- *Corresponding Author:
- Y. S. Xu
Department of Orthopedics, Inner Mongolia Medical University People’s Hospital, Hohhot, Inner Mongolia 010020, China
E-mail: xuyongshengnmg@aliyun.com
| Date of Received | 02 December 2021 |
| Date of Revision | 21 September 2022 |
| Date of Acceptance | 16 May 2023 |
| Indian J Pharm Sci 2023;85(3):835-840 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
To explore the function of curcumin on the cartilage cells, the cell proliferation and apoptosis by transforming growth factor-beta/suppressor of mothers against decapentaplegic/a disintegrin and metalloproteinase with thrombospondin motifs-7 axis was the objective of the study. The cell viability was detected by cell counting kit-8. The expression of transforming growth factor-beta, suppressor of mothers against decapentaplegic protein, a disintegrin and metalloproteinase with thrombospondin motifs-7, caspase-9, B-cell lymphoma 2, Bcl-2-associated X protein was determined by Western blot. The cartilage cells were treated with 1 mmol/l sodium nitroprusside for 24 h. Then, the cells were treated with different concentration of curcumin for 24 h. We found that 1 μmol/l curcumin could recover the cartilage cells proliferation. The transforming growth factor-beta and suppressor of mothers against decapentaplegic protein show high expression and a disintegrin and metalloproteinase with thrombospondin motifs-7 was low in the curcumin treatment group. Meanwhile, the apoptosis pathway was also detected. The caspase-9 and B-cell lymphoma 2 was higher in the curcumin treatment group than without curcumin group. However, the Bcl-2-associated X protein was lower. The cell viability, a disintegrin and metalloproteinase with thrombospondin motifs-7, caspase-9, B-cell lymphoma 2 and Bcl-2-associated X proteins also show no changes with or without curcumin when the transforming growth factor-beta was inhibited. We found that a disintegrin and metalloproteinase with thrombospondin