- *Corresponding Author:
- Mohammed Z. Nasrullah
Department of Pharmacology and Toxicology, Faculty of Pharmacy, King Abdulaziz University, Jeddah 21589, Saudi Arabia
E-mail: mnasrullah@kau.edu.sa
| Date of Received | 28 July 2023 |
| Date of Revision | 12 February 2024 |
| Date of Accepted | 02 August 2024 |
| Indian J Pharm Sci 2024;86(4):1522-1528 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
A lack of definitive research exists regarding the application of low molecular weight heparin during pregnancy in connection with in vitro fertilization. The objective of this analysis is to determine if low molecular weight heparin enhances the success of pregnancies in women who do not have thrombophilia and are undergoing in vitro fertilization treatment. A comprehensive search was conducted across four databases (PubMed/MEDLINE, ScienceDirect, Scopus, and the Cochrane Library) for articles written in English from 2000 to 2020. All research methodologies evaluating the influence of low molecular weight heparin during in vitro fertilization on live birth rate, clinical pregnancy rate, and miscarriage rate were included. Individual case reports and studies focusing on women with hereditary or acquired thrombophilia, hormonal, or autoimmune disorders were excluded. Out of 3490 screened studies, 5 studies satisfied the selection requirements (consisting of 4 randomized and 1 observational study). The review encompassed a total of 1006 women, with 520 receiving low-molecular-weight heparin and 486 belonging to the control group. No significant differences were observed between the low molecular weight heparin and control groups in any of the evaluated outcomes (live birth rate, clinical pregnancy rate, miscarriage rate). It is important to note that the duration and dosage of low molecular weight heparin exhibited considerable variation across the studies included. While it may have a potential benefit especially in terms of live birth rate and clinical pregnancy rate, the current evidence doesn’t imply a significant influence of low-molecular-weight heparin on in vitro fertilization treatment outcomes in terms of successful pregnancy rates for women without thrombophilia. Additional research is needed to evaluate the actual role of low molecular weight heparin as a treatment during in vitro fertilization procedures.
Keywords
Low molecular weight heparin, in vitro fertilization, systematic review, anticoagulant
Low Molecular Weight Heparin (LMWH) has become the preferred medication in pregnancy cases[1,2]. Women who face an increased risk of blood clot development during pregnancy or after childbirth (postpartum) are often administered LMWH. Moreover, the medication is utilized for other purposes, such as improving perinatal term outcomes[3] and as a vaginal stimulant to induce labor in pregnancies[4]. Systematic evaluations of various studies have demonstrated the safety and efficacy of LMWH use in pregnant women, as it neither crosses the placental barrier nor enters breast milk[5]. A study carried out in a Finnish University Hospital investigated the safety and effectiveness of LMWH during pregnancy and the postpartum period for 180 d following delivery. The study concluded that LMWH is safe for both the mother and fetus during pregnancy. Additionally, the study reported that the risk of postpartum Venous Thromboembolic Events (VTE) was highest in the 1st w following delivery and then rapidly decreased, indicating the need for careful evaluation of VTE risk in the immediate postpartum phase[6].
The role of LMWH in pregnancy has been examined in various studies, yielding inconsistent outcomes[7-9]. A cohort study conducted by Papadakis et al.[10] revealed that out of 818 pregnant women in Greece who were administered LMWH, 98.7 % experienced live births, with 7 VTE instances recorded antepartum and 10 postpartum. Despite demonstrating the safety and efficacy of LMWH, the study did not provide conclusive evidence for some indications. In contrast, a study involving women with a history of 2 consecutive early miscarriages, 1 late miscarriage, and no inherited thrombophilia found that LMWH did not enhance ongoing pregnancy or live birth rates[11].
