- *Corresponding Author:
- N. Thitilertdecha
School of Cosmetic Science, Green Cosmetic Technology Research Group, School of Cosmetic Science, Mae Fah Luang University, Chiang Rai 57100, Thailand
E-mail: nont.thi@mfu.ac.th
| Date of Received | 07 October 2021 |
| Date of Revision | 30 April 2022 |
| Date of Acceptance | 14 September 2022 |
| Indian J Pharm Sci 2022;84(5):1218-1226 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study aimed to evaluate the biological properties of Carissa carandas Linn. pulp and seed extracts as well as its efficacy in cosmetic application. The extracts of Carissa carandas pulp and seeds were analysed for their phenolic content, antioxidant and tyrosine inhibition activities. Emulgels containing the extract were also formulated and evaluated for safety and efficacy in the improvement on skin conditions. The Carissa carandas seed extract contained a higher amount of phenolic content and exhibited more potential in radical scavenging than Carissa carandas pulp extract. All extracts possessed similar potential in tyrosine inhibition activity. The results indicated that the phenolic compounds of Carissa carandas extracts were attributed to its radical scavenging property (R=0.998). The formulated emulgels with Carissa carandas seed extract were stable under accelerated conditions and regarded as safe based on the irritation test. The efficacy results proved that the Carissa carandas seed extract was effective in improving skin moisture; transepidermal water loss, erythema and elasticity as well as lightening the skin’s color. The results recommend that phenolic compounds of Carissa carandas extract could be utilized in cosmetics as an antiaging agent.
Keywords
Carissa carandas Linn., cosmetic efficacy, in vitro, antioxidant, phenolic compounds, tyrosinase
Skin damage, regarded as a cosmetic and dermatological concern, is a result of the natural aging process caused by both intrinsic and extrinsic factors. Wrinkle formation, loss of skin elasticity, excessive dryness and change in skin pigmentation are unfavorable clinical signs of skin aging[1,2]. Although retinoid compounds have been applied topically to reduce age/photo-aged skin, unpleasant effects, such as skin dryness from an increase in Transepidermal Water Loss (TEWL) and photosensitivity are considerable concerns[3]. Various polyphenols including their metabolites play an important role not only to enhance epidermal cell function but also protect against damage under oxidative stress, therefore ameliorating problems with skin aging. Well known potent antioxidants, such as anthocyanin’s from pigmented rice, could reduce inflammation and enhance collagen production[4]. Metabolites of pine bark’s condensed tannins, which are potential antioxidants, could inhibit Matrix Metalloproteinase (MMP)[5]. In addition, the hydroxyl group in polyphenols is an important functional group in inhibiting tyrosinase enzyme[6].
Carissa carandas Linn., commonly known as karanda, carunda or namdaeng belongs to the Apocynaceae family, is a tropical fruit widely distributed in South- East Asia, including Thailand. The Carissa carandas fruits commonly used as a source of dietary supplement due to its high nutritional value[7]. The Carissa carandas fruit has also been studied for its pharmacological potential, including its hepatoprotective[8], antibacterial, antifungal[9], anti-diabetic[10], anti-inflammatory[11] and antioxidant properties[12,13]. The phytochemical constituents of Carissa carandas fruit are amino acids, triterpenoids, alkaloids, phytosterols, ascorbic acid and phenolic compounds[14,15]. Among phytochemicals, Carissa carandas fruit is rich in phenolic, flavonoids and anthocyanin with potential antioxidant properties not only to neutralize hydroxyl radicals, superoxide radicals, nitric oxide but also chelate metal ions[16,17]. Agroindustry generates a vast amount of waste from fruits, including the seeds and peels, resulting in a detrimental effect on the environment. In past decades, these waste materials were studied for their potential application as nutritional supplements, food additives as well as ingredients in cosmetics. The presences of numerous phytochemicals, especially phenolic compounds, are also observed in the Carissa carandas seeds. The Carissa carandas fruits have many biological properties, nutritional and medicinal purposes have been revealed, however; there is less scientific information concerning the determination of phenolic compounds in Carissa carandas seeds. In addition, there are no published studies on the applications of Carissa carandas fruit extract as a cosmetic ingredient. Therefore, the Carissa carandas pulp and seeds were evaluated in this study for total phenolic content, antioxidant activity and tyrosinase inhibition activity. The application of Carissa carandas extract as a cosmetic ingredient as an emulgel was also evaluated for its safety and efficacy in anti-aging.
Materials and Methods
Materials and chemicals:
Carissa carandas Linn. fruit was collected from the Nakhon Ratchasima province of Thailand in September, 2015. The Carissa carandas fruit was separated into pulp and seeds, cleansed and dried with a circulating air dryer at 50º. The dried sample was powdered and then sieved through a sieve size with a size 250 mesh. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH.), L-3,4-Dihydroxyphenylalanine (L-DOPA), Folin- Ciocalteu’s reagent, gallic acid, 6-Hydroxy-2,5,7,8- Tetramethylchroman-2-Carboxylic Acid (Trolox), kojic acid and tyrosinase were obtained from Sigma-Aldrich (St. Louis, Missouri, United States of America (USA)). All other chemicals were analytical grade whereas other chemicals required for the cosmetic formulations were cosmetic grade.
