- *Corresponding Author:
- I. Alotibi
Health Information Technology Department, The Applied College, King Abdulaziz University, Jeddah 22254, Saudi Arabia
E-mail: ialotibi@kau.edu.sa
| This article was originally published in a special issue, “Modern Applications in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(3) Spl Issue “219-229” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Enterobacteriaceae bacteria are clinically important and lead to various diseases associated with high mortality and morbidity, including urinary tract infections, yet their treatment is a challenge due to increasing antibiotic resistance. This study examined the cytotoxicity effects and viability-inducing abilities of mecillinam and fosfomycin antibiotics in the extended-spectrum beta-lactamase Klebsiella pneumoniae strain. The immortalized normal human urothelial cell line was used and the urothelial cell line and bacteria using Klebsiella pneumoniae cultures were prepared. The bacterial association assays with the urothelial cell line were then conducted. The cytotoxic effects were examined using cytotoxicity assays of Klebsiella pneumoniae, cefotaxime-Munich, ampicillinase C, oxacillinase and transmission electron microscopy on the immortalized normal human urothelial cell line. The cytotoxicity of fosfomycin and mecillinam on cell line cytotoxic assays were measured by lactate dehydrogenase, a cell death marker and visualized by hematoxylin. Klebsiella pneumoniae strains showed significantly reduced association with urothelial cells than cefotaxime-Munich strains. The cefotaxime-Munich and ampicillinase C had a higher association with the urothelial cells than the oxacillinase and transmission electron microscopy strains. The cytotoxicity assays indicated that all Klebsiella pneumoniae strains had increased cytotoxicity after 24 or 12 h of incubation. Antibiotic resistance is increased by the bacterial strains that have genes encoding resistance, such as cefotaxime-Munich, ampicillinase C, transmission electron microscopy and oxacillinase.
Keywords
Urinary tract infection, extended-spectrum beta-lactamase, Escherichia coli, Klebsiella pneumoniae, fosfomycin, mecillinam
The Enterobacteriaceae family is Gram-negative bacteria that include Escherichia coli (E. coli), Klebsiella pneumoniae (K. pneumoniae), Salmonella, Shigella, Enterobacter and Citrobacter species. The differentiation of these microorganisms from other bacterial species is that they are catalase-positive, reduce nitrate to nitrite; oxidase negative and produce acid from glucose fermentation. They are also motile and non-spore-forming. The bacteria can be located anywhere, in places such as the human body, water and soil, and have a critical part in maintaining the intestinal flora of animals. Some of these organisms are not normally disease-causing organisms, although they cause opportunistic infections[1]. For instance, E. coli is a commensal inhabitant of the gastrointestinal tract of humans and animals and rarely causes diseases. It is also used widely in industrial and medical applications, such as recombinant Deoxyribonucleic Acid (DNA) technology[2]. However, it is responsible for a wide range of diseases, including enteritis, septicemia and Urinary Tract Infections (UTIs) and other clinical infections like neonatal meningitis. It causes significant mortality and morbidity[1].
K. pneumoniae is second to E. coli, the most common Gram-negative pathogen that can cause healthcare-associated infections such as meningitis, surgical or wound infections, pneumonia and bloodstream infections, as it readily colonizes human mucosal surfaces[3,4]. The bacteria also cause chronic genital ulcerative disease and rhinoscleroma, pyogenic liver abscesses and intra-abdominal infections, and ozena[5]. In addition, the bacteria have been associated with comorbidities such as diabetes, organ transplant morbidities and cancer[6].
The prevalence of bacterial-associated UTIs has increased over the years and these are major causes of morbidities and mortalities in patients with underlying conditions[7]. The urothelium tissue that lines the inner surface of the renal pelvis and the ureters is highly elastic and has electrical resistance. It has 3-5 layers of cells with a thick layer of glycoprotein that functions as a protective layer. The layer also forms a barrier to water, ions, solutes and the entry of pathogens. Besides, the urothelium is a key in the control of urine flow and modulation of the ion and metabolite levels in the urine. However, the persistence of the antibiotics in the urothelial cells leading to cytotoxicity alters the functioning of the urothelial cells[8].
The treatment of UTIs is by using antibiotics and commonly used ones include fosfomycin (a phosphonic acid derivative), ceftriaxone, cephalexin, trimethoprim or nitrofurantoin. Extended-spectrum penicillin antibiotics such as mecillinam can also be used[9]. These bacteria have varying mechanisms of action. However, bacterial resistance to penicillin’s is high. The resistance of the Enterobacteriaceae to antibiotics is propagated by drug efflux pumps, impermeability of the membranes and inducible chromosomal enzymes, and in this way, the resistance of the bacteria to various antibiotics is maintained. In particular, bacterial resistance to beta-lactamase antibiotics has increased over the last decade. The resistance is propagated by the continued expression and horizontal transmission of single genes that encode efficient drug modification enzymes[10]. In particular, K. pneumoniae produces Extended-Spectrum Βeta-Lactamases (ESBL) that have increased their resistance to several antimicrobials. Vading et al. conducted a study to evaluate the risk factors and prognosis in invasive infections caused by K. pneumoniae and E. coli in an area that recorded low antibiotic resistance. The researchers also compared the rates of antimicrobial resistance in various European countries, using the European Antimicrobial Resistance Surveillance Network (EARS-Net) data. 599 adult patients who were admitted to the Karolinska University Hospital with invasive K. pneumoniae infections were matched according to gender and age with patients with infections caused by E. coli in a retrospective review of medical records[3]. The results indicated that K. pneumoniae had higher 90 d mortality than E. coli, 26 % vs. 17 %, although there were no significant differences in the 7 d and 30 d mortality rates. 53 % and 38 % of patients with K. pneumoniae and E. coli infections had malignancy. The study also indicated high ESBL production by the bacteria, factors that continue to enhance antibiotic resistance.