In vitro Fertilization (IVF) has become an increasingly popular pregnancy technique. However, post-IVF pregnancy success rates among women remain low, ranging from 20 %-35 %[12]. A systematic review by Dentali et al.[13] suggested that LMWH might boost pregnancy and live birth rates for women undergoing IVF. Furthermore, a case where a woman diagnosed with Deep Vein Thrombosis (DVT) 5 d after IVF was treated with LMWH demonstrated an improvement in symptoms until delivery, with no additional complications arising[14]. Serious complications related to IVF treatment can be rare, with ovarian hyperstimulation syndrome being the most prevalent[15]. This syndrome may be linked to a higher risk of thromboembolism, which can be fatal. However, there is scarce information in the literature connecting ovarian hyperstimulation syndrome to the development of VTE and arterial thromboembolism. It has been noted that the risk of thromboembolism doubles in pregnant women following IVF[16]. Still, limited data is available regarding the actual incidence of thromboembolism and IVF. A Swedish systematic review clarifies that the antepartum risk of VTE doubles in pregnancies post-IVF, which is correlated to an extremely high risk of VTE during the first trimester following ovarian hyperstimulation syndrome. The authors suggest administering LMWH during the first trimester to IVF patients who experience ovarian hyperstimulation syndrome[17]. Despite the lack of concrete scientific evidence, the application of LMWH in pregnancy goes beyond VTE management and extends to addressing vascular gestational issues and enhancing IVF pregnancy outcomes. Nevertheless, uncertainties remain about LMWH usage in IVF pregnancies. This systematic review aimed to determine if LMWH improves pregnancy outcomes for women without thrombophilia undergoing IVF.
We conducted a systematic review of the effects of LMWH on pregnant women undergoing IVF by searching electronic databases including PubMed/MEDLINE, ScienceDirect, Scopus, and the Cochrane Library for studies published from January 2000 to October 2020. The search terms used were, 'heparin AND IVF' OR 'heparin AND In Vitro Fertilization'. Our systemic review was developed following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA). Our inclusion criteria covered English written randomized controlled trials, observational studies (both prospective and retrospective cohorts), clinical trials, and quasi-randomized studies that evaluated the influence of LMWH on Live Birth Rate (LBR), Clinical Pregnancy Rate (CPR), and Miscarriage Rates (MR) among women undergoing IVF. We excluded single case reports due to their limited statistical validity. Moreover, animal studies, articles without patient data, conference abstracts, review articles, and studies examining females with hereditary or acquired thrombophilia, hormonal, or autoimmune conditions were excluded. The selection process involved screening titles and abstracts to identify relevant articles for this systematic review. Subsequently, all articles were read, tabulated, and assessed by all authors (Table 1).
| Study | City, country | Duration, age of treated vs. controls | Study design, number of included patients treated vs. controls | Inclusion criteria | Exclusion criteria | Treatment | Outcome measures | Results |
|---|---|---|---|---|---|---|---|---|
| Urman et al.[18] | Istanbul, Turkey | January 2006 and May 2008 Mean age: 34.0±5.0 vs. 34.8±5.8 y |