Preparation of extracts from Carissa carandas Linn. samples:
In brief, the 10 g of each Carissa carandas Linn. sample were suspended in ethanol with liquid-solid ratio of 20:1 (ml:g) and then shaken with an orbital shaker (180 rpm) for 6 h at ambient temperature. After filtering each extract through Whatman® filter paper no.1, they were used for the analysis of total phenolic content, antioxidant and anti-tyrosinase properties. The selected extract solution was evaporated and lyophilized to obtain the extract powder for further used as an active ingredient in the emulgel formulations.
Determination of the total phenolic content:
The total phenolic content was estimated based on Folin-Ciocalteu’s assay[18]. Briefly, samples (1 ml) were mixed with deionized water (7 ml). After that, Folin- Ciocalteu’s reagent (0.5 ml) was added, followed by 200 g/l of sodium carbonate solution (1.5 ml). The absorbance of 760 nm was measured after the mixtures was shaken and incubated at ambient temperature for 1 h. The results were expressed as (mg Gallic Acid Equivalents (GAE)/g) sample.
Antioxidant assay:
The scavenging activity of DPPH. was evaluated based on the method previously described[19]. A 0.1 mmol/l DPPH. solution in ethanol (3 ml) was mixed with 1 mg/ml of sample solution (l ml). A control comprised of ethanol (1 ml) and DPPH. solution (3 ml). The absorbance was measured at 517 nm using an Ultraviolet-Visible (UV-Vis) spectrophotometer (SP- 880, MetertechInc) after the mixture was incubated at ambient temperature in dark conditions for 30 min. The DPPH. scavenging activity was calculated according to the following equation:
% DPPH. scavenging activity=[(A0-A1)/A0]×100 (1)
Where A0 was the absorbance of the control and A1 was the absorbance of the mixture containing the sample. The results were expressed as (mg Trolox Equivalents (TE)/g) sample.
Tyrosinase inhibition assay:
The inhibition of tyrosinase enzyme was evaluated based on the method described previously[20]. In brief, a 1 mg/ml of sample solution (20 μl) were mixed with a phosphate buffer (50 mM, pH 6.8, 110 μl) and 1000 U/ ml of tyrosinase (20 μl). A 10 mmol/l L-DOPA solution (50 μl) was added after the mixture was incubated at 37° for 10 min. After further incubation at 37° for 10 min, the absorbance was measured at 475 nm. The control, containing a phosphate buffer (50 mM, pH 6.8, 130 μl), 1000 U/ml of tyrosinase (20 μl) and 10 mM L-DOPA (50 μl) was prepared. The percent inhibition was calculated according to the following equation:
% Tyrosinase inhibition=[(A0-A1)/A0]×100 (2)
Where A0 was the absorbance of the control and A1 was the absorbance of the mixture containing the sample. The results were expressed as (mg Kojic Acid Equivalents (KE)/g) sample.
Formulations:
The formulations of the placebo (F1) and the experimental formulations (F2 and F3) were developed with the list of ingredients in Table 1. Several physicochemical parameters of the formulations, including pH, viscosity and color, were determined for their stability under accelerated conditions. A centrifugation test was performed at 5000 rpm at 25° for 30 min (Spectrafuge™, USA). Phase separation at the end of the centrifugation was an indicator of cosmetic instability. The stability under accelerated conditions through a heating-cooling cycle stability test was performed in three cycles which were stored at 4° and 45° for 48 h each. The physicochemical properties were assessed including pH value by pH meter (Mettler Toledo, Columbus, Ohio, USA) and viscosity by viscometer (Book field, Middleborough, Massachusetts, USA) (No.5, 160 rpm) were cosmetic grade (Namsiang, Thailand).
| Ingredients | Emulgel (% w/w) | ||
|---|---|---|---|
| F1 (Placebo) | F2 | F3 | |
| Deionized water | 77.2 | 77.15 | 77.1 |
| Carbopol ultrez 21 | 0.7 | 0.7 | 0.7 |
| Xanthan gum | 0.3 | 0.3 | 0.3 |
| Isopropyl myristate | 2 | 2 | 2 |
| Capric/caprylic triglyceride | 2 | 2 | 2 |
| Cyclopentasiloxane | 2 | 2 | 2 |
| Glyceryl monostearate | 0.4 | 0.4 | 0.4 |
| Cetyl alcohol | 1 | 1 | 1 |
| Propylene glycol | 10 | 10 | 10 |
| Polyethylene glycol-40 castor oil | 0.4 | 0.4 | 0.4 |
| Phenoxyethanol | 1 | 1 | 1 |
| Ethylhexyl p-methoxycinnamate | 2 | 2 | 2 |
| Triethanolamine | q.s. | q.s. | q.s. |
| C. carandas L. extract | - | 0.05 | 0.1 |
Table 1: The Ingredients of the Emulgel Formulations
Clinical treatment:
The ethical issues concerning this study were approved by the Ethics Committee of Mae Fah Luang University (REH-61038). A total of 15 healthy women of ages ranging between 20 y and 25 y old were enrolled into the clinical study. All volunteers who were eligible had no dermatological problems or history of skin allergy and were instructed not to use any skin treatment products on the test areas for 4 w prior to enrollment.
