- *Corresponding Author:
- Hayam Alrasheed
Department of Pharmacy Practice, College of Pharmacy, Princess Nourah Bint Abdulrahman University, Riyadh 11671, Saudi Arabia
E-mail: haalrasheed@pnu.edu.sa
| This article was originally published in a special issue, “Integrative Approaches in Biomedical Sciences for Drug Discovery and Development” |
| Indian J Pharm Sci 2024:86(6) Spl Issue “35-42” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Critical care units in hospitals can serve as the barometers for the proper usage of antibiotics. We conducted this study to evaluate the antimicrobial utilization and economic outcomes of implementing an antimicrobial stewardship program in critical care areas. This is a non-randomized, historical controlled, comparative study that was conducted in two phases such as the retrospective phase, where historical data of the individuals were collected, and the prospective interventional phase, where we enrolled adult intensive care unit individuals who prescribed the following targeted antimicrobials like caspofungin, voriconazole, amphotericin-B lipid complex, fluconazole, conventional amphotericin-B, tigecycline, meropenem, imipenem/cilastatin, colistin, vancomycin, ampicillin/sulbactam, piperacillin/ tazobactam, amikacin, ciprofloxacin, azithromycin, and ceftriaxone. A total of 88 individuals were included as a historical control before antimicrobial stewardship program implementation, and 122 individuals were enrolled in the interventional phase. Additionally, 1517 antimicrobial vials were consumed before antimicrobial stewardship program, and 1194 antimicrobial vials were consumed after implementing antimicrobial stewardship program. The p-value for the direct cost between the two groups was <0.01, with a 77 % cost saving in the antimicrobial stewardship program group compared to the control group. Antimicrobial stewardship program implementation positively impacted antimicrobial utilization and economic outcomes. However, further studies are needed to confirm the study findings.
Keywords
Antimicrobial stewardship program, intensive care unit, antimicrobials, economic outcomes, pathogens
Infectious diseases are generally one of the leading causes of death and increasing levels of morbidity among individuals in healthcare settings individuals[1]. Studies have generally found that individuals received antimicrobials 70 % of the time in the Intensive Care Unit (ICU)[2]. Inappropriate usage of antibiotics has become a significant public health issue; it is linked to the development of antibiotic-resistant pathogens, adverse events, and increased medical costs[3]. Extensive surveillance studies worldwide show that resistance to almost all antimicrobial classes is rising[4]. Additionally, antimicrobial resistance is estimated to increase global healthcare costs by one trillion United States of America (USA) dollars by 2050[5].
Antimicrobial resistance arises from inappropriate usage of antibiotics, which comprises overuse, misuse, underuse or abuse of antibiotics, failure to de-escalate, and over-prescription of broad-spectrum antibiotics[6]. Implementing an Antimicrobial Stewardship Program (ASP) is thought to be a fundamental way of reducing toxicity, minimizing resistance, and optimizing clinical outcomes[7]. This program has many strategies at different levels to promote appropriate antimicrobial usage and limit resistance through formulary restriction, treatment algorithm, dose optimization, guidelines adherence, and healthcare professional education[7-9].
Although implementing ASP is believed to be associated with better outcomes[7-11], many healthcare providers and administrators perceive it to be so expensive that there is no real value in the investment of implementing such a program. Numerous studies show that ASP reduces the development of infections due to resistant pathogens and decreases the costs without significant changes in the length of stay, mortality, and readmissions[7-11]. Only one study has investigated the impact of ASP on the Saudi population in the ICU setting[12]. However, this study measured only the appropriateness of antibiotics used before and after the institution’s implementation of ASP. Therefore, the antimicrobial utilization and economic outcomes of implementing ASP are needed to be rationally evaluated. Thus, we conducted a nonrandomized, historically controlled study to assess the antimicrobial utilization and the benefit of ASP on economic outcomes between individuals treated under ASP and those without ASP.