Randomized open-labelled pilot trial, N=150 (75 vs. 75) | Age ≤38 y. ≥2 failed fresh embryo transfer cycles. No hormonal, coagulation or immunological disorders detected. Normal uterine cavity Normal female and male peripheral karyotype |
Situations requiring anticoagulant treatment Congenital or acquired thrombophilia | Hormonal treatment, enoxaparin sodium 1mg/kg/d up to the 12th w of pregnancy if the test was positive | IR, CPR, LBR in treated vs. controls | IR: (24.5 % vs. 19.8 %; p=0.33) CRP: (45.3% vs. 38.7 %; p=0.41) LBR: (34.7 % vs. 26.7 %; p=0.29) |
| Noci et al.[19] | Pisa, Italy | May 2008 and December 2008, Mean age: 34.7±3.6 vs. 35.1±3.1 y | Pilot study, n=153 (73 vs. 80) | Age <40 y. First IVF /ICSI cycle no hormonal/endocrine, coagulation/haematological immunological disorders detected Absence of chronic diseases Normal uterine cavity No treatment with LMWH in the previous 3 mo |
Not meeting inclusion criteria | Hormonal treatment, dalteparin Sodium 2500 IU/d up to the 9th w of pregnancy if the test was positive | IR, CPR, LBR, MR in treated vs. controls | IR: (15 % vs. 12 %; p=NS) CPR: (26 % vs. 20 %; OR 1.30; 95 % CI 0.58-2.89; p=NS) LBR: (21 % vs. 16 %; OR 1.33; 95 % CI 0.54-3.27; p=NS) |
| Berker et al.[20] | Ankara, Turkey | June 2007 and October 2009, mean age: 31.3±4.9 vs. 31.2±5.0 y | Prospective quasi-randomized, controlled study, n=207 (104 vs. 103) | ≥2 consecutive failed cycles of (ICSI-ET) Normal uterine cavity No hormonal or coagulation disorders |
Coagulation disorders, situations requiring anticoagulant treatment abnormal uterine cavity | Hormonal treatment, enoxaparin sodium 40 mg/0.4 ml/d up to the 12th w of pregnancy if the test was positive | IR, CPR, LBR, MPR in treated vs. controls | IR CPR LBR MP in treated vs. controls IR: (22.6 % vs. 21.1 %; p=NS) CPR: (34.6 % vs. 33.9 %; p=NS) LBR: (30.7 % vs. 29.1 %; p=NS) MPR: (41.6 % vs. 42.8 %; p=NS) |
| Siristatidis et al.[21] | Athens, Greece | February 2012 to June 2017, median age: 36 (25-40) vs. 35 (22-40) y | Multicentral cohort study, N=230 (133 vs. 97) | Age 25-40 y History of ≥2 failed IVF/ICSI. Body Mass Index (BMI) 35 and 19, basal FSH 12 mIU/ml Poor ovarian response NO coagulation and/or autoimmune disorders primary or secondary subfertility |
History of endocrine or metabolic disorders, ovarian cystectomy or oophorectomy, abnormal endometrial cavity and/or receptivity. Hereditary or acquired thrombophilia | Hormonal treatment, enoxaparin sodium 3500 IU/d up to the 3rd trimester of pregnancy if the test was positive | CPR, LBR, MR in treated vs. controls | CPR: (24.8 % vs. 20.6 %; p=0.456) LBR: (17.3 % vs. 14.4 %; p=0.560) MR: (11.3% vs. 9.3%; p=0.624) |
| Lodigiani et al.[22] | Milan, Italy | November 2011 to December 2015 Age groups: ≤ 35 y: 36.3 % vs. 37.4 %; 36-38 y: 40.7 % vs. 39.7 %; 39-40 y: 22.9 % vs. 23 % | Prospective randomized controlled trial, N=266 (135 vs. 131) | Age: ≤40 and ≥18 y fresh IVF cycle. Absence of severe thrombophilia no hormonal/autoimmune disorders | ?Severe thrombophilia, hormonal or autoimmune or haematological disorder, contraindication for heparin chronic treatment with acetylsalicylic acid or steroids | Hormonal treatment, parnaparin sodium 4250 anti-Xa IU/0.4 ml and 6400 anti-Xa IU/0.6 ml for the whole cycle | CPR, LBR, MR in treated vs. controls | CPR: (21.5 % vs. 26.7 %; p=0.389) LBR: (18.5 % vs. 20.6 %; p=0.757) MR: (10.3 % vs. 22.9 %; p=0.319) |
Table 1: Characteristics of the included studies.