motifs-7 show over expression, the transforming growth factor-beta and suppressor of mothers against decapentaplegic protein show high expression in the curcumin treatment group, the cell viability, caspase-9, B-cell lymphoma 2 and Bcl-2-associated X proteins show no changes with or without curcumin. Curcumin can promote the cartilage cells proliferation via transforming growth factor-beta/suppressor of mothers against decapentaplegic/a disintegrin and metalloproteinase with thrombospondin motifs-7 axis and inhibit the cell apoptosis.
Keywords
Curcumin, osteoarthritis, a disintegrin and metalloproteinase with thrombospondin motifs-7, transforming growth factor-beta, apoptosis
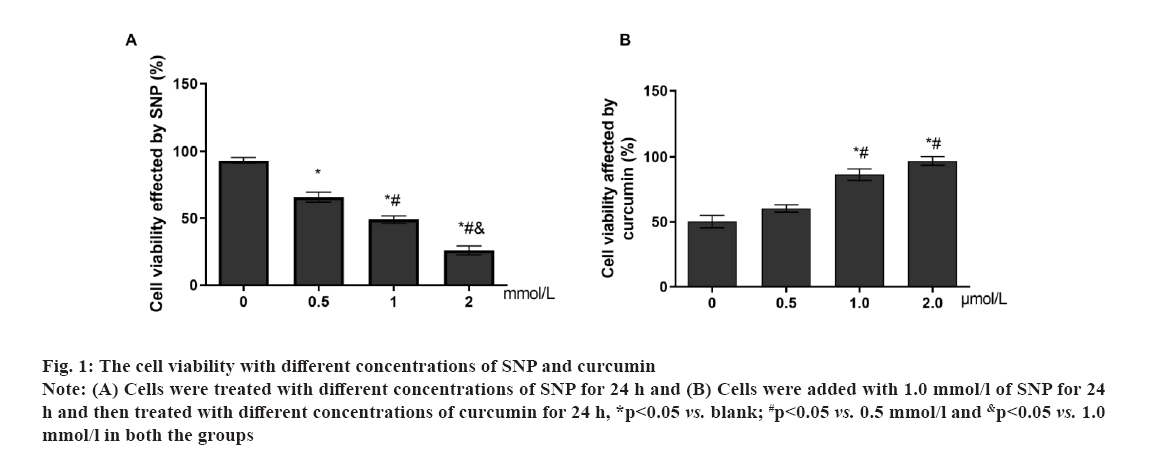
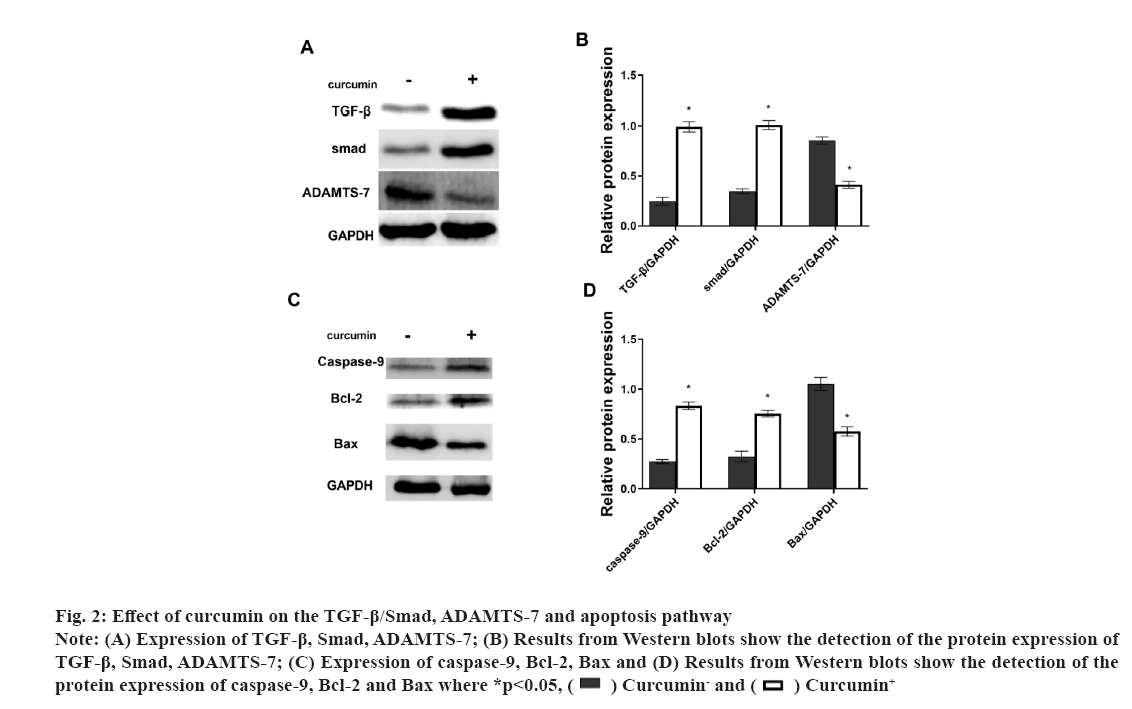
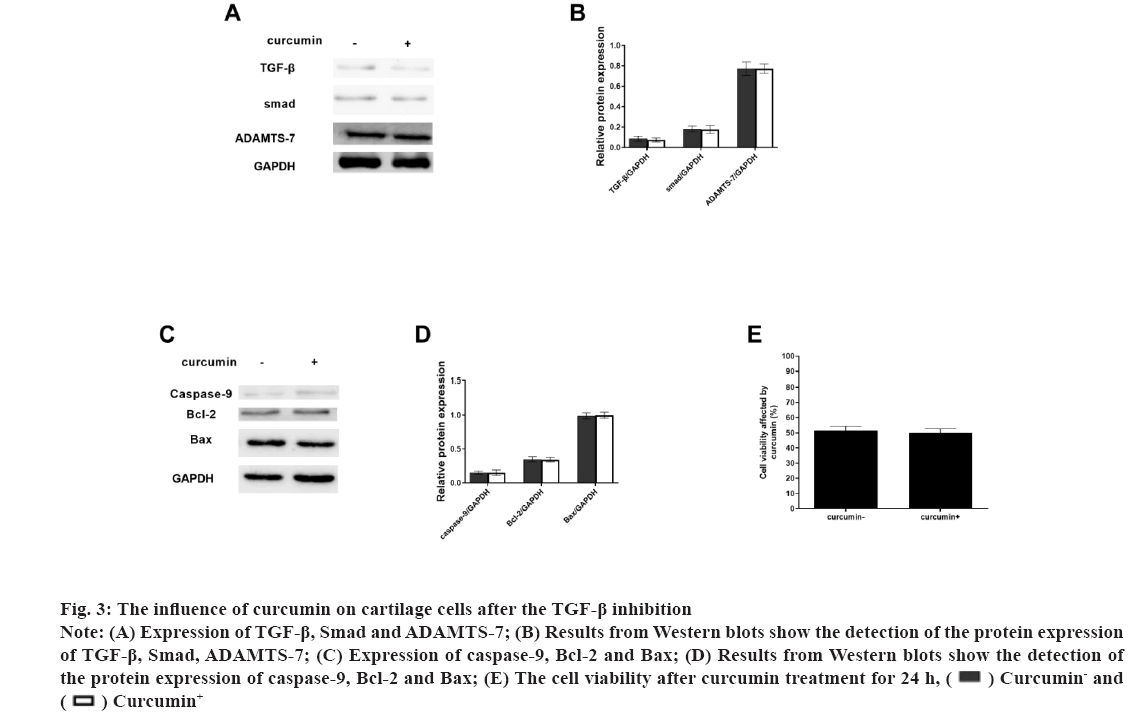
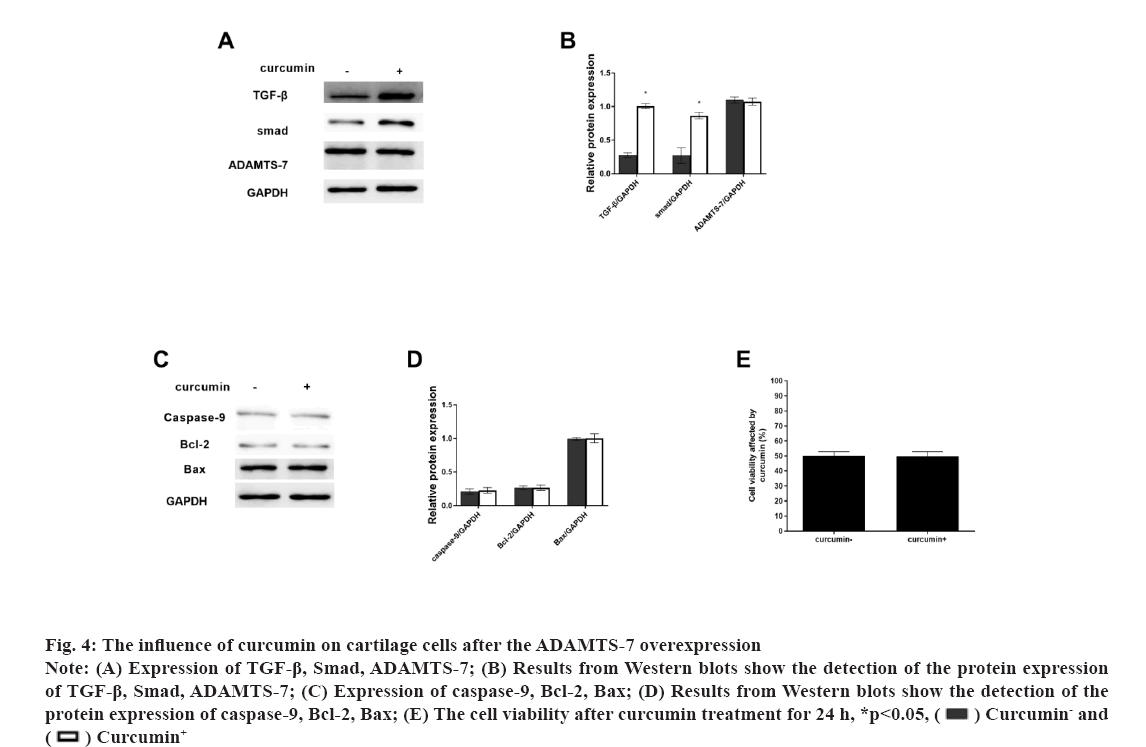
Osteoarthritis (OA) is a chronic pain disease with whole arthropathies such as synovitis, osteophyte formation etc[1-4]. OA showed the articular cartilage degeneration and persistent pain, causing disability, loss of function, decreased quality of life[5-7]. In OA, the destruction of articular cartilage is the most important change[8,9]. OA is mainly caused by the decomposition of extracellular matrix by degradation enzymes and the death of chondrocytes caused by apoptosis[10,11]. Curcumin (C21H20O6) is a natural polyphenolic compound extracted from the root of turmeric[12,13]. It has many pharmacological activities such as anti-diabetes, anti-aging, promote cell proliferation etc.[14,15]. Curcumin can operate in several different signalling pathways[13,16]. Curcumin has also shown significant antibacterial and antiviral effects[17-19]. The Transforming Growth Factor-beta (TGF-β) was involved in cell growth, differentiation and apoptosis[20,21] and A Disintegrin and Metalloproteinase with Thrombospondin Motifs-7 (ADAMTS-7) was expressed in bone and cartilage[22-24]. ADAMTS-7 negatively regulate endplate cartilage