The details of the study were shared with all of the volunteers before they signed the consent form. Formal, informed consent was obtained from volunteers before the study.
An irritation test of the experimental formulations and a placebo was performed utilizing the closed patch test on fifteen volunteers. To determine skin irritation, 10 g/l Sodium Lauryl Sulfate (SLS) was used as the positive control while deionized water was used as the negative control. The Finn chambers were individually loaded with the test samples and then closely placed on the inner upper forearm for 24 h. After the patch was removed, redness, edema or itching was subsequently observed after 30 min and 24 h. The level of skin irritation was interpreted and expressed as the Mean Irritation Index (MII)[21]. A single-blind study with a placebo was designed to study the efficacy of the formulations. The experimental formulations and the placebo were provided blindly to the volunteers for topical application twice a day (morning and evening) for each formulation (one square inch) on a UV exposure protected site on the inner forearm. During the study, the volunteers were asked not to use any topical products on the test areas. The measurement parameters, TEWL (Tewameter), skin moisture (Corneometer), melanin and erythema (Mexameter) and skin elasticity (Cutometer) were evaluated at the base lines of 14 d and 28 d. The condition of the measurement room was controlled at 50 %-60 % relative humidity and 25°. After the test areas were cleansed with mild soap, volunteers rested in a controlled room for 30 min prior to being measured for the skin parameters.
Statistical analysis:
The Statistical Package for Social Sciences (SPSS) Program version 21 (SPSS Inc, Chicago, Illinois, USA) was used for statistical analysis. The independent t-test and paired t-test were used to compare the differences between samples and differences between before and after the efficacy test. The statistical differences were considered significant at p<0.05.
Results and Discussion
In order to study the biological properties of Carissa carandas extracts and their applications in cosmetics, the extracts were evaluated for total phenolic content, DPPH. scavenging activity and tyrosinase inhibition activity. The extractive yields of Carissa carandas pulp and seeds were 35.27 %±0.53 % and 9.58 %±1.70 % (w/w), respectively.
An appropriate extract that contained a high amount of phenolic compound and exhibited potential biological activities was selected to be used as the active ingredient in the emulgel formulation. In addition, the formulation was tested for its safety and efficacy in anti-aging. The total phenolic content of the Carissa carandas extracts were estimated using Folin-Ciocalteu’s assay based on the capability of the compounds to transfer electrons. As can be seen in Table 2, the phenolic compounds were observed in both the pulp and seeds of Carissa carandas. It was found that the Carissa carandas seed extract possessed a higher amount of phenolic compounds when compared to the pulp extract (p<0.05) and these results were in agreement with previous study[22].
| Samples | Total phenolic content | DPPH radical scavenging | Tyrosinase inhibition |
|---|---|---|---|
| (mg GAE/g sample) | (mg TE/g sample) | (mg KE/g sample) | |
| C. carandas pulp extract | 16.18±2.21b | 16.86±2.31b | 25.32±4.81a |
| C. carandas seed extract | 52.20±1.15a | 182.76±2.15a | 18.77±0.27a |
Note: (a,b)means the column followed by different letters are significantly different (p<0.05)
Table 2: Phenolic Contents and Biological Properties of the Carissa Carandas Extracts
The Carissa carandas extracts were evaluated for their capability to scavenge DPPH. radicals. As the results show in Table 2, Carissa carandas seed extract exhibited more potential radical scavenging activity (182.76 mg TE/g sample) than the pulp extract (16.86 mg TE/g sample) (p<0.05). A higher potential in the reducing ability of the Carissa carandas seeds compared to Carissa carandas pulp has also been mentioned in prior research[14]. Considering the correlation between total phenolic content and DPPH. scavenging activity, it was a substantial correlation (R2=0.998) which was in agreement with previous study[23]. This result indicated that the phenolic compounds of Carissa carandas extracts potential attributed to DPPH. scavenging activity.
The inhibition of tyrosinase is one of the mechanisms which inhibit melanogenesis depressing melanin synthesis. Thus, the Carissa carandas pulp and seed extracts were evaluated for their capability to inhibit tyrosinase, indicating their potential capability as skin lightening agents. As can be seen in Table 2, the tyrosinase inhibition properties were found in both Carissa carandas pulp and seed extracts, ranging between 18.77-25.32 mg KE/g samples, however, there was no difference in their inhibition property (p>0.05). Based on total phenolic content and biological properties, the Carissa carandas seed extract was selected as the cosmetic ingredient in the emulgels in this study.