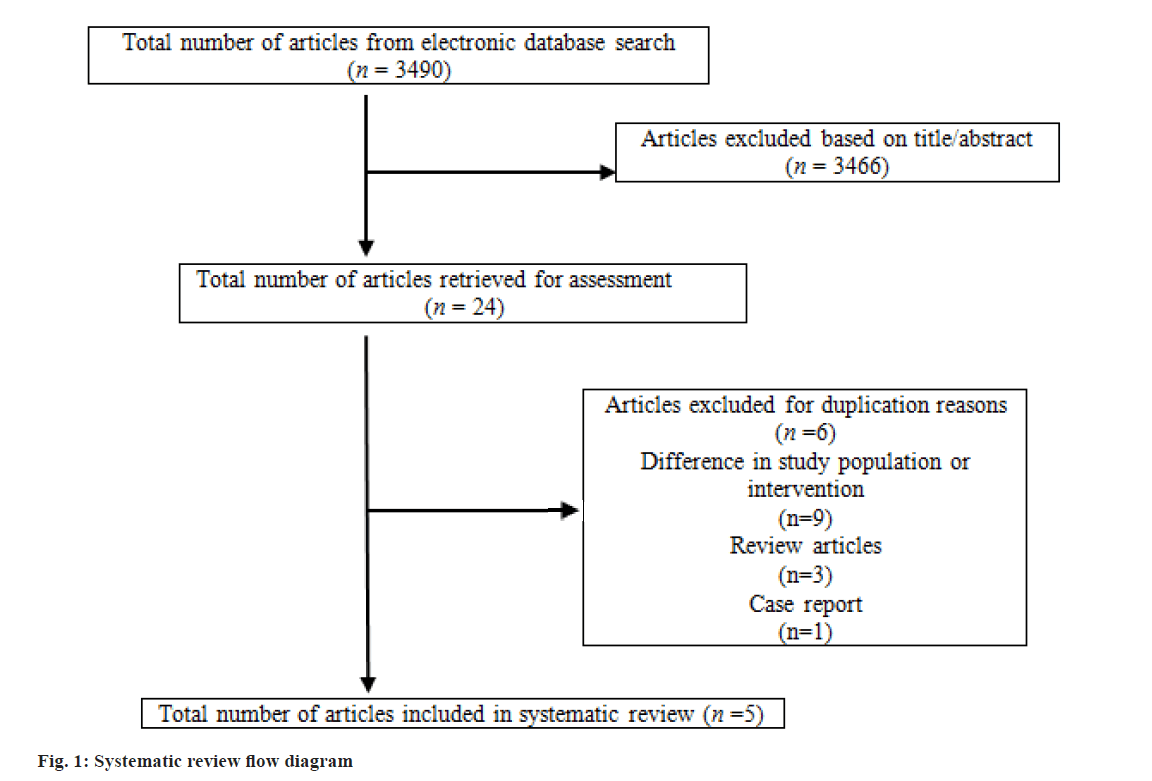
Control subjects were defined as women who met the inclusion criteria but did not receive LMWH treatment. In this investigation, LBR referred to the number of live births after 24 w of pregnancy. CPR was identified as the confirmation of a viable pregnancy via ultrasound 3-4 w following embryo transfer. MR was characterized as pregnancy loss occurring prior to 24 w of gestation. Approval from the Institutional Review Board was not necessary for this research, as it constitutes a secondary analysis. Out of the 3490 studies initially screened, 3466 were excluded based on their title or abstract. The remaining 24 studies were deemed suitable for full-text examination. After eliminating duplicate entries, 5 studies fulfilled the inclusion and exclusion criteria and were incorporated into this review. Fig. 1, displays the flow diagram for the systematic review process, while Table 1 outlines the features of the included studies.
The initiation and duration of the treatment varied across the studies (Table 1). One study commenced treatment the day following oocyte retrieval, and if the pregnancy test was positive, treatment continued until 12 w of pregnancy[18]. If the pregnancy test was negative, the treatment was halted. 2 studies started treatment on the day of oocyte retrieval and discontinued it with a negative pregnancy test or proceeded until either 9 w of pregnancy[19] or 12 w of pregnancy[20]. One study started treatment on the day of embryo transfer and carried on until the 3rd trimester of pregnancy[21]. Finally, one study began treatment for the entire cycle, from a day before the stimulation phase until the procedure's result, and if the pregnancy test was positive, treatment persisted until delivery or the pregnancy's conclusion[22].