Markedly, the beta (β)-lactamase producing bacteria are sensitive to β-lactamase inhibitors, although they cannot entirely be classified as ESBLs and are therefore called Ampicillinase C (AmpC) β-lactamases. The AmpC β-lactamases are clinically important cephalosporinases that mediate bacterial resistance to cephalosporins, penicillin’s and β-lactamase antibiotics and are encoded on the chromosomes of various Enterobacteriaceae, including the K. pneumoniae and E. coli[11]. Liu et al.[12], who investigated the genotype and phenotype of plasmid-mediated AmpC β-lactamase in K. pneumoniae and its role in its antibiotic resistance, noted increased resistance to cephalosporins among strains that encoded the cephalosporinases. Also, strains that had K. pneumoniae Carbapenemase-2 (KPC-2) genes were resistant to imipenem. There are also Cefotaxime-Munich (CTX-Ms) that are active against broad and narrow-extended spectrum penicillin’s, monobactams, and classical and extended-spectrum cephalosporins[13]. These plasmid-based encoded cefotaximases affect the action of cefotaxime antibiotics[14]. Canton et al.[15] note that the production of the CTX-Ms is propagated by the genetic mobilization units like the insertion sequences and the incorporation of genetic structures like the transposons and class-1 integrons. Oxacillinases (OXAs), are ESBLs encoded by plasmids and hydrolyze the β-lactam rings to make the bacteria resistant to cephalothin, ampicillin, oxacillin and cloxacillin[16]. Therefore, this study seeks to determine the cytotoxicity effects of fosfomycin and mecillinam antibiotics in urothelial cells. These antibiotics are commonly used in the treatment of K. pneumoniae-caused UTIs.
Materials and Methods
Urothelial cell line culture:
The immortalized Normal Human Urothelial Cell line (TRET-NHUC) was kindly provided by Professor Knowles, from the Cancer Research United Kingdom (UK) Clinical Centre at St. James’s University Hospital in Leeds[17]. TRET-NHUC cell line was provided as a frozen sample. That freezing medium (growth medium with 10 % Dimethyl Sulfoxide (DMSO) and 10 % Fetal Calf Serum (FSC)) was disposed by centrifugation at 1000 rpm for 4 min and the medium was discarded. The cells were re-suspended in 5 ml Keratinocyte Growth Medium-2 (KGM2) (purchased from Promo Cell) supplemented with bovine pituitary extract (0.004 mg/ml), epidermal growth factor (0.125 ng/ml), insulin (5 mg/ml), hydrocortisone (0.33 mg/ml), epinephrine (0.39 mg/ml), transferrin, holo (10 mg/ml) and Calcium chloride (CaCl2) (0.06 mM). Then, cells were passed into T25 flasks and incubated at 37° in humidified 95 % air and 5 % Carbon dioxide (CO2). Theoretically, cells become confluent within a week (1? 106 cells/well) and the medium was changed 3 times per week. In the next stage, the cells were ready to use or passed into other T25 or T75 flasks. All passages, used in the experiment, were between passages numbers 4 to 10.
Bacterial culture:
Five different K. pneumoniae strains (K. pneumoniae, CTX-M, AmpC, OXA and Transmission Electron Microscopy (TEM)) were used in the present. The strains were collected from the clinical microbiology laboratory at King Abdulaziz University Hospital by Dr. Dalia Marwan, Director of infection prevention and control. All K. pneumoniae strains were grown in Luria-Bertani (LB) broth overnight at 37° in an Oxygen (O2) incubator[18]. The Optical Density (OD) of bacteria in LB broth was measured by spectrophotometer with a wavelength of 595 nm and an OD of 0.22 was obtained (Wavelength 595, OD 0.22=~108 bacteria/ml). These strains were cultured onto Cystine Lactose Electrolyte Deficient (CLED) agar medium and refreshed every week.
Association assays:
The bacterial association assays with the urothelial cell line were performed following the protocol of Al-Sarraj [9]. The remaining initial bacterial solution with an OD of 0.22 was used for the association assay, which was carried out in 96-well plates where the urothelial cells have been grown (2?105 cells per 0.2 millilitres in each well) and until they became monolayer. Growth media was distributed in the plates and then 200 μl of KGM2 controls were added to the first well, 10-12 bacteria were added to each cell and left for 1 h at 37°. Wells were washed with 200 μl of Phosphate Buffered Saline (PBS). Cells were lysed with Sodium Dodecyl Sulfate (SDS) at 0.5 % final concentration. The number of bacteria associated with TRET-NHUC was calculated by this equation:
Percentage (%) of bacteria associated with cells=Number of bacteria recovered/Total number of urothelial cells?100.
Cytotoxic effect:
These assays were performed to determine the cytotoxic effect of K. pneumoniae, CTX-M, AmpC, OXA and TEM on the TRET-NHUC cell line. In brief, after the incubation of bacteria with the cell line, the supernatants were collected in Eppendorf tubes to be used for cytotoxic effects. The cytotoxic effect was assessed by three different methods. The first method was to stain the cells with hematoxylin staining and assessed them visually. The second method was measuring cell viability by staining with trypan blue to count viable and dead cells. The third method was by measuring Lactate Dehydrogenase (LDH) using a specific kit.
Cytotoxic effects of fosfomycin and mecillinam on cell line measured by LDH:
To determine the cytotoxicity of fosfomycin and mecillinam on cell line, cytotoxic assays were performed. The 96 well plates were inoculated with TRET-NHUC cells until confluence. Different concentrations (64, 32, 16 and 8 µg/ml) of fosfomycin and mecillinam were added on cell line and incubated for 12 h and 24 h at 37° in CO2 incubator to determine the cytotoxicity on the cell line.
The effect of mecillinam and fosfomycin on TRET-NHUC by trypan blue stain:
To determine the effect of mecillinam and fosfomycin on the urothelial cell line, viability assays were performed. The 96-well plates were prepared for TRET-NHUC cells with KGM2 medium until confluent in the tissue culture laboratory. Fosfomycin and mecillinam (32 µg/ml) were added on the infected urothelial cell line for different incubation times (12 h and 24 h) to check the viability of the cells. The medium was discarded after the incubation period and 100 µl of 0.2 % trypan blue was added for 15 min. After incubation time, trypan blue was discarded and cells were checked under the inverted microscope.
Cytotoxic effects of intracellular bacteria on cells measured by LDH:
LDH is a cell death marker to determine the cytotoxicity affecting bacteria in the presence and absence of fosfomycin and mecillinam. Cytotoxic effects were determined by measuring LDH released from cells in the supernatant (Cytotoxicity detection kitplus, Roche).
Cytotoxicity assays were performed. The cell line was inoculated in the 96 well plates. The bacteria (K. pneumoniae, CTX-M, AmpC, OXA and TEM) strains were added to cells for 1h to bind to the cell line. The extracellular bacteria were killed by imipenem. Then, wash once with PBS and 32 µg/ml of fosfomycin and mecillinam were added and incubated for different intervals (12 h and 24 h) at 37° in a CO2 incubator. The supernatants were collected and centrifuged (10 000 rpm for 5 min). Next, 100 µl of supernatants was added to 96-well plates. Moreover, the high and low controls were prepared as follows; the lysis buffer was added to the control cell with a growth medium to release a high amount of LDH, while low control was just controlled cells with a growth medium. Then, 50 µl of the reaction mixture was added to the supernatants and incubated for 15 min at room temperature in dark. Next, the stop solution was added to stop the reactions. In the end, the 96-well plates were shacked for 10 s and read by an Enzyme-Linked Immunosorbent Assay (ELISA) reader (wavelength 595). The cytotoxicity percentage was calculated as follows:
% Cytotoxicity=Sample value-Low control value/High control value-Low control value?100.