The products in this study were emulgel-based formulations. The preparation of the various emulgel formulations was conducted according to the ingredient list in Table 1. The placebo (F1) contained no Carissa carandas seed extract, while experimental formulations, F2 and F3, were mixed with 0.05 % and 0.10 % (w/w) extract, respectively. The formulations were evaluated for their stability. A preliminary stability test found that all formulations were stable, with no phase separation after a centrifugation test.
They were also tested for their stability under accelerated conditions through a heating-cooling cycle stability test. As can be seen in Table 3, the pH values of all formulations did not change significantly (p>0.05). The viscosity of all formulations slightly changed in a narrow, acceptable range during and after the test.
| Cycle | pH | Viscosity (cP) | ||||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F1 | F2 | F3 | |
| 0 | 5.53±0.02 | 5.52±0.01 | 5.58±0.02 | 1430±16 | 1480±46 | 2085±59 |
| 1 | 5.45±0.01 | 5.41±0.01 | 5.46±0.02 | 1292±24 | 1332±14 | 1900±91 |
| 2 | 5.46±0.01 | 5.41±0.02 | 5.49±0.03 | 1276±54 | 1333±40 | 1924±83 |
| 3 | 5.39±0.04 | 5.35±0.03 | 5.42±0.03 | 1243±46 | 1300±27 | 1908±90 |
Note: Values are expressed as mean±Standard Deviation (SD)
Table 3: Physicochemical Properties of the Formulations under the Heating-Cooling Cycle Stability Test
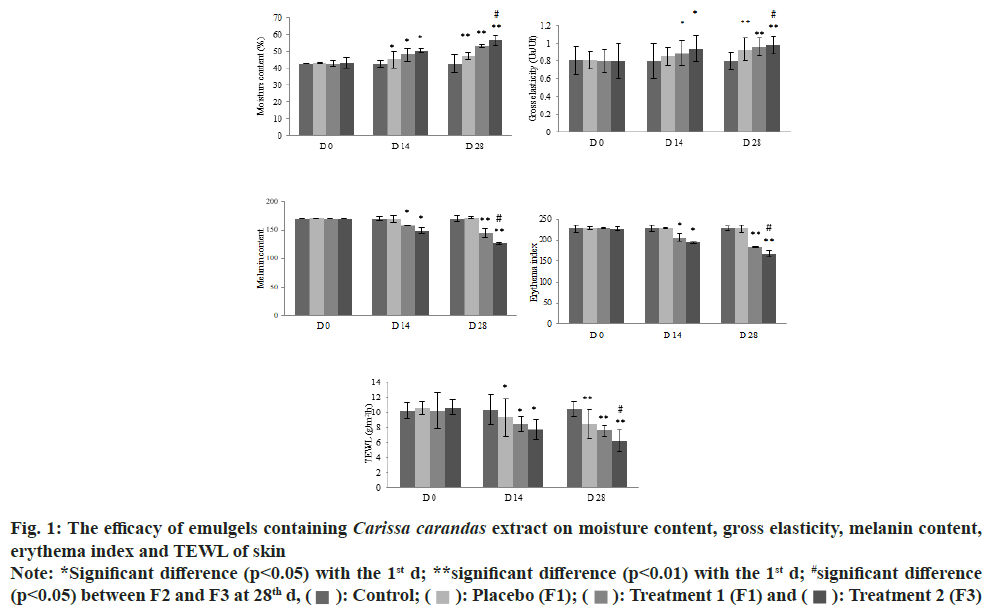
The clinical trial was carried out to evaluate the efficacy of the Carissa carandas emulgels in term of their improvement on skin parameters. Fifteen eligible volunteers who met the inclusion criteria of the study enrolled in the clinical trial. An irritation test based on closed patch test evaluated the safety of the products. The MII score was absolute zero, indicating Carissa carandas emulgel and placebo formulations were Non-Irritation (NI)/good cutaneous compatibility. All formulations were provided blindly to the volunteers who were asked to perform the skin analysis for 28 d. A skin measurement was performed at the base line and 14 d intervals to evaluate the skin parameters of the 12 volunteers who completed the study. The improvements in skin parameters were interpreted and expressed as the mean difference from the base line.
As the data shows in fig. 1 and Table 4, there was no improvement in any of the skin parameters for the control, skin treated with no product, during the study period (p>0.05). The formulas containing 0.10 % and 0.05 % (w/w) Carissa carandas extract as well as the placebo improved skin moisture and reduced TEWL in 14 d. The results showed that the Carissa carandas emulgels not only increased skin moisture and elasticity, but also decreased TEWL, as well as melanin and erythema values, in a dose-dependent manner (p<0.05). This improvement was also continuously observed from 14 d to 28 d. As can be seen in the Table 1, the placebo could enhance skin elasticity after 28 d however, emulgels containing Carissa carandas more greatly enhanced skin elasticity in a dose-dependent manner (p<0.05). These results were in agreement with those of all skin parameters. Although the placebo formula could ameliorate skin moisture, TEWL and skin elasticity, this formula was ineffective in reducing melanin and erythema values during the study, as shown in Table 4 (p>0.05). In considering its efficacy, formulas containing Carissa carandas extract at 0.10 % (w/w) were the most effective in improving skin parameters. This was followed by the 0.05 % formula and the placebo. Therefore, the Carissa carandas extract resulted in significant improvement in all skin parameters when compared with the base emulgel (placebo).