The type of LMWH employed differed among the included studies with enoxaparin being the most frequently administered LMWH (Table 1). The LMWH dosage received by participants varied as well (Table 1). One study utilized a dosage of 1 mg/kg/d, another administered 40 mg/0.4 ml of LMWH daily, another employed 2500 IU of LMWH per day, yet another used 3500 IU of LMWH daily, and finally, one study applied either 4250 anti-Xa IU/0.4 ml or 6400 anti-Xa IU/0.6 ml/d [18-22]. The studies incorporated into this systematic review examined the role of LMWH in women undergoing IVF treatment, though there were variations in the inclusion criteria among these studies (Table 1). Two studies explored the influence of LMWH on IVF treatment outcomes[19,22]. One study investigated the role of LMWH in Recurrent Implantation Failure (RIF) and excluded women with clotting disorders[20]. Furthermore, one study evaluated LMWH in sub-fertile women with unsuccessful IVF cycles and excluded those with thrombophilia, coagulation, and endocrine disorders[21]. The last study assessed the effect of LMWH administration during the luteal phase in patients with failed Intracytoplasmic Sperm Injection (ICSI)[18].
All 5 studies in this review evaluated LBR among the study participants. 4 of these studies were randomized trials[18-20,22]. LBRs were not statistically significant in women taking LMWH compared to the control groups. LBRs in LMWH compared to control were 34.7 % vs. 26.7 %, p=0.29; 21 % vs. 16 %, p=Non-statistically Significant (NS); 30.7 % vs. 29.1 %, p=NS; and 18.5 % vs. 20.6 %, p=0.757, respectively. One observational study also reported LBR as an outcome[21]. The LBR outcome in the observational study was also not statistically significant between the 2 groups. LBR was 17.3 % in the LMWH group, compared to 14.4 % in the control group, p=0.56. Each of the 5 studies in this review reported CPR as an outcome measure. In 4 randomized trials, higher CPR was observed in the LMWH-treated group compared to the control group[18-20,22], but the results did not reach statistical significance. CPR values were 45.3 % vs. 38.7 %, p= 0.41; 26 % vs. 20 %, p=NS; 34.6 % vs. 33.9 %, p=NS; and 21.5 % vs. 26.7 %, p=0.389 in the LMWH group compared to control group, respectively. One observational study also reported CPR as an outcome[21]. The CPR outcomes for this study were not statistically significant between women receiving LMWH and the control group. CPR was 24.8 % in the LMWH group, compared to 20.6 % in the control group, p= 0.456.
Out of the 5 studies, only 3 reported MR as an outcome[19,21,22]. In one study,[19] a higher MR was observed in the LMWH group (21 %) compared to the control group (19 %), p=NS. Conversely, another study[22] found a lower MR in the LMWH group (10.3 %) compared to the control group (22.9 %), but the difference was not statistically significant, p=0.319. The observational study also did not report a statistically significant difference in MR between the LMWH group (11.3 %) and the control group (9.3%), p=0.624[21].
In this analysis, we examined whether or not LMWH enhances pregnancy outcomes for women without thrombophilia undergoing IVF. The investigation encompassed four randomized and one observational trial, evaluating the impact of LMWH on LBR, CPR, and MR. The results of our systematic review indicated that, for non-thrombophilic women, LMWH slightly improved LBR and CBR but it did not reach a significant margin compared to control group. The Impact of LMWH on MR was conflicting and also did not reach a statistical significance. To our understanding, only a limited number of studies have explored the effects of LMWH on pregnancy rates for women undergoing IVF. Women with a history of thrombophilia were excluded to determine any potential benefits of LMWH on outcomes due to its non-anticoagulant properties. This review aimed to assess the implications of LMWH's effectiveness in non-thrombophilic IVF pregnancies. As indicated by multiple studies, LMWH has demonstrated potential benefits. Administering a prophylactic dose of LMWH to non-thrombophilic women undergoing IVF has shown promise in increasing the likelihood of successful embryo transfer, implantation rate, and pregnancy rate[19].