Materials and Methods
Study design:
This study was a non-randomized, historicalcontrolled, comparative study. The study was conducted in two phases; a retrospective phase, where historical data of the individuals were collected, and a prospective interventional phase, where ICU adult individuals were enrolled under an ASP. The study was conducted at the Security Forces Hospital (SFH) in Saudi Arabia. SFH is a tertiary hospital with a 600 bed capacity and 23 adult ICU beds for medical and surgical individuals.
Criteria:
The included subjects were adult critical care individuals who received the following antimicrobials; caspofungin, voriconazole, amphotericin-B lipid complex, fluconazole, conventional amphotericin-B, tigecycline, meropenem, imipenem/cilastatin, colistin, vancomycin, ampicillin/sulbactam, piperacillin/tazobactam, amikacin, ciprofloxacin, azithromycin, and ceftriaxone. These antimicrobials were targeted based on their spectrum of activity, susceptibility patterns in the unit, cost, misuse, or overuse. Individuals who were excluded received only one dose of the antimicrobials or died before completing the course of antimicrobials.
Data collection:
The historical-controlled cohort included individuals who received critical care at SFH for 1 y before the ASP implementation and met the inclusion and exclusion criteria. ASP implantation was started based on the program guidelines. The ASP team comprised one infectious disease/critical care consultant, one critical care fellow, and one clinical pharmacist. On Sunday to Thursday, the team prospectively audited the initial orders if they were initiated within 24 h and weekly if the duration of therapy exceeded 7 d. The intervention was made on the antimicrobials mentioned above. During the second phase, which is the interventional phase, individuals data were collected for 1 y on the same targeted antimicrobials. The interventions were made by prospective audit and feedback. This strategy was created by reviewing individuals who were on the targeted antimicrobials and offering feedback either written on the patient’s chart or verbally by discussing the intervention with the team taking care of the patient. ASP techniques used in this study included de-escalation of empirical therapy, escalation, dose optimization, and shifting from parenteral to oral therapy[2,10].
For all individuals, information collected included their age, gender, and type of infection at ICU admission. In addition, the type of antibiotic prescribed, the number of vials consumed, and the antibiotic costs were also recorded. Institutional Review Board (IRB) approval was obtained from the SFH-Research Ethical Committee for conducting this study. This study was exempt because it is a secondary data analysis. Patient-identifiable information was removed and obscured before the analysis was initiated. The analysis did not allow the identification of any individual patient.
Study’s perspective:
In order to measure resources from multiple perspectives, we did our assessment from the perspective of the hospital and the provider of care, specifically the formulary committee perspective.
Measurement and valuing resources data:
In SFH, medical and pharmacy data are integrated into a single database. Therefore, detailed clinical data and measures were obtained retrospectively from hospital medical records, while resources consumed during hospitalization were obtained from patient medical records, the institution’s administrative purchasing databases, and hospital cost-accounting systems. The data were collected at weekly intervals. Additionally, the SFH routinely carefully validated and verified its resource databases for missing data elements and coding procedures.