differentiation[25]. In this study, to explore the function of curcumin on the cartilage cells, the cell proliferation and apoptosis were detected and the role of the TGF-β and ADAMTS-7 was also shown. Unless otherwise specified, all chemicals and reagents in the study were purchased from the Sigma Chemical Company (St. Louis, Missouri (MO), United States of America (USA). Immunoglobulin G (IgG) antibodies, Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH), TGF-β, Suppressor of mothers against decapentaplegic (Smad) protein, ADAMTS-7, caspase-9, B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X (Bax) proteins were purchased from the cell signal technology company, USA. Cell cultures used in the study were as follow. The cartilage cells (ScienCell, Carlsbad, California (CA), USA) were cultured under the standard conditions (37°, 5 % Carbon dioxide (CO2) and humidified air). Cell viability and proliferation were evaluated using a Cell Counting Kit-8 (CCK-8) (Dojindo Laboratories, Kumamoto, Japan). The absorbance at 450 nm was determined (Bio-Tek Instruments, Winooski, Virginia Tech, USA). Cell growth rate was calculated using the following equation: Percentage (%) growth rate=(Mean experimental absorbance/mean control absorbance)×100, each test was repeated five times. Radioimmunoprecipitation Assay (RIPA) lysis buffer (Beyotime, China) was used to obtain the protein and a Bicinchoninic Acid (BCA) kit was used to determine the protein content (Beyotime, Haimen, China). Protein samples were isolated through 10 % Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and transferred to Polyvinylidene Fluoride (PVDF) membrane, followed by blocking with 5 % nonfat milk for 1 h at 37°. After blocking, primary antibodies were added (overnight at 4°). The membranes were washed and species-compatible peroxidase-conjugated secondary antibodies were added. Enhanced Chemiluminescence (ECL) (Pierce, Rockford, Illinois (IL), USA) was used to detect membrane-bound