A systematic review by Sennström et al.[17] indicated that the risk of thromboembolism doubled after IVF due to physiological changes during pregnancy and ovarian stimulation. Dentali et al.[13] found that LMWH might increase the number of pregnancies and live births in women undergoing IVF. However, a contrasting study suggested that low-dose heparin and/or aspirin as adjunct therapy in non-screened thrombophilic IVF pregnant women did not enhance live birth rates[23]. Two controlled studies utilizing LMWH with varying criteria, types, and doses demonstrated similar outcomes, highlighting LMWH's crucial impact on LBR[19,22]. In contrast, three controlled studies employed the same inclusion criteria but differed in LMWH type and dose[18,20,21]. Berker et al.[20] could not establish any beneficial effect of LMWH on pregnancy outcomes, including women who experienced RIF. However, Urman et al.[18] found that LMWH increased live births by 30 %. Siristatidis et al.[21], which involved women with two or more IVF failure cycles, provided no evidence of LMWH's effect on LBR.
The evaluation of LMWH focused on LBR, CPR, and MR. All 5 studies in this review considered LBR and CPR as outcomes. Nonetheless, none of the studies demonstrated statistical significance. LBR and CPR were higher in the LMWH-treated group than in the control group[18-21]. In contrast, Lodigiani et al.[22] reported lower LBR and CPR in the LMWH-treated group compared to the control group, which may be due to the small sample size and the trials premature conclusion. In the study by Urman et al.[18], researchers observed a roughly 30 % improvement in LBR following LMWH administration, implying a clinically significant trend. However, the authors reported that LMWH exhibited a non-significant trend toward increased LBR/embryo transfer and clinical pregnancy rate in women experiencing their first IVF cycle[19].
In a meta-analysis, researchers examined the impact of LMWH on LBRs and Implantation Rates (IRs) in women experiencing RIF while undergoing IVF[24]. 2 separate studies assessing LMWH effects in women without coagulation disorders demonstrated significant improvement in LBR with LMWH groups[18,20]. The aforementioned meta-analysis study revealed that adding LMWH to IVF/ICSI treatment increased LBR by 79 % in women with 3 RIFs. The employment of LMWH did not yield a statistically significant difference in MR. However, only 3 studies in this review reported MR as an outcome[19,21,22], suggesting that more research is necessary to substantiate scientific evidence for using LMWH to reduce MR. Lodigiani et al.[22] found a lower MR in the LMWH group compared to the control group, while Noci et al.[19] and Siristatidis et al.[21] reported higher MR in the LMWH group. These findings align with those of Yang et al.[25] and Seshadri et al.[26], where no difference in miscarriage rates was identified. However, Potdar et al.[24] observed a 78 % reduction in the miscarriage rate for the intervention group.
The most frequently reported side effects of LMWH include pain, redness, bruising, and allergic reactions at the injection site. However, the most significant side effect is bleeding. Among the 4 studies[18,19,21,22], only minor bruising around the LMWH injection sites was reported as a side effect. In another retrospective study involving pregnant women receiving LMWH, side effects were minimal, but some maternal and infant complications were identified[27]. The strength of this systematic review lies in its comprehensive search strategy, incorporating all pertinent studies from 2000 to 2020, which resulted in a large number of publications with varying exclusion and inclusion criteria. On the other hand, the limitations stem from clinical variations among the studies. These differences can be attributed to disparate study designs, inclusion criteria, and LMWH dosages. The study's limitations also include the heterogeneity of LMWH administration's start time, duration, and dosage. Consequently, the results should be interpreted cautiously.
In summary, our systematic review demonstrated that the current literature has not adequately evaluated the role of LMWH in women undergoing IVF treatment. Based on the published literature, it is challenging to definitively identify which patients might benefit from LMWH therapy. To conclusively determine the role of LMWH in non-thrombophilic pregnant women undergoing IVF, large prospective studies are necessary and could draw upon this study's findings. Furthermore, therapeutic guidelines and essential education on the appropriate use of LMWH should be made available to healthcare professionals.