Source of valuation:
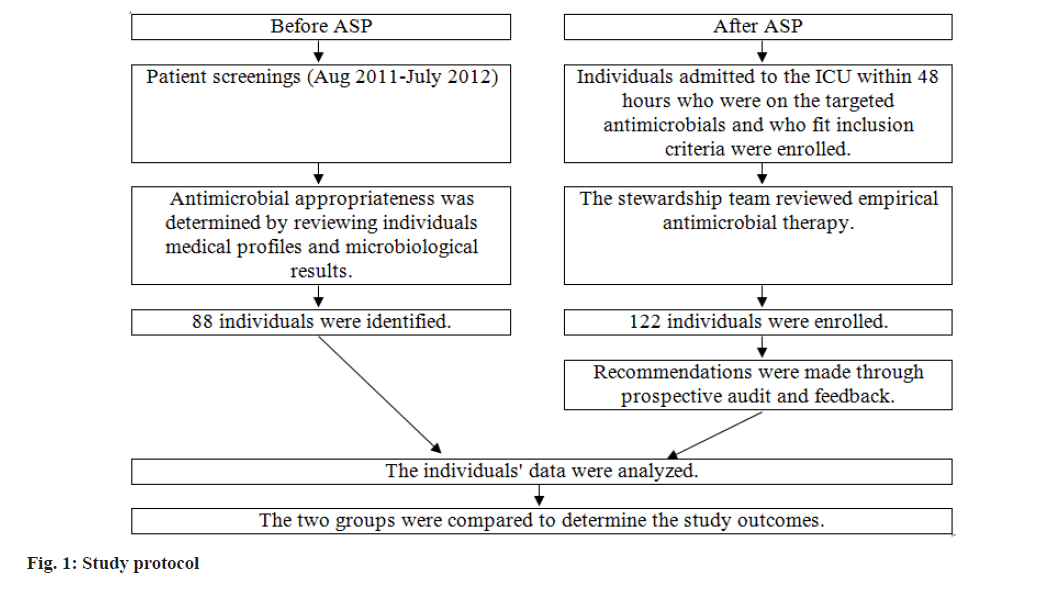
To calculate the medication cost, we used the cost to the institution, the Average Wholesale Price (AWP), as a proxy of cost data for the valuation source. To avoid errors in the measurement of outcomes, misclassification errors, and errors in past characteristics, we validated our results by assigning one individual to check files to prevent the factors potentially blurring the accuracy of the effect of the ASP intervention. The study protocol was illustrated in fig. 1.
Empirical therapy: The therapy initiated before identifying the causative pathogens, usually a broad-spectrum antimicrobial combination, is used. Typically, it takes 24-48 h to have the full susceptibility results after obtaining the patient’s specimen[12,13].
De-escalation: Discontinuation or changing the empirical antimicrobial therapy to a more targeted one based on culture results and antimicrobial sensitivity[7,13].
Escalation: Addition of a new antimicrobial or changing to a broader spectrum one upon the result of the antimicrobial sensitivity or individuals’ clinical status[13].
Dose optimization: Dosing is based on the patient’s characteristics, infection site, causative microorganism, drug pharmacokinetics, and pharmacodynamics[4,7].
Parenteral to oral: Conversion of parenteral antimicrobials to the oral route with excellent bioavailability in those individuals whose conditions allow it[4,7].
Statistical analysis:
Analyses were conducted using Statistical Analysis System (SAS), version 9.3 (SAS Institute Inc., Cary, North Carolina, USA). Categorical variables were summarized using frequency and percentage. Normally distributed continuous variables were summarized using mean and Standard Deviation (SD), and non-normally distributed continuous variables were summarized using median, minimum, and maximum. Comparison of patient characteristics before and after ASP was conducted using a twosample t-test, Wilcoxon rank sum test, or Fisher’s exact test, as appropriate. To assess the significance level, a p-value of 0.01 with a two-tailed significance level was used.
Results and Discussion
A total of 88 individuals were included as a historical control before ASP implantation, while 122 individuals were enrolled in the interventional phase. The baseline patient characteristics of the study population are presented in Table 1. There was no statistically significant difference in age, gender, and type of infection among the study groups.