antibodies[26]. Protein quantification was performed using Clinx Chemi Analysis software (ChemiScope 6000, Shanghai, China). Experiments were performed in triplicate. Cell transfection was explained clearly in this study. The small interfering Ribonucleic Acids (siRNAs) of TGF-β and overexpression plasmid of ADAMTS-7 were obtained from GenePharma (Shanghai, China). LipofectamineTM 2000 was used to transfect plasmids or oligonucleotides into cells according to the protocols. Comparisons between groups were made using GraphPad Prism version 5 software (GraphPad Software, La Jolla, CA, USA). One-way Analysis of Variance (ANOVA) with Newman-Keuls Post-hoc test was used to determine differences in gene expression levels. Replicates were included in the statistical model. A 95 % confidence level (p<0.05) was deemed to be statistically significant. Data are shown as mean±Standard Deviation (SD). The selection optimum concentration of curcumin treatment on the cartilage cell viability was shown here. To obtain the OA model on the cartilage cells in vitro, the Sodium Nitroprusside (SNP) was used and the 1 mmol/l SNP can inhibit the cartilage cell proliferation at about 50 % after 24 h (fig. 1A). Hence, the 1 mmol/l SNP was used. The cartilage cells were treated with 1 mmol/l SNP for 24 h. Then, the cells were treated with different concentration of curcumin for 24 h. We found that, 1 μmol/l curcumin could recover the cartilage cells proliferation (fig. 1B). Data are presented as mean±SD. The independent sample t test was employed in comparison between groups. The experiments were repeated three times. The influence of curcumin on the TGF-β/Smad, ADAMTS-7 and apoptosis pathway in cartilage cell was shown in fig. 2. To explore the influence of curcumin on the TGF-β/Smad, ADAMTS-7 in cartilage cell, these protein expressions were detected by Western blot. The cartilage cells were treated with 1 mmol/l SNP for 24 h. Then, the cells were treated with 1 μmol/l curcumin