem cell differentiation and its application in tissue engineering and regenerative medicine. Stem Cell Rev Rep 2023:1-20.
[Crossref] [Google Scholar] [PubMed]
- Zare A, Salehpour A, Khoradmehr A, Bakhshalizadeh S, Najafzadeh V, Almasi-Turk S, et al. Epigenetic modification factors and microRNAs network associated with differentiation of embryonic stem cells and induced pluripotent stem cells toward cardiomyocytes: A review. Life 2023;13(2):569.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Zhang J, Zhu X, Jiao W, Yang Y, Wu Y, et al. Metformin enhances neural precursor cells migration and functional recovery after ischemic stroke in mice. Exp Brain Res 2023;241(2):505-15.
[Crossref] [Google Scholar] [PubMed]
- Pan JP, Xie JL, Huang LY, Wu QZ, Tang DF, Jin Q, et al. miR-29a-3p promotes the regulatory role of eicosapentaenoic acid in the NLRP3 inflammasome and autophagy in microglial cells. Kaohsiung J Med Sci 2023;39(6):565-75.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Li W, Hao M, Yang Y, Xu Y. Hypoxia-treated umbilical mesenchymal stem cell alleviates spinal cord ischemia-reperfusion injury in SCI by circular RNA circOXNAD1/miR-29a-3p/FOXO3a axis. Biochem Biophys Rep 2023;34:101458.
[Crossref] [Google Scholar] [PubMed]
- Xi T, Wang R, Pi D, Ouyang J, Yang J. The p53/miR-29a-3p axis mediates the antifibrotic effect of leonurine on angiotensin II-stimulated rat cardiac fibroblasts. Exp Cell Res 2023;426(1):113556.
[Crossref] [Google Scholar] [PubMed]
- Chen H, Ren L, Ma W. Mechanism of SOX10 in ferroptosis of hippocampal neurons after intracerebral hemorrhage via the miR-29a-3p/ACSL4 axis. J Neurophysiol 2023;129(4):862-71.
[Crossref] [Google Scholar] [PubMed]
- Liu Z, Yang Z, He L. Effect of miR-29a-3p in exosomes on glioma cells by regulating the PI3K/AKT/HIF-1α pathway. Mol Med Rep 2023;27(3):1-2.
[Crossref] [Google Scholar] [PubMed]
- Wang S, Li N, Meng X. Silencing of the circ-Arhgap5 RNA protects neuronal PC12 cells against injury and depends on the miR-29a-3p/Rock1 axis. Metab Brain Dis 2023;38(4):1285-96.
[Crossref] [Google Scholar] [PubMed]
- Luo ML, Pan L, Wang L, Wang HY, Li S, Long ZY, et al. Transplantation of NSCs promotes the recovery of cognitive functions by regulating neurotransmitters in rats with traumatic brain injury. Neurochem Res 2019;44:2765-75.
[Crossref] [Google Scholar] [PubMed]
- Duan H, Li X, Wang C, Hao P, Song W, Li M, et al. Functional hyaluronate collagen scaffolds induce NSCs differentiation into functional neurons in repairing the traumatic brain injury. Acta Biomater 2016;45:182-95.
[Crossref] [Google Scholar] [PubMed]
- Shao R, Zhang L, Yang H, Wang Y, Zhang Z, Yue J, et al. Autophagy activation promotes the effect of iPSCs-derived NSCs on bladder function restoration after spinal cord injury. Tissue Cell 2021;72:101596.
[Crossref] [Google Scholar] [PubMed]
- Kong D, Feng B, Amponsah AE, He J, Guo R, Liu B, et al. hiPSC-derived NSCs effectively promote the functional recovery of acute spinal cord injury in mice. Stem Cell Res Ther 2021;12(1):172.
[Crossref] [Google Scholar] [PubMed]
- Li X, Peng Z, Long L, Lu X, Zhu K, Tuo Y, et al. Transplantation of Wnt5a-modified NSCs promotes tissue repair and locomotor functional recovery after spinal cord injury. Exp Mol Med 2020;52(12):2020-33.
[Crossref] [Google Scholar] [PubMed]
- Han T, Song P, Wu Z, Wang C, Liu Y, Ying W, et al. Inflammatory stimulation of astrocytes affects the expression of miRNA-22-3p within NSCs-EVs regulating remyelination by targeting KDM3A. Stem Cell Res Ther 2023;14(1):52.
[Crossref] [Google Scholar] [PubMed]
- Cao Y, Liu H, Zhang J, Dong Y. Circular RNA cZNF292 silence alleviates OGD/R-induced injury through up-regulation of miR-22 in rat neural stem cells (NSCs). Artif Cells Nanomed Biotechnol 2020;48(1):594-601.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Wang H, Zhan L, Li Q, Li Y, Wu G, et al. LncRNA FER1L4 promotes differentiation and inhibits proliferation of NSCs via miR-874-3p/Ascl2. Am J Transl Res 2022;14(4):2256.
[Google Scholar] [PubMed]
- Hu G, Xia Y, Zhang J, Chen Y, Yuan J, Niu X, et al. ESC-sEVs rejuvenate senescent hippocampal NSCs by activating lysosomes to improve cognitive dysfunction in vascular dementia. Adv Sci 2020;7(10):1903330.
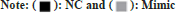 .
.