| Parameters | Formulations | |||
|---|---|---|---|---|
| Control | F1 | F2 | F3 | |
| Moisture | ||||
| 14th d | 0.119±0.597d | 2.062±0.979c | 5.145±2.581b | 7.562±2.701a |
| (-1.070, 1.400) | (0.67, 3.70)* | (0.80, 9.59)* | (4.27, 12.29)* | |
| 28th d | 0.205±0.476d | 4.250±1.378c | 10.241±3.247b | 13.537±2.687a |
| (-0.460, 1.330) | (2.40, 6.94)*# | (5.55, 16.24)*# | (8.25, 17.16)*# | |
| Elasticity | ||||
| 14th d | -0.012±0.023d | 0.039±0.068b | 0.091±0.034b | 0.143±0.077a |
| (-0.055, 0.027) | (-0.069, 0.138) | (0.014, 0.132)* | (0.025, 0.256)* | |
| 28th d | -0.011±0.034d | 0.119±0.069b | 0.159±0.045ab | 0.182±0.072a |
| (-0.061, 0.036) | (0.025, 0.236)*# | (0.095, 0.236)*# | (0.084, 0.283)*# | |
| Melanin | ||||
| 14th d | -0.174±1.821c | 0.585±2.489c | -12.583±13.083b | -22.19±14.101a |
| (-2.830, 4.140) | (-3.27, 4.39) | (-40.00, 6.00)* | (-47.55, -6.04)* | |
| 28th d | -0.254±1.626c | 0.905±6.033c | -25.296±15.429b | -44.577±18.198a |
| (-2.700, 3.4700) | (-5.80, 19.03) | (-55.94, -5.56)*# | (-77.90, -23.50)*# | |
| Erythema | ||||
| 14th d | 0.585±2.489c | -0.451±3.518c | -22.304±8.426b | -33.259±14.734a |
| (-3.270, 4.390) | (-5.80, 4.57) | (-36.64, -9.75)* | (-60.47, -8.40)* | |
| 28th d | 0.335±2.405c | -0.314±4.374c | -45.642±19.473b | -58.853±22.161a |
| (-3.270, 4.100) | (-10.59, 5.16) | (-90.67, -22.05)*# | (-91.90, -23.53)*# | |
| TEWL | ||||
| 14th d | -0.149±0.826d | -1.253±0.673c | -1.814±0.611b | -2.927±0.791a |
| (-1.630, 1.200) | (-2.730, -0.170)* | (-2.540, -0.800)* | (-4.540, -1.940)* | |
| 28th d | -0.212±0.778d | -2.105±0.574c | -2.713±0.738b | -4.414±1.095a |
| (-1.750, 0.730) | (-3.430, -1.330)*# | (-4.300, -1.670)*# | (-6.540, -2.800)*# | |
Note: *Statistical difference when compared with the 1st d; #statistical difference when compared with the 14th d and (a-d)means the row followed by different letters are significantly different (p<0.05)
Table 4: The Changes In Skin Moisture, Skin Elasticity, Melanin, Erythema And Tewl Using Different Emulgel Formulations Containing Carissa Carandas, Placebo And Control
Fig. 1: The efficacy of emulgels containing Carissa carandas extract on moisture content, gross elasticity, melanin content,
erythema index and TEWL of skin Note: *Significant difference (p<0.05) with the 1st d; **significant difference (p<0.01) with the 1st d; #significant difference
(p<0.05) between F2 and F3 at 28th d,  : Control;
: Control;  : Placebo (F1);
: Placebo (F1);  : Treatment 1 (F1) and
: Treatment 1 (F1) and  : Treatment 2 (F3)
: Treatment 2 (F3)
Skin plays many important roles for the human body. The most exposed part of the skin, the epidermal skin layers, act as a protective barrier from environmental stimuli, pathogens, chemicals, damaging irradiation and water loss. Skin’s strength and flexibility are provided by the dermal skin layer according to its Extracellular Matrix (ECM), such as collagen, elastin fibers, etc. Aged skin has morphological changes resulting in a lowered rate of cell renewal, a weakened protective barrier, as well as in clinical signs including wrinkle formation, skin laxity and excessive dryness[2,24]. Phenolic compounds could slow down the skin aging process through many approaches. These include enhancing cell proliferation[25] and increasing ECM synthesis[26] while lowering the degradation of ECM through the inhibition of MMPs[27].