for 24 h. The TGF-β and Smad show higher expression in the curcumin treatment group (p<0.05) and the ADAMTS-7 has lower expression in the curcumin treatment group (fig. 2A and fig. 2B). Meanwhile, the apoptosis pathway was also detected. The caspase-9 and Bcl- 2 was higher in the curcumin treatment group than without curcumin group. However, the Bax was lower (fig. 2C and fig. 2D). The measurement data are represented as mean±SD. The independent sample t test was used for comparisons between groups. The experiments were repeated three times. The influence of curcumin on the TGF-β/Smad, ADAMTS-7 and apoptosis pathway in cartilage cells after the TGF-β inhibition was shown in fig. 3. To certify the influence of TGF-β on the cartilage cell when the curcumin was added, the TGF-β was silenced. The cartilage cells were treated with 1 mmol/l SNP for 24 h. Then, the cells were treated with 1 μmol/l curcumin for 24 h and the Smad expression in two groups was low with no difference. ADAMTS-7 in the two groups has no difference (fig. 3A and fig. 3B). The caspase-9, Bcl- 2 and Bax also show no changes in two groups (fig. 3C and fig. 3D). The cell viability also shows no changes in two groups (fig. 3E). The measurement data are represented as mean±SD. The independent sample t-test was used for comparisons between groups. The experiments were repeated three times. The influence of curcumin on the TGF-β/Smad, ADAMTS-7 and apoptosis pathway in cartilage cell after the ADAMTS-7 overexpression was shown in fig. 4. When the ADAMTS-7 is overexpressed, the TGF-β and Smad has higher expression in the curcumin treatment group (fig. 4A and fig. 4B). However, the caspase-9, Bcl-2 and Bax also show no changes in the two groups (fig. 4C and fig. 4D). The cell viability also shows no changes in the two groups (fig. 4E). The measurement data are presented as mean±SD. The independent sample t test was used for comparisons between groups. The experiments were repeated three times.