Conflict of interests:
The authors declared no conflict of interests.
References
- Lindqvist PG, Bremme K, Hellgren M, Swedish society of obstetrics and gynecology (SFOG) working group on Hemostatic disorders (Hem?ARG). Efficacy of obstetric thromboprophylaxis and long?term risk of recurrence of venous thromboembolism. Acta Obstet Gynecol Scand 2011;90(6):648-53.
[Crossref] [Google Scholar] [PubMed]
- Patel JP, Hunt BJ. Where do we go now with low molecular weight heparin use in obstetric care? J Thromb Haemost 2008;6(9):1461-7.
[Crossref] [Google Scholar] [PubMed]
- Gris JC, Mercier E, Quéré I, Lavigne-Lissalde G, Cochery-Nouvellon E, Hoffet M, et al. Low-molecular-weight heparin versus low-dose aspirin in women with one fetal loss and a constitutional thrombophilic disorder. Blood 2004;103(10):3695-9.
[Crossref] [Google Scholar] [PubMed]
- Ekman-Ordeberg G, Åkerud A, Dubicke A, Malmström A, Hellgren M. Does low molecular weight heparin shorten term labor? Acta Obstet Gynecol Scand 2010;89(1):147-50.
[Crossref] [Google Scholar] [PubMed]
- Greer IA, Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: A systematic review of safety and efficacy. Blood 2005;106(2):401-7.
[Crossref] [Google Scholar] [PubMed]
- Galambosi P. Safety and efficacy of low-molecular-weight heparin related to pregnancy and risk of thromboembolism in the postpartum-period. 2017.
- Donnelly J, Byrne J, Murphy K, McAuliffe F. Obstetric outcome with low molecular weight heparin therapy during pregnancy. Ir Med J 2012;105(1):27-29.
[Google Scholar] [PubMed]
- Isma N, Svensson PJ, Lindblad B, Lindqvist PG. The effect of low molecular weight heparin (dalteparin) on duration and initiation of labour. J Thromb Thrombolysis 2010;30:149-53.
[Crossref] [Google Scholar] [PubMed]
- Knol HM, Schultinge L, Veeger NJ, Kluin-Nelemans HC, Erwich JJ, Meijer K. The risk of postpartum hemorrhage in women using high dose of low-molecular-weight heparins during pregnancy. Thromb Res 2012;130(3):334-8.
[Crossref] [Google Scholar] [PubMed]
- Papadakis E, Pouliakis A, Aktypi Α, Christoforidou A, Kotsi P, Αnagnostou G, et al. Low molecular weight heparins use in pregnancy: a practice survey from Greece and a review of the literature. Thromb J 2019;17:1-4.
[Crossref] [Google Scholar] [PubMed]
- Schleussner E, Kamin G, Seliger G, Rogenhofer N, Ebner S, Toth B, et al. Low-molecular-weight heparin for women with unexplained recurrent pregnancy loss: A multicenter trial with a minimization randomization scheme. Ann Intern Med 2015;162(9):601-9.
[Crossref] [Google Scholar] [PubMed]
- Nygren KG, Andersen AN. Assisted reproductive technology in Europe, 1998. Results generated from European registers by ESHRE. Hum Reprod 2001;16(11):2459-71.
[Crossref] [Google Scholar] [PubMed]
- Dentali F, Grandone E, Rezoagli E, Ageno W. Efficacy of low molecular weight heparin in patients undergoing in vitro fertilization or intracytoplasmic sperm injection. J Thromb Haemost 2011;9(12):2503-6.
[Crossref] [Google Scholar] [PubMed]
- Seong SW, Park JH, Shin SK, Jin SA, Park YK, Choi SW. A case with upper extremity deep vein thrombosis after in vitro fertilization. J Cardiovasc Ultrasound 2010;18(3):98-100.