The solvents used in phenolic extraction are important factor influencing the efficiency of extraction. Alcohols, such as methanol and ethanol, are solvents normally used for a broad range of phytochemical extraction, especially phenolic compounds. Alcohols are common solvents used for phenolic extraction due to their broad range of phytochemical solubility. Carissa carandas pulp and seeds have generated a high extractive yield, ranging from 18.11 % to 20.05 %[22]. Phytochemical contents, especially phenolic, can be affected by many extraction variables, including extraction time, liquid to solid ratio, temperature and solvent system. Most of the Carissa carandas fruit phenolic compounds are phenolic acids and flavonoids[23] while other phytoconstituents are triterpenoids, phytosterols, volatile compounds and amino acids[28]. Among protective phytoconstituents, phenolic compounds play an important role to ameliorate free radical and oxidative stress. The antioxidant properties of Carissa carandas fruit extract strongly correlate to phenolic compounds[23], however, there has been little study into the comparison of phenolic content between pulp and seeds of Carissa carandas fruit.
The total phenolic content and antioxidant properties of Carissa carandas pulp and seed extracts were estimated by Folin-Ciocalteu’s assay and DPPH. scavenging assay, respectively. Considering its phenolic content, Carissa carandas seed extract could be considered as an alternative active ingredient of Carissa carandas fruit. This aligned with previous findings that a higher content of phenolic compounds with potential antioxidant properties were observed in the Carissa carandas seed extract[22]. The phenolic content identified in Carissa carandas fruit are vanillin acid, protocatechuic acid, t-cinnamic acid, ferulic acid, chlorogenic acid, caffeic acid, rutin, myricetin, catechin, quercetin and apigenin[23]. Although there is little information known about the identified phenolic compounds and other phytoconstituents in Carissa carandas seeds, there was no toxicity to dermal fibroblast of Carissa carandas extract observed. This suggested that the utilization of Carissa carandas seed extract was safe[13]. There was a negative correlation (R²=-0.716 and -0.752, respectively) in the consideration of tyrosinase inhibition activity with total phenolic content and DPPH. scavenging activity. This indicated that phenolic compounds may not attribute to the tyrosinase inhibition activity observed and the mechanisms of both assays may be different. However, phenolic compounds including flavonoids exhibit anti-tyrosinase activity which depends on the number of hydroxyl functional groups are present in their molecule[29]. Considering this phenomenon, different phenolic constituents in Carissa carandas pulp and seeds may influence their tyrosinase inhibition property.
Skin aging is caused by excessive free radical accumulation and inflammation which adversely result in diminishing the skin’s function as a protective barrier. Clinical signs of aging skin are wrinkle formation, loss of elasticity, excessive dryness and change in skin pigmentation[1,29]. This study also evaluated the efficacy of emulgels containing Carissa carandas phenolic extract. A large amount of Reactive Oxygen Species (ROS) production as an aging factor is attributed to the activation of Activator Protein-1 (AP-1) which suppresses Transforming Growth Factor-Beta (TGF-β) receptors and then alleviates the expression of pro-collagen synthesis[30]. In contrast, the expression of MMPs, such as collagenase and lactase are up-regulated which then lead to the degradation of many ECM. Therefore, radical scavenging properties could directly retard the aging process. The inhibitory effect of natural compounds against MMPs also reduces the rates of ECM degradation. The methanolic extract of Carissa carandas fruit has been revealed to inhibit collagenase and lactase[31]. The proliferation and differentiation of epidermal melanocytes to stimulate melanin synthesis through melanogenesis are also down-regulated by the inhibition of ROS and many inflammatory mediators[32]. Tyrosinase is a melanogenic enzyme which catalyzes the rate-limiting step for the melanogenesis process. Carissa carandas seed extract suppresses oxidative stress[22], ameliorates inflammation by inhibiting inducible nitric oxide synthase and cyclooxygenase-2[11] and according to this study, hinders the function of tyrosinase. The reduction of melanin content in the skin might be attributed to the tyrosinase inhibition property of Carissa carandas seed extract. Carissa carandas seeds also contained 9Z, 12Z-Octadecadienoic acid and 9Z-Octadecenoic acid and unsaturated fatty acids[33]. These fatty acids could suppress pigmentation by inhibiting melanin synthesis through stimulating tyrosinase ubiquitination and the proteolytic degradation of tyrosinase[34]. Carissa carandas pulp and seed extracts were also reported to inhibit nitric oxide, one of the mediators causing skin erythema[35]. Skin dryness, a clinical sign of aged skin, can be improved by using humectants and emollients. The principal function of emollients is to maintain skin hydration and the skin’s barrier function by creating an inert barrier over the skin’s surface, restoring epidermal differentiation, lipid lamellae and trapping moisture in the skin. The emollients in the formulations, e.g. isopropyl myristate, could improve skin hydration, skin barrier and skin elasticity. Humectants such as propylene glycol could increase skin moisture based on their hygroscopic property[36,37]. According to this supporting data, it can be stated that Carissa carandas seed extract could be utilized as an alternative cosmeceutical agent in cosmetics. This study assessed the feasibility of Carissa carandas pulp and seeds for use as an anti-aging ingredient in cosmetics. Total phenolic content and biological anti-aging activities through antioxidant and tyrosinase inhibition were determined. The Carissa carandas seeds are a potential source of phenolic compounds which may attribute to their antioxidant properties. The extracts also exhibit tyrosinase inhibition. Thus, the Carissa carandas seed extract was used to formulate anti-aging emulgels which were stable under accelerated conditions. The products proved safe and effective in improving skin barrier, moisture, elasticity and erythema. Therefore, residual Carissa carandas seeds which are normally wasted can be considered a potential active ingredient in anti-aging cosmetics. We are continuing our attempts to enhance the extract’s efficiency through the extraction condition and specifying the marker compound to standardize the extract.