| Clinical characteristics | Historical control, total, % (n=88) | ASP group, total, % (n=122) | #p value |
|---|---|---|---|
| Age, mean in years (SDh) | 53.5 (22.2) | 54.9 (21.1) | 0.64 |
| Gender | |||
| Male | 55 (62.5 %) | 71 (58.2 %) | 0.43 |
| Type of infection/diagnosis | |||
| Multiple infections | 23 (26.1 %) | 30 (24.5 %) | 0.87 |
| Pneumonia | 21 (23.9 %) | 35 (28.7 %) | |
| Unspecified sepsis | 6 (6.8 %) | 10 (8.2 %) | |
| Surgical site | 6 (6.5 %) | 7 (5.7 %) | |
| Bloodstream | 7 (7.9 %) | 4 (3.0 %) | |
| Disseminated systemic infection | 7 (7.6 %) | 8 (6.6 %) | |
| Skin and soft tissue | 4 (4.4 %) | 7 (5.2 %) | |
| Urinary tract | 6 (6.5 %) | 10 (8.2 %) | |
| Bone and joint | 3 (3.3 %) | 5 (3.7 %) | |
| Gastrointestinal | 5 (5.4 %) | 7 (5.2 %) |
Note: Data are presented as numbers with percentages unless otherwise indicated. #p<0.01 shows a significant difference between the two groups, SDη: Standard Deviation
Table 1: Baseline Patient Characteristics
In the historical control group, 88 individuals consumed around 1517 antimicrobial vials; colistin 80 mg, voriconazole 200 mg, caspofungin 70 mg, imipenem/cilastatin 500 mg, tigecycline 50 mg, piperacillin/tazobactam 4.5 gm, amphotericin-B 50 mg, and ceftriaxone 1 gm were the most common antimicrobials prescribed. In the ASP interventional group, 122 individuals consumed around 1194 antimicrobial vials. There were different antimicrobials listed as the top prescribed antimicrobials after ASP implementation, including amphotericin-B lipid complex 50 mg, voriconazole 200 mg, fluconazole 50 mg, ciprofloxacin 200 mg, and ampicillin/sulbactam 1.5 gm. Generally, the use of broad-spectrum antimicrobials such as tigecycline 50 mg, imipenem/cilastatin 500 mg, colistin 80 mg, piperacillin/tazobactam 4.5 gm, amphotericin-B 50 mg, and ceftriaxone 1 gm was decreased after the implementation of ASP; the use of amphotericin-B lipid complex 50 mg, voriconazole 200 mg, fluconazole 50 mg, ciprofloxacin 200 mg, and ampicillin/sulbactam 1.5 gm was increased (Table 2).
| Drug | Number of individuals, n (%) | Consumption, median (min, max) | Cost (SR)/vial, median (min, max) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before ASP (n=88) | After ASP (n=122) | #p value | Before ASP | After ASP | #p value | Before ASP | After ASP | #p value | |
| Caspofungin 50 mg | 30 (34.1 %) | 27 (22.1 %) | 0.08 | 7.5 (123) | 4 (132) | 0.05 | 17376 (2317, 53 286) | 9267 (2317, 74 137) | 0.06 |
| Tigecycline 50 mg | 23 (26.1 %) | 17 (13.9 %) | 0.04 | 25 (1116) | 17 (559) | 0.03 | 4731 (189, 21 935) | 3217 (946, 11 166) | 0.03 |