Cell visualizing by haematoxylin:
Cytotoxic effects were assessed visually by hematoxylin staining. Briefly, cells were fixed by acetic acid and methanol 1:1 for 15 min, after which supernatants were collected. Then, hematoxylin staining was added to the cells to stain for 30 min. In the end, the staining cells were assessed visually and counted under the microscope.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) v.22 was used to analyze all the data. For significant differences between groups, one-way Analysis of Variance (ANOVA) was utilized. A p value of 0.05 was considered statistically significant.
Results and Discussion
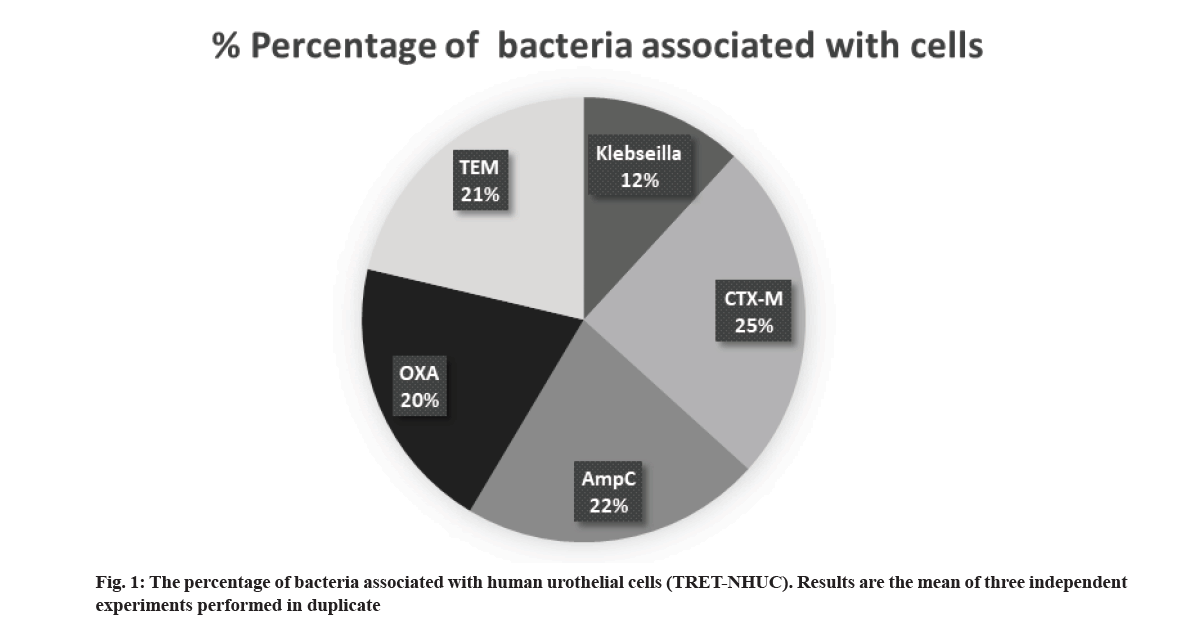
Association assays were performed to determine the ability of K. pneumoniae, CTX-M, AmpC, OXA and TEM to interact with urothelial cells. Our findings revealed that K. pneumoniae strains exhibited significantly lesser association with urothelial cells than CTX-M strains (fig. 1) where the association percentage of K. pneumoniae with urothelial cells was ~12 %, the associated percentage of ESBL-positive strains with urothelial cells lies between ~20 % and 25 %. CTX-M and AmpC were found to have a higher association rate with human urothelial cells. OXA and TEM strains have a lesser association than CTX-M and AmpC. Native K. pneumoniae has the least association with urothelial cells than other strains.
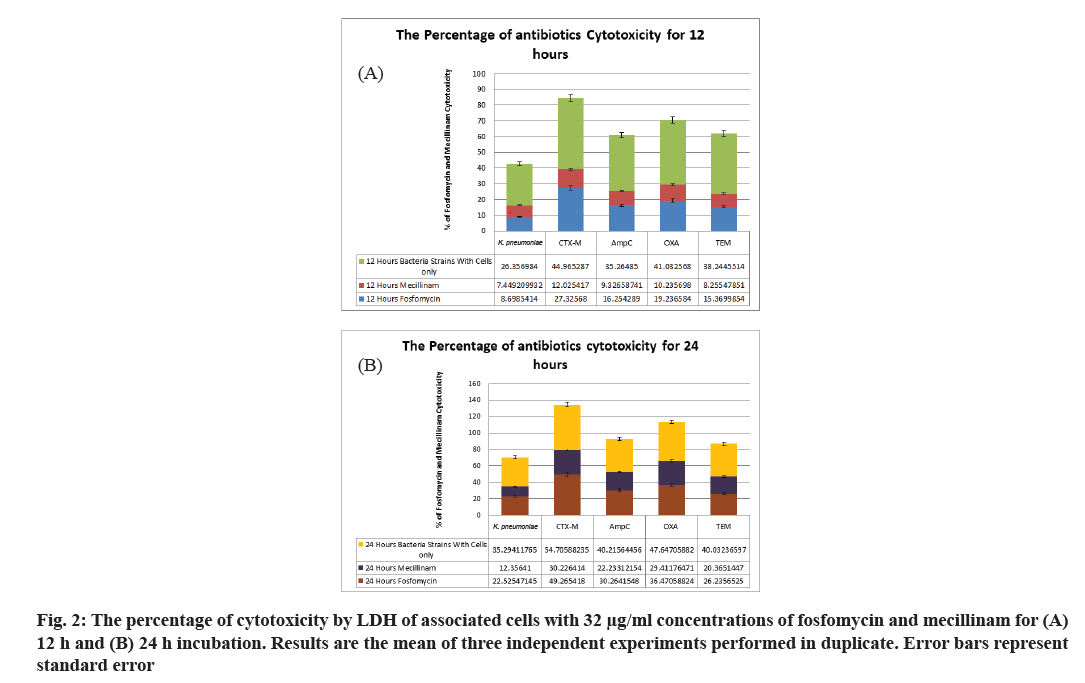
According to the previous experiments, the cytotoxicity assays were performed to study the effect of bacterial strains under investigation on the urothelial cell line in the presence and absence of the two antibiotics. Results showed that ESBL-negative K. pneumoniae increased its cytotoxicity from 10 % to 20 %, which show an increase of 10 %, 24 h after incubation. In contrast, although the cytotoxicity of K. pneumoniae CTX-M at 12 h after incubation was 26 %, it increased significantly with an additional 12 h of incubation. The AmpC strain had increased cytotoxicity at 12 h of incubation, but its increase after another 12 h was higher than that obtained for the TEM strain (fig. 2).