Acknowledgement:
The research was financially supported by Mae Fah Luang University. The authors are happy to acknowledge the School of Cosmetic Science, Mae Fah Luang University and its many facilities. Finally, the authors greatly appreciated Miss Satja Phosri and Miss Tanaporn Jungrajang for their kindness and support during the study.
Ethical approval:
The ethical issues of this study were approved by the Ethics Committee of Mae Fah Luang University, with approval number REH-61038 and follow the declaration of Helsinki.
Conflict of interests:
The authors declared no conflict of interests in this study.
References
- Naylor EC, Watson RE, Sherratt MJ. Molecular aspects of skin ageing. Maturitas 2011;69(3):249-56.
[Crossref] [Google Scholar] [PubMed]
- White-Chu EF, Reddy M. Dry skin in the elderly: Complexities of a common problem. Clin Dermatol 2011;29(1):37-42.
[Crossref] [Google Scholar] [PubMed]
- Kim H, Koh J, Baek J, Seo Y, Kim B, Kim J, et al. Retinyl retinoate, a novel hybrid vitamin derivative, improves photoaged skin: A double-blind, randomized-controlled trial. Skin Res Technol 2011;17(3):380-5.
[Crossref] [Google Scholar] [PubMed]
- Palungwachira P, Tancharoen S, Phruksaniyom C, Klungsaeng S, Srichan R, Kikuchi K, et al. Antioxidant and anti-inflammatory properties of anthocyanins extracted from Oryza sativa L. in primary dermal fibroblasts. Oxid Med Cell Longev 2019;2019:2089817.
[Crossref] [Google Scholar] [PubMed]
- Grimm T, Schäfer A, Högger P. Antioxidant activity and inhibition of matrix metalloproteinases by metabolites of maritime pine bark extract (pycnogenol). Free Radic Biol Med 2004;36(6):811-22.
[Crossref] [Google Scholar] [PubMed]
- Zuo AR, Dong HH, Yu YY, Shu QL, Zheng LX, Yu XY, et al. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups. Chin Med 2018;13(1):1-51.
[Crossref] [Google Scholar] [PubMed]
- Sakunkan S. Carissa carandas L.: The fruit mentioned in Thai literature that has many health benefits. Khon Kaen Agr J 2016;44(3):557-66.
- Saher S, Narnawre S, Patil J. Evaluation of phytochemical and pharmacological activity of Carissa carandas L. fruits at three different stages of maturation. Drug Res 2020;70(2/3):80-5.
[Crossref] [Google Scholar] [PubMed]
- Mishra CK, Pattnaik AK, Asha R, Sasmal D, Nema RK. Antifungal and antibacterial activity of Carissa carandas Linn. Int J Plant Sci 2009;4(2):564-8.
- Itankar PR, Lokhande SJ, Verma PR, Arora SK, Sahu RA, Patil AT. Antidiabetic potential of unripe Carissa carandas Linn. fruit extract. J Ethnopharmacol 2011;135(2):430-3.
[Crossref] [Google Scholar] [PubMed]
- Weerawatanakorn M, Pan MH. Phytochemical components of Carissa carandas and the inhibitory effects of fruit juice on inducible nitric oxide synthase and cyclooxygenase-2. J Food Biochem 2017;41(3):e12343.
- Sarma A, Sarmah P, Kashyap D, Dutta S, Mahanta M. Antioxidant activity and nutraceutical property of the fruits of an ethno-medicinal plant: Carissa carandas L. found in Brahmaputra valley agro-climatic condition. J Pharm Sci Res 2015;7(2):55-7.
- Sudjaroen Y. In vitro antioxidant, antibacterial and cytotoxicity activities from Karanda (Carissa carandas L.) fruit extracts. Int J Green Pharm 2017;11(1):189-93.
- Kumar V, Tarpada P, Sadariya K, Goswami S. Comparative phytochemical and antioxidant activities of methanol and petroleum ether extract of Carissa carandas leaves, fruit and seed. Vivechan Int J Res 2017;8:70-5.
- Tesfaye T, Ravichadran YD. Traditional uses pharmacological action and phytochemical analysis of Carissa carandas Linn.: A review. Nat Prod Chem Res 2018;6(5):1000334.
- Kachhwaha P, Gehlot HS. Changes in phytonutrients and antioxidant properties of Cordia myxa and Carissa carandas fruit during ripening. Indian J Plant Physiol 2015;20(1):72-8.