| Amphotericin-B lipid complex 50 mg | 20 (22.7 %) | 16 (13.1 %) | 0.1 | 7.5 (360) | 13.5 (342) | 0.08 | 5378 (2151, 43 020) | 9680 (2151, 30 114) | 0.08 |
| Voriconazole 200 mg | 13 (14.8 %) | 3 (2.5 %) | <0.01 | 24 (4108) | 40 (244) | <0.01 | 9000 (1500, 40 500) | 750 (750, 16 500) | <0.01 |
| Caspofungin 70 mg | 30 (34.1 %) | 14 (11.5 %) | <0.01 | 1 (12) | 1 (14) | <0.01 | 2947 (2947, 5894) | 2947 (2947, 11 787) | <0.01 |
| Meropenem 1 g | 80 (90.9 %) | 62 (50.8 %) | <0.01 | 15 (0.7, 222) | 9.1 (0.5, 167.5) | 0.21 | 1436 (67, 21 245) | 873 (48, 16 030) | <0.01 |
| Fluconazole 50 mg | 22 (25.0 %) | 14 (11.5 %) | 0.02 | 3.8 (1.0, 15.5) | 16 (4140) | <0.01 | 353 (93, 1442) | 1488 (372, 13 020) | 0.03 |
| Vancomycin 1 g | 79 (89.8 %) | 91 (74.6 %) | <0.01 | 7 (0.5, 62) | 6 (0.75, 50.5) | 0.56 | 240 (17, 2128) | 206 (26, 1734) | <0.01 |
| Imipenem/cilastatin 500 mg | 8 (9.1 %) | 32 (26.2 %) | <0.01 | 28 (4100) | 12.5 (177) | <0.01 | 882 (126, 3150) | 394 (32, 2426) | <0.01 |
| Colistin 80 mg | 43 (48.9 %) | 34 (27.9 %) | <0.01 | 32 (1396) | 17 (1148) | <0.01 | 532 (17, 6585) | 283 (17, 2461) | <0.01 |
| Piperacillin/tazobactam 4.5 g | 22 (25.0 %) | 60 (49.2 %) | <0.01 | 18.25 (1, 104) | 12.25 (1183) | <0.01 | 1027 (26, 5850) | 689 (56, 10 294) | <0.01 |
| Amphotericin-B 50 mg | 16 (18.2 %) | 8 (6.6 %) | 0.02 | 5.8 (1.0, 11.0) | 4.2 (1.0, 43.2) | <0.01 | 348 (60, 660) | 252 (60, 2592) | <0.01 |
| Ciprofloxacin 200 mg | 30 (34.1 %) | 16 (13.1 %) | 0.01 | 20 (290) | 22.5 (260) | <0.01 | 400 (40, 1800) | 450 (40, 1200) | <0.01 |
| Ampicillin/sulbactam 1.5 g | 10 (11.4 %) | 6 (4.9 %) | 0.14 | 18.3 (4156) | 34 (4208) | <0.01 | 328 (72, 2796) | 609 (72, 3727) | 0.09 |
| Azithromycin 500 mg | 20 (22.7 %) | 28 (23.0 %) | >0.99 | 5 (112) | 5 (114) | 0.91 | 273 (55, 654) | 273 (55, 763) | 0.91 |
| Ceftriaxone 1 g | 20 (22.7 %) | 41 (33.6 %) | 0.12 | 9 (140) | 6 (118) | <0.01 | 161 (18, 714) | 107 (18, 321) | 0.17 |
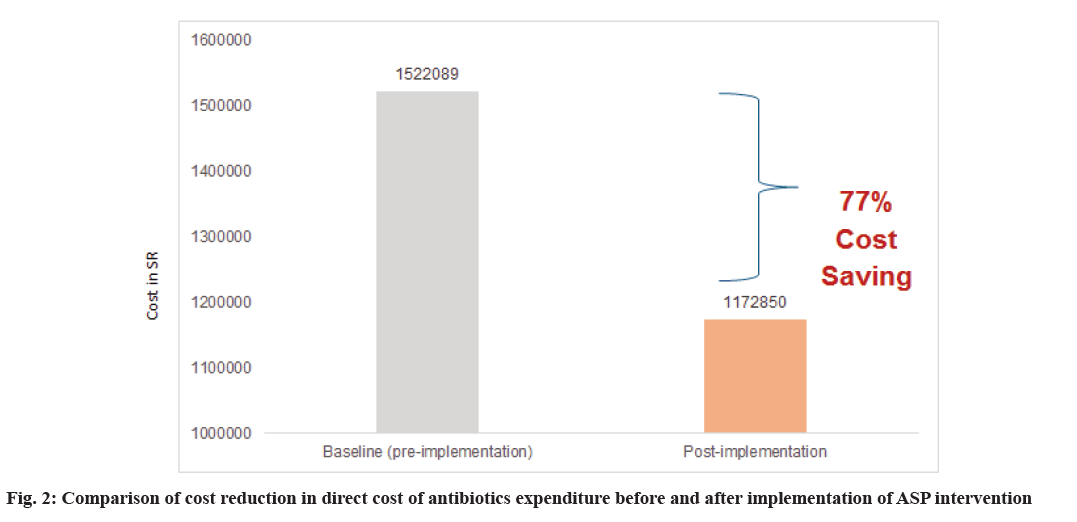
| Total cost (SR) | 1522089.33 | 1172850.57 | <0.01 | ||||||
Note: Data are presented as numbers with percentages unless otherwise indicated, #p<0.01 shows a significant difference between the two groups
Table 2: Comparison of the Antimicrobial Expenditure before and after ASP Implementation
The direct total cost of antimicrobials was significantly lower in the ASP interventional group, with a p<0.01. The ASP group had a 77 % cost saving compared to the control group (fig. 2). After ASP implementation, the team conducted a total of 422 interventions, mostly de-escalation (Table 3).