Fig 1: The cell viability with different concentrations of SNP and curcumin
Note: (A) Cells were treated with different concentrations of SNP for 24 h and (B) Cells were added with 1.0 mmol/l of SNP for 24 h and then treated with different concentrations of curcumin for 24 h, *p<0.05 vs. blank; #p<0.05 vs. 0.5 mmol/l and &p<0.05 vs. 1.0 mmol/l in both the groups
Fig 2: Effect of curcumin on the TGF-β/Smad, ADAMTS-7 and apoptosis pathway
Note: (A) Expression of TGF-β, Smad, ADAMTS-7; (B) Results from Western blots show the detection of the protein expression of TGF-β, Smad, ADAMTS-7; (C) Expression of caspase-9, Bcl-2, Bax and (D) Results from Western blots show the detection of the protein expression of caspase-9, Bcl-2 and Bax where *p<0.05,  Curcumin- and
Curcumin- and  Curcumin+
Curcumin+
Fig 3: The influence of curcumin on cartilage cells after the TGF-β inhibition
Note: (A) Expression of TGF-β, Smad and ADAMTS-7; (B) Results from Western blots show the detection of the protein expression of TGF-β, Smad, ADAMTS-7; (C) Expression of caspase-9, Bcl-2 and Bax; (D) Results from Western blots show the detection of the protein expression of caspase-9, Bcl-2 and Bax; (E) The cell viability after curcumin treatment for 24 h,  Curcumin- and
Curcumin- and  Curcumin+
Curcumin+
Fig 4: The influence of curcumin on cartilage cells after the ADAMTS-7 overexpression
Note: (A) Expression of TGF-β, Smad, ADAMTS-7; (B) Results from Western blots show the detection of the protein expression of TGF-β, Smad, ADAMTS-7; (C) Expression of caspase-9, Bcl-2, Bax; (D) Results from Western blots show the detection of the protein expression of caspase-9, Bcl-2, Bax; (E) The cell viability after curcumin treatment for 24 h, *p<0.05,  Curcumin- and
Curcumin- and  Curcumin+
Curcumin+
OA is the most common chronic bone and joint disease worldwide. OA give a big social burden on public health[27,28]. In worldwide, 303 million adults were affected. OA has high prevalence; however there is no drug to treat it. In the in vivo test, the SNP was always used to make the OA model in the cartilage cells[10,29]. Hence, we also used the SNP to affect the cartilage cells. Meanwhile, we found that curcumin can promote the cartilage cells proliferation. Curcumin has important biological functions and future application prospects[17,30]. TGF-β/Smad pathway was involved in cell growth, differentiation, migration and apoptosis[20,31]. In this study, TGF-β played a key role in the curcumin promoting the cartilage cells proliferation. TGF-β could promote the cell proliferation. Smad3 is the primary molecule involved in TGF-β signaling. ADAMTS-7 is expressed in the bone, cartilage, synovium, tendon and ligament[25]. ADAMTS-7 was shown to facilitate vascular smooth muscle cell migration, thereby promoting neointima formation following vascular injury[24,32,33]. We found that curcumin could promote ADAMTS-7 expression and this may be useful to help the cartilage cells proliferation and OA reduction. In conclusion, curcumin can promote the cartilage cells proliferation via TGF-β/Smad/ADAMTS-7 axis and inhibit the cell apoptosis.
Acknowledgements:
This work was supported by Inner Mongolia Natural Science Foundation (No. 2021MS08036) and Million Project Science and Technology of Inner Mongolia Medical University (No. YKD2018KJBW (LH) 063).
Conflict of interests:
The authors declared no conflict of interest.
References
- Butler JJ, Azam MT, Weiss MB, Kennedy JG, Walls RJ. Supramalleolar osteotomy for the treatment of ankle osteoarthritis leads to favourable outcomes and low complication rates at mid-term follow-up: A systematic review. Knee Surg Sports Traumatol Arthrosc 2023;31(2):701-15.
[Crossref] [Google scholar] [PubMed]
- Cuffaro D, Ciccone L, Rossello A, Nuti E, Santamaria S. Targeting aggrecanases for osteoarthritis therapy: From zinc chelation to exosite inhibition. J Med Chem 2022;65(20):13505-32.
[Crossref] [Google scholar] [PubMed]
- Han S. Osteoarthritis year in review 2022: Biology. Osteoarthritis Cartilage 2022;30(12):1575-82.
[Crossref] [Google scholar] [PubMed]
- Jiang L, Zhu X, Rong J, Xing B, Wang S, Liu A, et al. Obesity, osteoarthritis and genetic risk: The rs182052 polymorphism in the ADIPOQ gene is potentially associated with risk of knee osteoarthritis. Bone Joint Res 2018;7(7):494-500.
[Crossref] [Google scholar] [PubMed]
- Mills ES, Becerra JA, Yensen K, Bolia IK, Shontz EC, Kebaish KJ, et al. Current and future advanced imaging modalities for the diagnosis of early osteoarthritis of the hip. Orthop Res Rev 2022;14:327-38.