- Ondee S. Antioxidant and antiproliferative activities of Carissa carandas Linn. fruits. Thai Cancer J 2019;39(1):7-15.
- Waterman PG, Mole S. Analysis of phenolic plant metabolites. Oxford, Boston: Blackwell Scientific Publications; 1994.
- Devasagayam TP, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. Free radicals and antioxidants in human health: Current status and future prospects. J Assoc Physicians India 2004;52:794-804.
[Google Scholar] [PubMed]
- Mendes E, Perry MD, Francisco AP. Design and discovery of mushroom tyrosinase inhibitors and their therapeutic applications. Expert Opin Drug Discov 2014;9(5):533-54.
[Crossref] [Google Scholar] [PubMed]
- An SM, Ham H, Choi EJ, Shin MK, An SS, Kim HO, et al. Primary irritation index and safety zone of cosmetics: Retrospective analysis of skin patch tests in 7440 Korean women during 12 years. Int J Cosmet Sci 2014;36(1):62-7.
[Crossref] [Google Scholar] [PubMed]
- Buachoon N. Antioxidant activity of total phenolic from seeds and fruits of Carissa carandas. VRU Res Dev J Sci Technol 2018;13(2):53-63.
- Azeez S, Karunakaran G, Tripathi PC, Shivashankara KS, Roy TK. Evaluation of antioxidant activity, total phenolics and phytochemical content of selected varieties of karonda fruits (Carissa carandas). Indian J Agric Sci 2016;86(6):815-22.
- Kuwazuru O, Miyamoto K, Yoshikawa N, Imayama S. Skin wrinkling morphology changes suddenly in the early 30s. Skin Res Technol 2012;18(4):495-503.
[Crossref] [Google Scholar] [PubMed]
- Addis R, Cruciani S, Santaniello S, Bellu E, Sarais G, Ventura C, et al. Fibroblast proliferation and migration in wound healing by phytochemicals: Evidence for a novel synergic outcome. Int J Med Sci 2020;17(8):1030.
[Crossref] [Google Scholar] [PubMed]
- Agyare C, Lechtenberg M, Deters A, Petereit F, Hensel A. Ellagitannins from Phyllanthus muellerianus (Kuntze) Exell.: Geraniin and furosin stimulate cellular activity, differentiation and collagen synthesis of human skin keratinocytes and dermal fibroblasts. Phytomedicine 2011;18(7):617-24.
[Crossref] [Google Scholar] [PubMed]
- Yodkeeree S, Thippraphan P, Punfa W, Srisomboon J, Limtrakul P. Skin anti-aging assays of proanthocyanidin rich red rice extract, oryzanol and other phenolic compounds. Nat Prod Commun 2018;13(8):967-72.
- Singh AK, Uppal GK. A review on Carissa carandas g phytochemistry, ethnogpharmacology, and micropropagation as conservation strategy. Asian J Pharm Clin Res 2015;8(3):26-30.
- Conner EM, Grisham MB. Inflammation, free radicals and antioxidants. Nutrition 1996;12(4):274-7.
[Crossref] [Google Scholar] [PubMed]
- Lephart ED. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res Rev 2016;31:36-54.
[Crossref] [Google Scholar] [PubMed]
- Khuanekkaphan M, Khobjai W, Noysang C, Wisidsri N, Thungmungmee S. Bioactivities of Karanda (Carissa carandas Linn.) fruit extracts for novel cosmeceutical applications. J Adv Pharm Technol Res 2021;12(2):162-8.
[Crossref] [Google Scholar] [PubMed]
- Fu C, Chen J, Lu J, Yi L, Tong X, Kang L, et al. Roles of inflammation factors in melanogenesis. Mol Med Rep 2020;21(3):1421-30.
[Crossref] [Google Scholar] [PubMed]
- Singh S, Bajpai M, Mishra P. Carissa carandas L.–phyto-pharmacological review. J Pharm Pharmacol 2020;72(12):1694-714.
[Crossref] [Google Scholar] [PubMed]
- Ando H, Watabe H, Valencia JC, Yasumoto KI, Furumura M, Funasaka Y, et al. Fatty acids regulate pigmentation via proteasomal degradation of tyrosinase: A new aspect of ubiquitin-proteasome function. J Biol Chem 2004;279(15):15427-33.
[Crossref] [Google Scholar] [PubMed]
- Jampa O, Panthong S, Itharat A. Phytochemical constituents, anti-microbial, anti-inflammatory and cytotoxic activities of Carissa carandas L. fruit and seed extracts. Thammasat Med J 2019;19(4):654-66.
- Proksch E, Lachapelle JM. The management of dry skin with topical emollients–recent perspectives. J Deutsch Dermatol Ges 2005;3(10):768-74.
[Crossref] [Google Scholar] [PubMed]
- Voegeli D. The role of emollients in the care of patients with dry skin. Nurs Stand 2007;22(7):64-8.
[Google Scholar] [PubMed]