| ASP interventions | Total 422 |
|---|---|
| De-escalation | 289 |
| Escalation | 67 |
| Change dose | 74 |
| IV to PO | 12 |
Table 3: Number of ASP Interventions Conducted for the ASP Group
This non-randomized, historical-controlled comparative study investigated the drug utilization and economic impact of an ASP in the ICU setting. The study found that implementing ASP improves the use of antimicrobials and reduces costs. In fact, we believe that the positive outcomes reported also reflect the rigorous process of care and intense practices and processes provided in our healthcare system’s ICU.
More appropriate use of antimicrobials was observed in the ASP intervention period compared to the historical control period. Additionally, the implementation of ASP was associated with less antimicrobial utilization and cost, similar to the results reported by Amer et al. 2013[12]. Generally, the appropriate use of antimicrobials, as demonstrated in our study, could optimize the patient’s treatment plans and outcomes, minimizing long-term antimicrobial resistance.
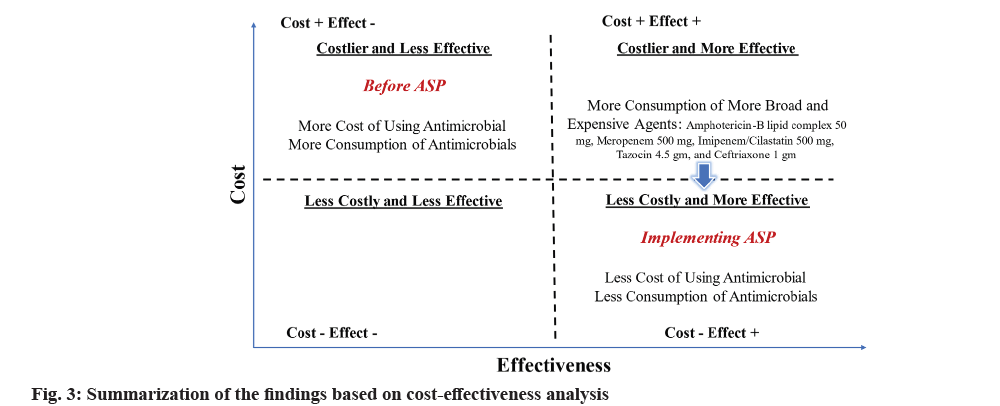
Although the objective of this study was not to conduct a cost-effectiveness analysis, we tried to summarize our findings using such an analytical approach, as shown in fig. 3. We strongly believe that reducing antimicrobial expenditure, having a lower MDR rate, and having less inappropriate antimicrobial use, as described in our findings, are surrogate outcomes for positive clinical response to antimicrobial therapy, an essential outcome of drug use evaluation and patient outcome studies.
Earlier literature suggested that antimicrobial stewardship interventions could improve patient outcomes and minimize antibiotic resistance[7-10]. Evidence of a possible linkage between ASP and enhanced clinical outcomes of individuals comes from the study by Nowak et al.[10]. These researchers showed that ASP decreases infections caused by resistant pathogens and reduces antimicrobial expenditure. Additional evidence from another study supports these findings by showing the strong correlation between ASP and reduced pharmacy costs[3]. Several economic analyses were investigated, including the primary economic endpoint; cost reduction in antibiotic expenditure gained in implementing ASP intervention relative to no intervention group. Consistent with the previous studies, we noticed that ASP reduces the cost of antimicrobial use[3,11].