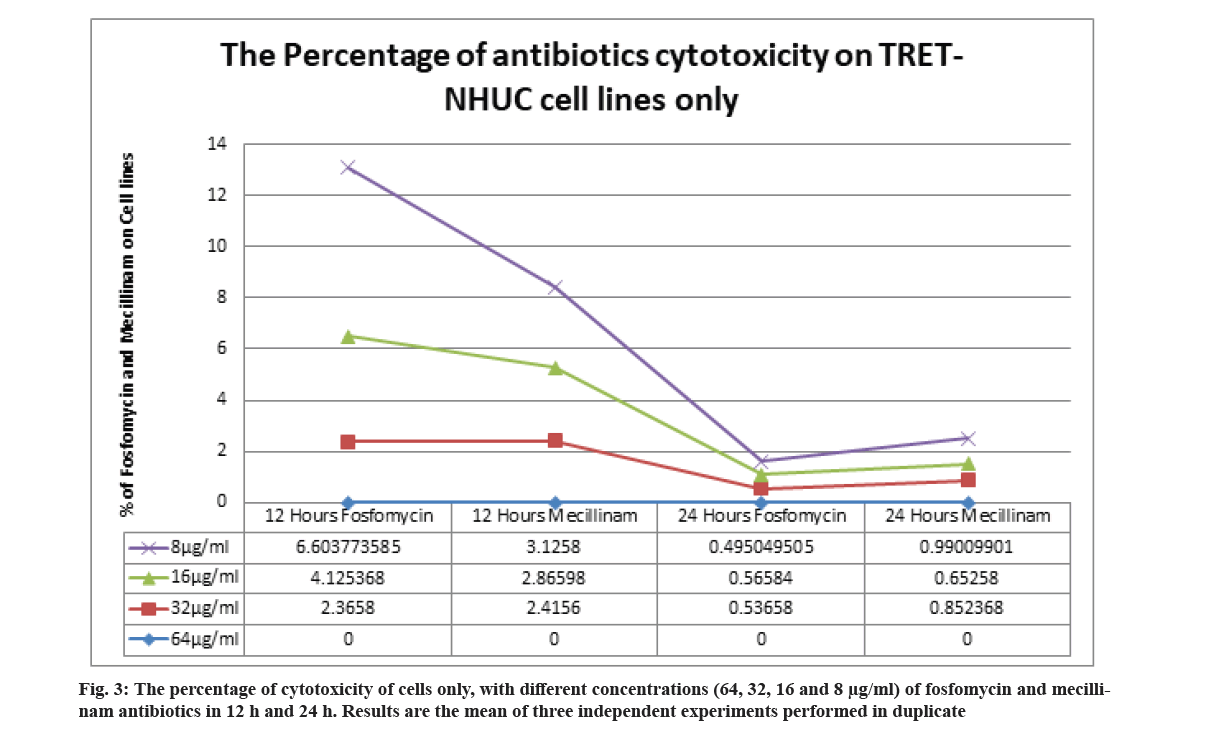
The cytotoxic effects of fosfomycin and mecillinam on the TRET-NHUC cell line were determined after 12 h and 24 h of incubation. The different concentrations of fosfomycin and mecillinam have obtained a very low percentage of cytotoxicity (0-~7 %) on the cell line (fig. 3).
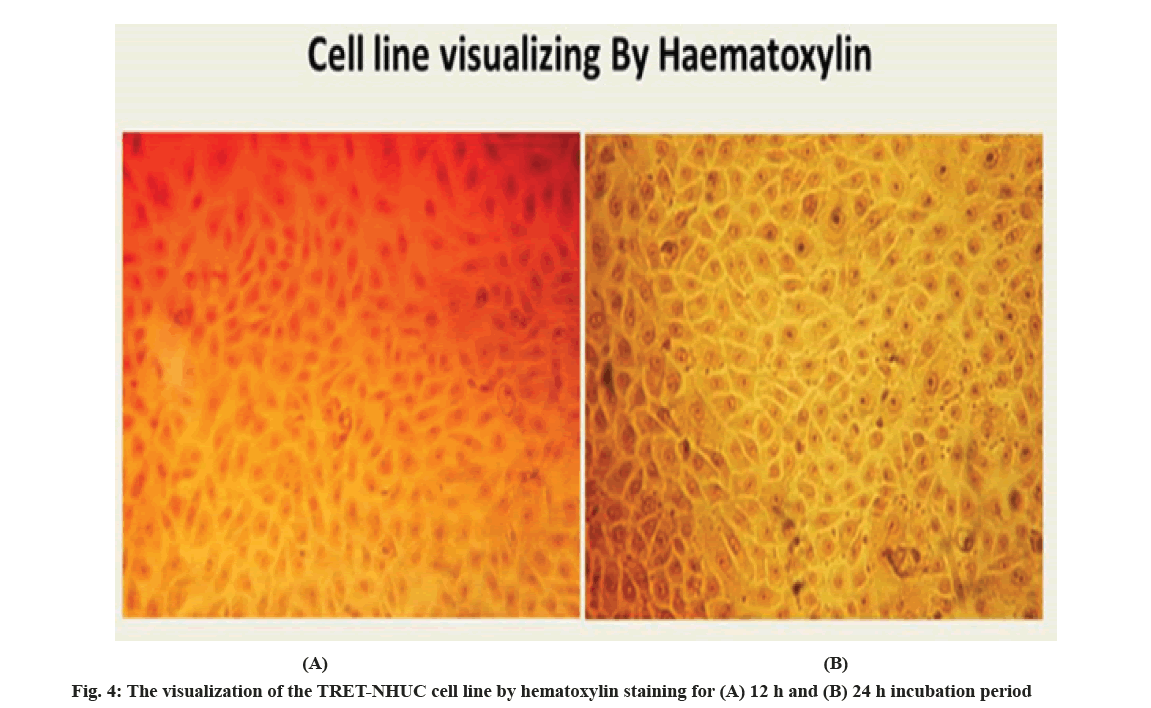
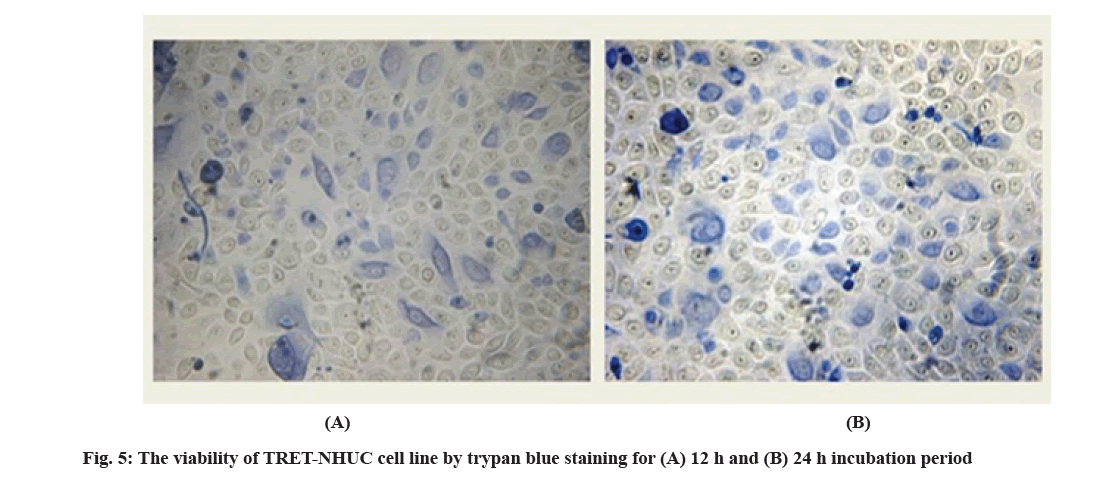
Although, 0.2 % trypan blue staining was performed for the infected TRET-NHUC for 12 h and 24 h incubation, it was observed that the viability of the cells decreased as the incubation time increased. More dead cells were observed in the 24 h of incubation samples. On another hand, the viability of the urothelial cell lines without bacteria strain, stained with haematoxylin is shown in fig. 4 and stained with 0.2 % trypan blue is shown in fig. 5.