[Crossref] [Google Scholar] [PubMed]
- Bates SM. Anticoagulation and in vitro fertilization and ovarian stimulation. Hematology Am Soc Hematol Educ Program 2014;2014(1):379-86.
[Crossref] [Google Scholar] [PubMed]
- Rova K, Passmark H, Lindqvist PG. Venous thromboembolism in relation to in vitro fertilization: An approach to determining the incidence and increase in risk in successful cycles. Fertil Steril 2012;97(1):95-100.
[Crossref] [Google Scholar] [PubMed]
- Sennström M, Rova K, Hellgren M, Hjertberg R, Nord E, Thurn L, et al. Thromboembolism and in vitro fertilization-a systematic review. Acta Obstet Gynecol Scand 2017;96(9):1045-52.
[Crossref] [Google Scholar] [PubMed]
- Urman B, Ata B, Yakin K, Alatas C, Aksoy S, Mercan R, et al. Luteal phase empirical low molecular weight heparin administration in patients with failed ICSI embryo transfer cycles: A randomized open-labeled pilot trial. Hum Reprod 2009;24(7):1640-7.
[Crossref] [Google Scholar] [PubMed]
- Noci I, Milanini MN, Ruggiero M, Papini F, Fuzzi B, Artini PG. Effect of dalteparin sodium administration on IVF outcome in non-thrombophilic young women: A pilot study. Reprod Biomed Online 2011;22(6):615-20.
[Crossref] [Google Scholar] [PubMed]
- Berker B, Ta?k?n S, Kahraman K, Ta?k?n EA, Atabeko?lu C, Sönmezer M. The role of low-molecular-weight heparin in recurrent implantation failure: A prospective, quasi-randomized, controlled study. Fertil Steril 2011;95(8):2499-502.
[Crossref] [Google Scholar] [PubMed]
- Siristatidis C, Dafopoulos K, Salamalekis G, Galazios G, Christoforidis N, Moustakarias T, et al. Administration of low-molecular-weight heparin in patients with two or more unsuccessful IVF/ICSI cycles: A multicenter cohort study. Gynecol Endocrinol 2018;34(9):747-51.
[Crossref] [Google Scholar] [PubMed]
- Lodigiani C, Dentali F, Banfi E, Ferrazzi P, Librè L, Quaglia I, et al. The effect of parnaparin sodium on in vitro fertilization outcome: A prospective randomized controlled trial. Thromb Res 2017;159:116-21.
[Crossref] [Google Scholar] [PubMed]
- Akhtar MA, Eljabu H, Hopkisson J, Raine-Fenning N, Quenby S, Jayaprakasan K. Aspirin and heparin as adjuvants during IVF do not improve live birth rates in unexplained implantation failure. Reprod Biomed Online 2013;26(6):586-94.
[Crossref] [Google Scholar] [PubMed]
- Potdar N, Gelbaya TA, Konje JC, Nardo LG. Adjunct low-molecular-weight heparin to improve live birth rate after recurrent implantation failure: A systematic review and meta-analysis. Hum Reprod Update 2013;19(6):674-84.
[Crossref] [Google Scholar] [PubMed]
- Yang XL, Chen F, Yang XY, Du GH, Xu Y. Efficacy of low-molecular-weight heparin on the outcomes of in vitro fertilization/intracytoplasmic sperm injection pregnancy in non-thrombophilic women: A meta-analysis. Acta Obstet Gynecol Scand 2018;97(9):1061-72.
[Crossref] [Google Scholar] [PubMed]
- Seshadri S, Sunkara SK, Khalaf Y, El-Toukhy T, Hamoda H. Effect of heparin on the outcome of IVF treatment: A systematic review and meta-analysis. Reprod Biomed Online 2012;25(6):572-84.
[Crossref] [Google Scholar] [PubMed]
- de Sancho MT, Khalid S, Christos PJ. Outcomes in women receiving low-molecular-weight heparin during pregnancy. Blood Coagul Fibrinolysis 2012;23(8):751-5.
[Crossref] [Google Scholar] [PubMed]