During and after the implementation of ASP intervention, we observed migration of resources due to the substitution or drop of some antibiotics done with de-escalation and escalation. For example, after ASP implementation, the use of tigecycline 50 mg, imipenem/cilastatin 500 mg, colistin 80 mg, piperacillin/tazobactam 4.5 gm, amphotericin-B 50 mg, and ceftriaxone 1 gm had decreased. In comparison, the use of amphotericin-B lipid complex 50 mg, voriconazole 200 mg, fluconazole 50 mg, ciprofloxacin 200 mg, ampicillin/sulbactam 1.5 gm had increased. This migration of resources was observed with the significantly lower antimicrobial cost in the intervention group. It is also possible that using some antibiotics was associated with an increase of other agents or a change in resources needed in ICU or other areas, such as nursing time or physician time, that were not being measured in this study. Although we did follow personnel costs associated with preparing and administering therapies, our findings may imply that pharmacy and nursing drug dispensing time can be reduced, reflecting that implementing ASP may increase the system’s efficiency, one of the primary targets of improvement in the quality of care. Secondly, other economic direct medical resources, use of procedures, practice style, the concomitant process of care, interventions, and parameters (such as per dose costs of administration, frequency of dosage needed to control, health care providers’ time as hours of work, hospital days, number of laboratory services and diagnostic and monitoring tests, the amount of time spent providing care relative to the total time at work, amount of the nursing time used, the number of intensive care days, number of exacerbations, and the drugs used) were not considered. Other uncalculated indirect and intangible costs, such as the cost of property on lease, volunteer time, and other free services, were not accessible. The possibility of these measurement errors in statistically uncontrolled factors can influence our study’s final interpretation of clinical and economic outcomes. They can introduce potential external validity biases that limit the generalizability of our findings to other settings.
Although conducting such an investigation in one center may limit the results’ generalizability, our method design provides a relatively acceptable internal validity and high scientific rigor for several reasons. First, both groups’ intensity and quality of care were relatively equal. Secondly, our design allows the observation and collection of actual outcomes and everyday resource use data that accurately reflect practical clinical practice patterns vs. ideal conditions.
This observation renders a realistic focus on the studied population, minimizes patient biases, and gives great confidence in the drawn conclusion. The study findings provide medical care results-assessing antimicrobial utilization and economic outcomes simultaneously and therefore provide supportive and transparent information that helps justify the value of implementing the ASP in all hospital departments. The study also provides sufficient information that eases defending subsequent providers’ decisions, Pharmacy and Therapeutics Committee protocols and formulary decisions, and prospective health care managers’ policies. Our findings also demonstrate the excellent value of money in the costs invested and saved regarding drug expenditure and how deviation from accepted recommendations impacts clinical outcomes. All these factors maximize the external validity of our practice site and may assist in the more efficient and appropriate use of healthcare resources.
Although the study analysis was not designed to control confounding variables, absolute attribution of our findings to the ASP can be challenging for several reasons. First, our study design is vulnerable to inherent patient selection bias due to differences in study groups. In addition, the hosting of other factors, such as patient differences in severities of illness and comorbidities, the difference in the use of nonantimicrobial drugs and procedures, and different time intervals between the control and the intervention groups in our design can affect the interpretation of our findings and demonstrate substantial difference in the conclusions drawn. Furthermore, we believe the groups are incomparable in all the unknown unmeasured prognostic factors. For example, it was difficult to escape subjective judgments in clinical case improvement in that the value of a benefit may vary across individuals (providers or individuals). Also, other possibilities may occur as part of routine practice; for example, nurses might not adhere to antimicrobial administrations, individuals may have their dose or therapy changed or discontinued by the clinician, or they might experience adverse events.
Although we did not conduct a sensitivity analysis, we believe that the quality of care and discount rate did not vary widely and were relatively stable over the years of our investigations. Nevertheless, sensitivity analysis can help put the resource use associated with antimicrobial utilization into perspective, increasing confidence in the accuracy of study results and the drawing of conclusions.