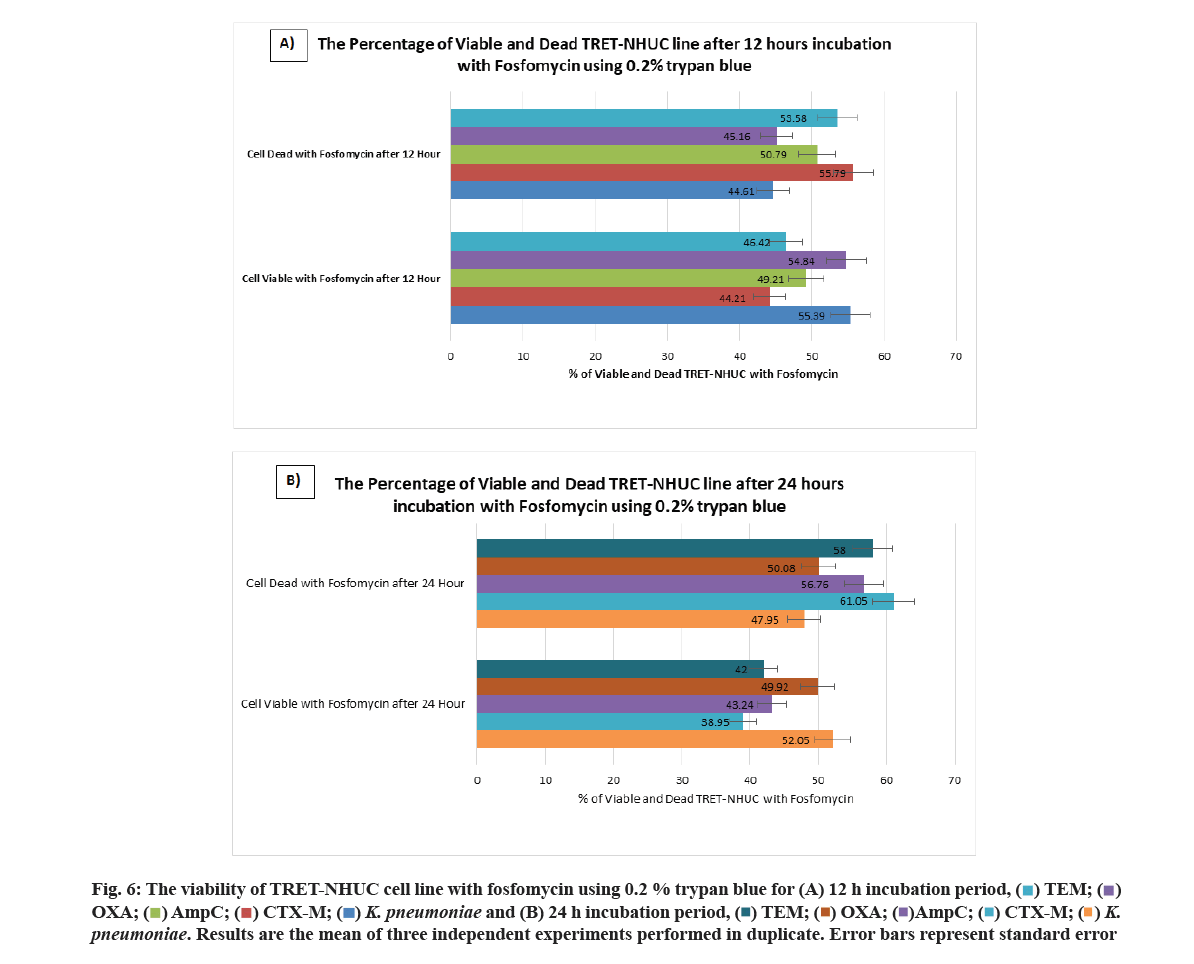
Finally, according to the viability of cells used 0.2 % trypan blue was added. The charts showed the percentage of viable and dead TRET-NHUC lines 12 h incubation with fosfomycin (fig. 6A) and 24 h incubation with fosfomycin (fig. 6B). The charts showed the percentage of viable and dead urothelial cell lines after 12 h incubation with mecillinam (fig. 7A) and 24 h incubation with mecillinam (fig. 7B).
The results of this study sought to determine the cytotoxicity effects on K. pneumoniae infected urothelial tissues by fosfomycin and mecillinam antibiotics indicated that the K. pneumoniae strains showed significantly reduced association with urothelial cells than CTX-M strains. The CTX-M and AmpC had a higher association with the urothelial cells than the OXA and TEM strains. The native K. pneumoniae had the least association with urothelial cells than other strains. The cytotoxicity assays indicated that all K. pneumoniae strains had increased cytotoxicity after 24 h or 12 h of incubation. The cytotoxic effects of the antibiotics were determined after 12 h and 24 h of incubation indicating increased cytotoxicity that led to cell death and reduced cell viability of the TRET-NHUC cell line.