[Crossref] [Google scholar] [PubMed]
- Shang H, Hao Y, Hu W, Hu X, Jin Q. Association between ADIPOQ gene variants and knee osteoarthritis in a Chinese population. Biosci Rep 2019;39(3):1-6.
[Crossref] [Google scholar] [PubMed]
- Zhao XX, Xie WQ, Xiao WF, Li HZ, Naranmandakh S, Bruyere O, et al. Perlecan: Roles in osteoarthritis and potential treating target. Life Sci 2022;312:121190.
[Crossref] [Google scholar] [PubMed]
- Yang J, Liu W. The role of AIM2 inflammasome in knee osteoarthritis. J Inflamm Res 2022;15:6453-61.
[Crossref] [Google scholar] [PubMed]
- Pirayeh N, Kazemi K, Rahimi F, Mostafaee N, Shaterzadeh-Yazdi MJ. The effect of balance training on functional outcomes in patients with knee osteoarthritis: A systematic review. Med J Islam Repub Iran 2022;36(1):1-15.
[Crossref] [Google scholar] [PubMed]
- Hart DA. Osteoarthritis as an umbrella term for different subsets of humans undergoing joint degeneration: The need to address the differences to develop effective conservative treatments and prevention strategies. Int J Mol Sci 2022;23(23):1-15.
[Crossref] [Google scholar] [PubMed]
- Rodríguez-Merchán EC. Intraarticular injections of mesenchymal stem cells in knee osteoarthritis: A review of their current molecular mechanisms of action and their efficacy. Int J Mol Sci 2022;23(23):1-15.
[Crossref] [Google scholar] [PubMed]
- Racz LZ, Racz CP, Pop LC, Tomoaia G, Mocanu A, Barbu I, et al. Strategies for Improving bioavailability, bioactivity, and physical-chemical behavior of curcumin. Molecules 2022;27(20):1-29.
[Crossref] [Google scholar] [PubMed]
- Huminiecki L. Evidence for multilevel chemopreventive activities of natural phenols from functional genomic studies of curcumin, resveratrol, genistein, quercetin, and luteolin. Int J Mol Sci 2022;23(23):1-17.
[Crossref] [Google scholar] [PubMed]
- Kesharwani P, Johnston TP, Sahebkar A. Anticancer potential of curcumin-cyclodextrin complexes and their pharmacokinetic properties. Int J Pharm 2022;631:122474.
[Crossref] [Google scholar] [PubMed]
- Ming T, Tao Q, Tang S, Zhao H, Yang H, Liu M, et al. Curcumin: An epigenetic regulator and its application in cancer. Biomed Pharmacother 2022;156:113956.
[Crossref] [Google scholar] [PubMed]
- Lu KH, Lu PW, Lin CW, Yang SF. Curcumin in human osteosarcoma: From analogs to carriers. Drug Discov Today 2022;28(2):103437.
[Crossref] [Google scholar] [PubMed]
- Saeedi F, Farkhondeh T, Roshanravan B, Amirabadizadeh A, Ashrafizadeh M, Samarghandian S. Curcumin and blood lipid levels: An updated systematic review and meta-analysis of randomised clinical trials. Arch Physiol Biochem 2022;128(6):1493-502.
[Crossref] [Google scholar] [PubMed]
- Kodentsova VM, Risnik DV, Sarkisyan VA, Frolova YV. Adequate and clinically effective levels of curcumin consumption. Vopr Pitan 2022;91(5):6-15.
[Crossref] [Google scholar] [PubMed]
- Bozkurt O, Kocaadam-Bozkurt B, Yildiran H. Effects of curcumin, a bioactive component of turmeric, on type 2 diabetes mellitus and its complications: An updated review. Food Funct 2022;13(23):11999-2010.
[Crossref] [Google scholar] [PubMed]
- Hiew LF, Poon CH, You HZ, Lim LW. TGF-β/smad signalling in neurogenesis: Implications for neuropsychiatric diseases. Cells 2021;10(6):1-17.