Our study provides a valuable starting point for continual evaluation of ASP interventions and can be used as a springboard to move from the traditional prescribing pattern. As historically controlled studies are not always considered conclusive for economic evaluation, utilizing more rigorous longitudinal designs will likely provide more precise estimates of the studied outcomes. Observing the impact of ASP intervention on other intermediate outcomes, such as the prevalence of antimicrobial resistance, would be worth studying in the future. Also, it would be promising to measure health-related quality of life, such as symptom-free days. Moreover, a cost-benefit analysis may be conducted where monetary value can be placed on all outcomes. Additionally, it is crucial in the future to assess the impact of such programs on the entire range of direct and indirect tangible and intangible costs and benefits, such as productivity, cost of pain and suffering, and other aspects of one’s quality of life and satisfaction.
Our study showed that ASP implantation improved antimicrobial utilization across the ASP and control groups. Furthermore, the prevalence of broadspectrum antimicrobial use was significantly higher in the control group. For economic analysis, implementing ASP resulted in 77 % cost savings compared to the control group. Further studies are needed to confirm our study’s findings.
Acknowledgments:
The author would like to express sincere gratitude to all individuals who helped to collect the data and make this research possible. Also, many thanks to Princess Nourah bint Abdulrahman University Researchers Supporting Project (No: PNURSP2024R485), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Ethical approval:
The IRB of SFH-Research Ethical Committee, Riyadh, Saudi Arabia, approved and exempted this study. Additionally, informed consent was obtained from the university students who agreed to participate in the online survey, as participation was voluntary, and no identifiable information was obtained.
Conflict of interests:
The authors declared no conflict of interests.
References
- World Health Organization. The top 10 causes of death. 2020.
- Kollef MH, Fraser VJ. Antibiotic resistance in the intensive care unit. Ann Intern Med 2001;134(4):298-314.
[Crossref] [Google Scholar] [PubMed]
- Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Singal B. Clinical and economic outcomes from a community hospital's antimicrobial stewardship program. Am J Infect Control 2013;41(2):145-8.
[Crossref] [Google Scholar] [PubMed]
- Owens Jr RC. Antimicrobial stewardship: Concepts and strategies in the 21st century. Diagn Microbiol Infect Dis 2008;61(1):110-28.
[Crossref] [Google Scholar] [PubMed]
- O'neill JI. Antimicrobial resistance: Tackling a crisis for the health and wealth of nations. Rev Antimicrob Resist 2014.
- Hand K. Antibiotic stewardship. Clin Med 2013;13(5):499.
[Crossref] [Google Scholar] [PubMed]
- Dellit TH, Owens RC, McGowan JE, Gerding DN, Weinstein RA, Burke JP, et al. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007;44(2):159-77.
[Crossref] [Google Scholar] [PubMed]
- Averbuch D, Orasch C, Cordonnier C, Livermore DM, Mikulska M, Viscoli C, et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: Summary of the 2011 4th European conference on infections in leukemia. Haematologica 2013;98(12):1826.
[Crossref] [Google Scholar] [PubMed]
- Doron S, Davidson LE. Antimicrobial stewardship. Mayo Clin Proc 2011;86(11):1113-23.
[Crossref] [Google Scholar] [PubMed]
- Nowak MA, Nelson RE, Breidenbach JL, Thompson PA, Carson PJ. Clinical and economic outcomes of a prospective antimicrobial stewardship program. Am J Health Syst Pharm 2012;69(17):1500-8.
[Crossref] [Google Scholar] [PubMed]
- Nathwani D, Varghese D, Stephens J, Ansari W, Martin S, Charbonneau C. Value of hospital Antimicrobial Stewardship Programs (ASPs): A systematic review. Antimicrob Resist Infect Control 2019;8:1-3.
[Crossref] [Google Scholar] [PubMed]
- Amer MR, Akhras NS, Mahmood WA, Al-Jazairi AS. Antimicrobial stewardship program implementation in a medical intensive care unit at a tertiary care hospital in Saudi Arabia. Ann Saudi Med 2013;33(6):547-54.
[Crossref] [Google Scholar] [PubMed]
- Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother 2010;54(11):4851-63.
[Crossref] [Google Scholar] [PubMed]