The cytotoxicity assays determine the loss of the cellular and intercellular functions of different cells and are indications of the injuries to cells and tissues. These assays are indicators of tissue injury and are based on different cell functions, including membrane permeability, enzyme activity, Adenosine Triphosphate (ATP) production, adherence of cells, production of co-enzymes and nucleotide uptake activity. The fosfomycin and mecillinam antibiotics that are used in the treatment of UTIs were shown to lead to the cytotoxicity of the TRET-NHUC cell line at different concentrations of 64, 32, 16 and 8 µg/ml, and the viability or death of the cells was indicated by the LDH cell death marker. Mecillinam is an extended-spectrum penicillin antibiotic that binds to penicillin-binding protein-2 and is effective against Gram-negative bacteria. In high concentrations, the antibiotic affects the structure of the cells and these become large and round, although, in standard pressure and temperature, cell division is not affected. However, increased temperatures in high mecillinam concentrations affect the cell division of cells, leading to their lysis[19]. Mecillinam also induces a lethal malfunctioning of the Rod system and Prostaglandin (PG) synthesis that increases its toxicity to cells. This explains why the incubation of the TRET-NHUC cells treated with mecillinam led to increased cell death and reduced numbers of viable cells after 12 h and 24 h.
Similarly, fosfomycin antibiotic is a phosphonic acid derivative that inhibits the synthesis of the cell wall and peptidoglycans in the bacteria through the formation of spheroplast in high osmolarity media. The antibiotic inhibits the flow of the N-acetylamino sugars to the cell wall and stops the action of key enzymes[20]. In high concentrations, the antibiotic affects the impermeability of the bacterial cells and accumulates in the cytoplasm. Studies have shown that the toxicity of fosfomycin is reduced, when it is used in combination with other antibiotics, as it does not bind to plasma proteins and is cleared by the kidneys[21]. Besides, fosfomycin is a water-soluble antibiotic whose absorption occurs through a non-saturable passive diffusion and a saturable carrier-mediated mechanism. The urothelium tissue provides a barrier against the entry of pathogens and disruption of the barrier leads to increased pathogen penetration, leading to the development of UTIs. Also, the disruption of the barrier affects the absorption of the antibiotic, leading to its high concentration in urine. In vivo, cells use oxygen for respiration, but the cultured cells rely on glycolysis, which is catalyzed by cytosolic LDH enzyme, which may be anaerobic due to the absence of haemoglobin[22]. The reduced mitochondrial activity leads to the reduction of pyruvate to lactate by Nicotinamide Adenine Dinucleotide Hydrogen (NADH). Therefore, impaired mitochondrial action in the cultured cell lines can explain the cytotoxic effects noted in the TRET-NHUC cell lines. The action of these antibiotics, fosfomycin and mecillinam on the K. pneumoniae bacteria affected cellular metabolism and increased the cytotoxic effects on the cells.
The results of this study also showed a reduced association of K. pneumoniae strains with the urothelial cells than CTX-M and AmpC strains. The CTX-M and AmpC had a higher association with the urothelial cells than the OXA and TEM strains. The native K. pneumoniae had the least association with urothelial cells than other strains. These results showed a high prevalence of multidrug resistance and ESBL carriage of these strains, highlighting the high antimicrobial resistance in the K. pneumoniae bacterial strains. These results indicated that the most predominant ESBL strains were CTX-M and AmpC. The ESBLs are known for their resistance to the third-generation cephalosporins and resistance to clavulanic acid and are encoded by plasmids. The CTX-M, AmpC, OXA and TEM bacterial strains have elevated resistance against specific antibiotics[23]. For instance, AmpC β-lactamases are clinically important cephalosporinases that mediate bacterial resistance to cephalosporins, penicillin’s and β-lactamase antibiotics and are encoded on the chromosomes of various Enterobacteriaceae[24]. The CTX-Ms are active against broad and narrow-extended spectrum penicillin’s, monobactams and classical and extended-spectrum cephalosporins and have genetic mobilization units that increase their resistance. Markedly, CTX-Ms are the most common ESBL encoding genes[25-27]. The OXAs are encoded by plasmids and hydrolyze the β-lactam rings to make the bacteria resistant to various antibiotics.
The results of this study showed an increasing prevalence of antibiotic-resistant ESBL strains that were consistent with those obtained by Alqasim et al.[28]. Alqasim et al. noted an increasing prevalence of multidrug resistance and ESBL in various uropathogenic isolates from 100[28]. Their results indicated the high antimicrobial resistance in the antibiotics that are commonly used in the treatment of UTIs, highlighting the need for the revision of clinical guidelines and policies for optimal therapy against UTIs. Besides, the results that showed that the CTX-M and AmpC strains are predominant and were consistent with the results obtained by Liu et al.[12], who observed a high prevalence of AmpC K. pneumoniae strains from the 130 isolates they used. Similarly, Mansouri et al.[29] determined the presence of the CTX-M and AmpC strains in clinical isolates of the K. pneumoniae producing ESBLs and noted a high prevalence of these strains. Tan et al.[30] also noted a high prevalence of AmpC strains, 93.7 %, in clinical isolates obtained from 255 patients with UTIs. However, the study by Feizabadi et al.[31] indicated that TEM K. pneumoniae strains were the most prevalent when compared to CTX-M and AmpC’s, although this was attributed to the low ESBL resistance in Iran at the time of the study.
The Enterobacteriaceae family is responsible for a wide range of diseases, including UTIs and is therefore clinically important. In particular, K. pneumoniae causes healthcare-associated infections such as meningitis, surgical or wound infections, pneumonia and bloodstream infections, as it readily colonizes human mucosal surfaces. However, the treatment of these infections is hindered by antibiotic resistance, which is propagated by the continued expression and horizontal transmission of single genes that encode efficient drug modification enzymes. This study examined the cytotoxicity effects and viability-inducing abilities of mecillinam and fosfomycin antibiotics in ESBL and K. pneumoniae strains. The results obtained showed that the cytotoxicity of the antibiotics increased with incubation and after 12 h or 24 h, the number of viable cells were lower than the number of dead cells. Besides, the association of the K. pneumoniae strains with urothelial cells was higher than the association of CTX-M, AmpC, TEM and OXA strains with the urothelial cells. These results indicated the high antibiotic resistance using single genes and the need to implement clinical guidelines and policies that ensure reduced antibiotic resistance and improved patient outcomes.
Author’s contributions:
This work was carried out in collaboration among all authors. Faisal Mohammed Basher Al-Sarraj designed the manuscript, wrote the protocol and supervised the work. Ibrahim Alotibi carried out all the methods and results searches and wrote the first and final draft of the manuscript. All authors read and approved the final manuscript.