[Crossref] [Google scholar] [PubMed]
- Chalkia A, Gakiopoulou H, Theochari I, Foukas PG, Vassilopoulos D, Petras D. TGF-β1/Smad signalling in proliferative glomerulonephritis associated with autoimmune diseases. Mediterr J Rheumatol 2022;33(2):176-84.
[Crossref] [Google scholar] [PubMed]
- Yu H, Zhu Y. Expression of ADAMTS-7 and ADAMTS-12 in the nucleus pulposus during degeneration of rat caudal intervetebral disc. J Vet Med Sci 2012;74(1):9-15.
[Crossref] [Google scholar] [PubMed]
- Gao YX, Yu CA, Lu JH, Gao HM, Li G, Kong W, et al. ADAMTS-7 expression increases in the early stage of angiotensin II-induced renal injury in elderly mice. Kidney Blood Press Res 2013;38(1):121-31.
[Crossref] [Google scholar] [PubMed]
- Zhang Y, Lin J, Wei F. The function and roles of ADAMTS-7 in inflammatory diseases. Mediators Inflamm 2015;2015:1-11.
[Crossref] [Google scholar] [PubMed]
- Li JK, Cheng L, Zhao YP, Guo YJ, Liu Y, Zhang W, et al. ADAMTS-7 exhibits elevated expression in cartilage of osteonecrosis of femoral head and has a positive correlation with TNF-α and NF-κB P65. Mediators Inflamm 2015;2015:1-10.
[Crossref] [Google scholar] [PubMed]
- Kang H, Tong C, Li C, Luo J. miR-497 plays a key role in Tanshinone IIA-attenuated proliferation in OCI-AML3 cells via the MAPK/ERK1/2 pathway. Cytotechnology 2020;72:427-32.
[Crossref] [Google scholar] [PubMed]
- Mei Y, Williams JS, Webb EK, Shea AK, MacDonald MJ, Al-Khazraji BK. Roles of hormone replacement therapy and menopause on osteoarthritis and cardiovascular disease outcomes: A narrative review. Front Rehabil Sci 2022;3:1-11.
[Crossref] [Google scholar] [PubMed]
- Park J, Lee SY. A review of osteoarthritis signaling intervention using small-molecule inhibitors. Medicine 2022;101(32):e29501.
[Crossref] [Google scholar] [PubMed]
- Whittaker JL, Losciale JM, Juhl CB, Thorlund JB, Lundberg M, Truong LK, et al. Risk factors for knee osteoarthritis after traumatic knee injury: A systematic review and meta-analysis of randomised controlled trials and cohort studies for the OPTIKNEE Consensus. Br J Sports Med 2022;56(24):1406-21.
[Crossref] [Google scholar] [PubMed]
- Vargas-Mendoza N, Madrigal-Santillán E, Álvarez-González I, Madrigal-Bujaidar E, Anguiano-Robledo L, Aguilar-Faisal JL, et al. Phytochemicals in skeletal muscle health: Effects of Curcumin (from Curcuma longa Linn) and Sulforaphane (from Brassicaceae) on muscle function, recovery and therapy of muscle atrophy. Plants 2022;11(19):1-24.
[Crossref] [Google scholar] [PubMed]
- Saad HK, Abd Rahman AA, Ab Ghani AS, Taib WR, Ismail I, Johan MF, et al. Activation of STAT and SMAD signaling induces hepcidin re-expression as a therapeutic target for β-Thalassemia patients. Biomedicines 2022;10(1):1-17.
[Crossref] [Google scholar] [PubMed]
- Li T, Peng J, Li Q, Shu Y, Zhu P, Hao L. The mechanism and role of ADAMTS protein family in osteoarthritis. Biomolecules 2022;12(7):1-28.
[Crossref] [Google scholar] [PubMed]
- Wu W, Wang H, Yu C, Li J, Gao Y, Ke Y, et al. Association of ADAMTS-7 levels with cardiac function in a rat model of acute myocardial infarction. Cell Physiol Biochem 2016;38(3):950-8.
[Crossref] [Google scholar] [PubMed]