Acknowledgements:
I would like to thank the staff of the Microbiology lab in Biology Department at King Abdul-Aziz University for the help during the collection of data.
Conflict of interests:
The authors declared no conflict of interest.
References
- Allocati N, Masulli M, Alexeyev MF, Di Ilio C. Escherichia coli in Europe: An overview. Int J Environ Res Public Health 2013;10(12):6235-54.
[Crossref] [Google scholar] [PubMed]
- Yoon SH, Jeong H, Kwon SK, Kim JF. Genomics, biological features and biotechnological applications of Escherichia coli B: “Is B for better?!”. In: Lee SY editor. Systems biology and biotechnology of Escherichia coli. New York: Springer; 2009. p. 1-7.
- Vading M, Nauclér P, Kalin M, Giske CG. Invasive infection caused by Klebsiella pneumoniae is a disease affecting patients with high comorbidity and associated with high long-term mortality. PLoS One 2018;13(4):e0195258.
[Crossref] [Google scholar] [PubMed]
- Paczosa MK, Mecsas J. Klebsiella pneumoniae: Going on the offense with a strong defense. Microbiol Mol Biol Rev 2016;80(3):629-61.
[Crossref] [Google scholar] [PubMed]
- Chi X, Berglund B, Zou H, Zheng B, Börjesson S, Ji X, et al. Characterization of clinically relevant strains of extended-spectrum β-lactamase-producing Klebsiella pneumoniae occurring in environmental sources in a rural area of China by using whole-genome sequencing. Front Microbiol 2019;10:1-13.
[Crossref] [Google scholar] [PubMed]
- Meatherall BL, Gregson D, Ross T, Pitout JD, Laupland KB. Incidence, risk factors and outcomes of Klebsiella pneumoniae bacteremia. Am J Med 2009;122(9):866-73.
[Crossref] [Google scholar] [PubMed]
- Odoki M, Almustapha Aliero A, Tibyangye J, Nyabayo Maniga J, Wampande E, Drago Kato C, et al. Prevalence of bacterial urinary tract infections and associated factors among patients attending hospitals in Bushenyi district, Uganda. Int J Microbiol 2019;2019:1-8.
[Crossref] [Google scholar] [PubMed]
- Lewis AJ, Richards AC, Mulvey MA. Invasion of host cells and tissues by uropathogenic bacteria. 2nd ed. In: Urinary tract infections: Molecular pathogenesis and clinical management; 2017. p. 359-81.
- Al-Sarraj F. The effect of antibiotics and photodynamic therapy on extended-spectrum beta-lactamase (ESBL) positive of Escherichia coli and Klebsiella pneumoniae in urothelial cells. Saudi J Biol Sci 2021;28(10):5561-7.
[Crossref] [Google scholar] [PubMed]
- Iredell J, Brown J, Tagg K. Antibiotic resistance in Enterobacteriaceae: Mechanisms and clinical implications. BMJ 2016;352.
[Crossref] [Google scholar] [PubMed]
- Jacoby GA. AmpC β-lactamases. Clin Microbiol Rev 2009;22(1):161-82.
[Crossref] [Google scholar] [PubMed]
- Liu XQ, Liu YR. Detection and genotype analysis of AmpC β?lactamase in Klebsiella pneumoniae from tertiary hospitals. Exp Ther Med 2016;12(1):480-4.
[Crossref] [Google scholar] [PubMed]
- Maleki N, Tahanasab Z, Mobasherizadeh S, Rezaei A, Faghri J. Prevalence of CTX-M and TEM β-lactamases in Klebsiella pneumoniae isolates from patients with urinary tract infection, Al-Zahra hospital, Isfahan, Iran. Adv Biomed Res 2018;7.
[Crossref] [Google scholar] [PubMed]
- Ali T, Ali I, Khan NA, Han B, Gao J. The growing genetic and functional diversity of extended spectrum beta-lactamases. Biomed Res Int 2018;2018:1-14.
[Crossref] [Google scholar] [PubMed]
- Cantón R, González-Alba JM, Galán JC. CTX-M enzymes: Origin and diffusion. Front Microbiol 2012;3:110.
[Crossref] [Google scholar] [PubMed]
- Laws M, Shaaban A, Rahman KM. Antibiotic resistance breakers: Current approaches and future directions. FEMS Microbiol Rev 2019;43(5):490-516.
[Crossref] [Google scholar] [PubMed]
- Chapman EJ, Hurst CD, Pitt E, Chambers P, Aveyard JS, Knowles MA. Expression of hTERT immortalises normal human urothelial cells without inactivation of the p16/Rb pathway. Oncogene 2006;25(36):5037-45.
[Crossref] [Google scholar] [PubMed]
- Alsam S, Jeong SR, Sissons J, Dudley R, Kim KS, Khan NA. Escherichia coli interactions with Acanthamoeba: A symbiosis with environmental and clinical implications. J Med Microbiol 2006;55(6):689-94.
[Crossref] [Google scholar] [PubMed]
- Cho H, Uehara T, Bernhardt TG. Beta-lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery. Cell 2014;159(6):1300-11.
[Crossref] [Google scholar] [PubMed]
- González MJ, Da Cunda P, Notejane M, Zunino P, Scavone P, Robino L. Fosfomycin tromethamine activity on biofilm and intracellular bacterial communities produced by uropathogenic Escherichia coli isolated from patients with urinary tract infection. Pathog Dis 2019;77(3):ftz022.
[Crossref] [Google scholar] [PubMed]
- Dijkmans AC, Ortiz Zacarías NV, Burggraaf J, Mouton JW, Wilms EB, van Nieuwkoop C, et al. Fosfomycin: Pharmacological, clinical and future perspectives. Antibiotics 2017;6(4):1-17.
[Crossref] [Google scholar] [PubMed]
- Duewelhenke N, Krut O, Eysel P. Influence on mitochondria and cytotoxicity of different antibiotics administered in high concentrations on primary human osteoblasts and cell lines. Antimicrob Agents Chemother 2007;51(1):54-63.
[Crossref] [Google scholar] [PubMed]
- Low YM, Yap PS, Abdul Jabar K, Ponnampalavanar S, Karunakaran R, Velayuthan R, et al. The emergence of carbapenem resistant Klebsiella pneumoniae in Malaysia: Correlation between microbiological trends with host characteristics and clinical factors. Antimicrob Resist Infect Control 2017;6(1):1-13.
[Crossref] [Google scholar] [PubMed]
- Ye Q, Wu Q, Zhang S, Zhang J, Yang G, Wang H, et al. Antibiotic-resistant extended spectrum β-lactamase and plasmid-mediated AmpC-producing enterobacteriaceae isolated from retail food products and the Pearl River in Guangzhou, China. Front Microbiol 2017;8:96.
[Crossref] [Google scholar] [PubMed]
- Bevan ER, Jones AM, Hawkey PM. Global epidemiology of CTX-M β-lactamases: Temporal and geographical shifts in genotype. J Antimicrob Chemother 2017;72(8):2145-55.
[Crossref] [Google scholar] [PubMed]
- Naseer U, Sundsfjord A. The CTX-M conundrum: Dissemination of plasmids and Escherichia coli clones. Microb Drug Resist 2011;17(1):83-97.
[Crossref] [Google scholar] [PubMed]
- Hall JM, Ingram PR, O'Reilly LC, Inglis TJ. Temporal flux in β-lactam resistance among Klebsiella pneumoniae in Western Australia. J Med Microbiol 2016;65(5):429-37.
[Crossref] [Google scholar] [PubMed]
- Alqasim A, Abu Jaffal A, Alyousef AA. Prevalence of multidrug resistance and extended-spectrum β-lactamase carriage of clinical uropathogenic Escherichia coli isolates in Riyadh, Saudi Arabia. Int J Microbiol 2018;2018:1-9.
[Crossref] [Google scholar] [PubMed]
- Mansouri S, Neyestanaki DK, Shokoohi M, Halimi S, Beigverdi R, Rezagholezadeh F, et al. Characterization of AmpC, CTX-M and MBLs types of β-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli producing extended spectrum β-lactamases in Kerman, Iran. Jundishapur J Microbiol 2014;7(2):e8756.
[Crossref] [Google scholar] [PubMed]
- Tan TY, Ng LS, He J, Koh TH, Hsu LY. Evaluation of screening methods to detect plasmid-mediated AmpC in Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis. Antimicrob Agents Chemother 2009;53(1):146-9.
[Crossref] [Google scholar] [PubMed]
- Feizabadi MM, Delfani S, Raji N, Majnooni A, Aligholi M, Shahcheraghi F, et al. Distribution of bla TEM, bla SHV, bla CTX-M genes among clinical isolates of Klebsiella pneumoniae at Labbafinejad Hospital, Tehran, Iran. Microb Drug Resist 2010;16(1):49-53.
[Crossref] [Google scholar] [PubMed]
 K. pneumoniae and (B) 24 h incubation period,
K. pneumoniae and (B) 24 h incubation period,  K .pneumoniae. Results are the mean of three independent experiments performed in duplicate. Error bars represent standard error
K .pneumoniae. Results are the mean of three independent experiments performed in duplicate. Error bars represent standard error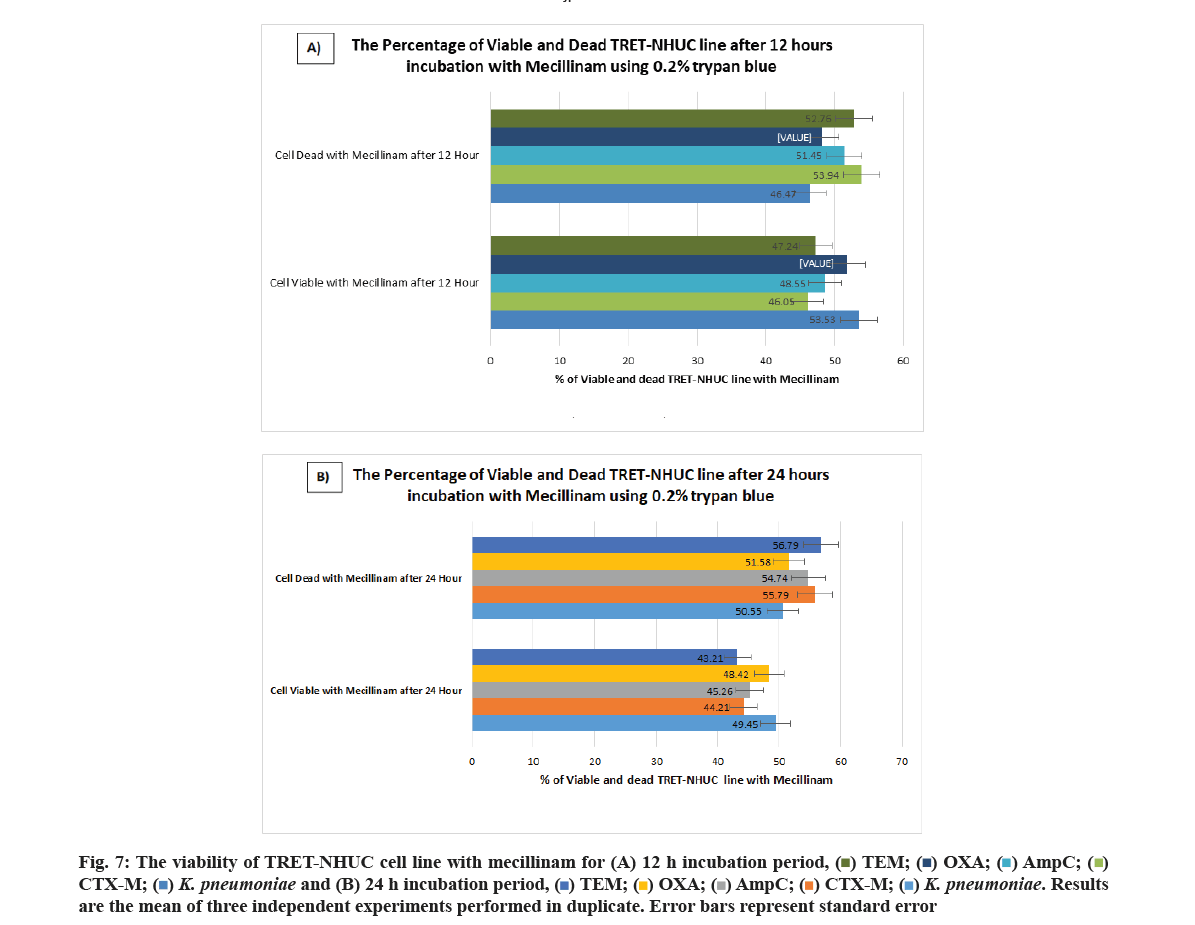
 and (B) 24 h incubation period,
and (B) 24 h incubation period,  K. pneumoniae. Results are the mean of three independent experiments performed in duplicate. Error bars represent standard error
K. pneumoniae. Results are the mean of three independent experiments performed in duplicate. Error bars represent standard error



