- *Corresponding Author:
- H. J. Zhao
Institute of Pharmacology, College of Pharmaceutical Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310053
E-mail: zhj@zcmu.edu.cn
| Date of Submission | 06 June 2016 |
| Date of Revision | 06 September 2016 |
| Date of Acceptance | 17 September 2016 |
| Indian J Pharm Sci 2016;78(5):602-607 |
This is an open access article distributed under terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Epithelial injury caused by reactive oxygen species including hydrogen peroxide plays a critical role in the pathogenesis of gastric disorders. Tetrahydroxystilbene glucoside is an active component extracted from Polygonum multiflorum, a famous traditional Chinese herb. This study aimed to evaluate the protective effect of tetrahydroxystilbene glucoside on hydrogen peroxide-induced oxidative stress on non-malignant gastric epithelial cells and to see if these protective actions can be extended to gastric cancer cells. The results from MTT assay showed that incubating gastric epithelial cells and gastric cancer cells with 200 μΜ hydrogen peroxide for 24 h significantly decreased cell viability, whereas pre-incubating cells with 10 μΜ tetrahydroxystilbene glucoside for 24 h protected the cells against hydrogen peroxide cell damage, and more significantly in gastric epithelial cells. Using 2′,7′-dichlorofluorescin diacetate, we found that tetrahydroxystilbene glucoside inhibited the production of intracellular reactive oxygen species in gastric epithelial cells and gastric cancer cells. In addition, it induced the expression of antioxidant enzymes hemeoxygenase-1 and NADPH quinone oxidoreductase-1. These results demonstrated that tetrahydroxystilbene glucoside exerted stronger cytoprotective effect against peroxide-induced cell death in gastric epithelial cells than gastric cancer cells and the cytoprotective mechanisms might be through decreasing oxidative stress and activating the expression of hemeoxygenase-1 and NADPH quinone oxidoreductase-1.
Keywords
Tetrahydroxy stilbene glucoside, GES-1 cells, SGC-7901 cells, H2O2
The impact of gastric diseases on human health, such as chronic gastritis, duodenal, gastric ulceration and adenocarcinoma, remains a big issue deserving worldwide attention. Oxidative stress is the major gastric pathologic feature and plays an essential role in the multiple progressions of gastric diseases [1]. The excessive generation of reactive oxygen species (ROS) is associated with cell death and generation of ROS is associated with cell death an keep cellular homeostasis, excessive ROS can cause damage to lipids and proteins as well as single stranded DNA breaks [2] and gastric epithelium is exposed to higher ROS lever than other tissues [3,4]. Therefore, it is necessary for cells to effectively counteract ROS generation by triggering their own defensive mechanisms with the help of antioxidants. Hemeoxygenase-1 (HO-1) is a highly inducible, stress-responsive protein (also called heat shock protein 32), which catalyzes the first and ratelimiting step in heme degradation, a potent oxidant [5]. Indeed, ample evidence currently supports the notion that HO-1 serves to provide potent cytoprotective effects in many in vitro and in vivo models of oxidantinduced cellular and tissue injury. The NADPHquinone oxidoreductase 1 (NQO1) is a predominantly cytosolic enzyme which provides cells with multiple layers of protection against oxidative stress, including the direct detoxification of highly reactive quinones [6,7].
Polygonum multiflorum, a traditional Chinese medicinal herb, has been widely used as a tonic and antiaging agent [8]. Tetrahydroxystilbene glucoside (TSG) is an active component extracted from P. multiflorum. Recently, TSG has received a great deal of attention owing to its biological properties, including antioxidative [9], antiatherosclerotic [10] and antiinflammatory [11] activities. However, whether TSG has a protective activity against oxidative stress related injury to gastric epithelial cells remains unknown. TSG is the water-soluble active component. Therefore, TSG may well penetrate the cells with free diffusion. Some reports found that TSG was mainly absorbed in the stomach, but the absorption rate was reduced. So its absorption in the stomach may be by active transport. Lv et al. [12] discovered that TSG was rapidly absorbed into the body fluids and widely distributed throughout the body, with great efficiency of utility, followed by quick elimination. Sun et al. [13] preliminarily determined that the metabolites of TSG were glucuronic acid combination. Wang et al. [14] found that bile excretion might be the main drainage way of TSG and its metabolites.
Herein, in the present study, we investigated whether TSG protected ROS induced damage on human gastric epithelial cell line (GES-1) with oxidative stress mediated by hydrogen peroxide (H2O2) via the antioxidant enzymes HO-1 and NQO1. More importantly, we demonstrated whether TSG performed the same cytoprotective effect on gastric cancer cell line SGC-7901, thus weakening the antitumor effect of other drugs through decreasing ROS generation.
Materials and Methods
TSG (purity above 98%) was purchased from Putian Biological Technology Co., Ltd. (Beijing, China). 2′,7′-dichlorofluorescin diacetate (DCFH-DA), MTT (3- [4,5-dimehyl-2-thiazolyl]-2,5-diphenyl-2Htetrazolium bromide) and H2O2 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco's modified Eagle's medium DMEM/F12 and trypsin ethylenediamine tetra acetic acid (EDTA) were purchased from Gibco Invitrogen (Carlsbad, CA, USA).
Cell culture
GES-1 and gastric cancer cell line SGC-7901 were purchased from Bogoo (Shanghai, China) and cultured in DMEM/F12 medium, supplemented with 10% heat inactivated fetal bovine serum (FBS, Gibco) and 1% penicillin/streptomycin (Gibco). All the cells were incubated at 37° in humidified 5% CO2, 95% air atmosphere.
Cell proliferation assay
Inhibition of cell proliferation by TSG was measured by the MTT assay. GES-1 cells and SGC-7901 cells were plated in 96-well plates at a density of 4000 cells per well. After incubation with medium for 24 h, cells were incubated with indicated concentrations of TSG for 24 h. Then, MTT was added to cell cultures at a final concentration of 5 mg/ml for 4 h at 37°, after that the media were carefully removed. The adherent cells were solubilized with 100 μl of DMSO. Absorbance was measured at 570 nm using a microplate reader (BIO-RAD, California, USA).
Measurement of ROS
Generation of intracellular ROS was detected using the non-fluorescent probe DCFH-DA. DCFH-DA, a nonfluorescent substance, passively diffuses into cells and is deacetylated by esterases to form non-fluorescent DCFH. In the presence of ROS, DCFH reacts with ROS to form the fluorescent product DCF, which is trapped inside the cell [12]. The cells were treated with H2O2 for 4 h after being pretreated with or without TSG for 24 h. Then cells were harvested and incubated with 5 μM DCFH-DA at 37° for 30 min and analyzed by flow cytometry (Millipore, Bedford, USA).
Quantitative real time polymerase chain reaction
After treating with TSG, the total RNA was extracted with Trizol (Invitrogen, California, USA), using the standard method. cDNA synthesis was performed with 1 μg of total RNA, using the One Step Prime Script miRNA cDNA Synthesis Kit (TaKaRa, Dalian, China) according to the manufacturer’s instructions [13]. A quantitative real-time polymerase chain reaction (qRT-PCR) was performed on the CFX 96 Touch TM Real-Time PCR Detection System (BIO-RAD, California, USA), using the miScript SYBR Green PCR Kit (TaKaRa, Dalian, China) according to the manufacturer’s protocol. Glyceraldehyde-3 phosphate dehydrogenase (GAPDH) was used as the endogenous control. The gene-specific primers of human HO-1 and NQO1 were listed in Table 1. Data were presented as mean±SD. Statistical analysis was performed using one-way ANOVA with P<0.05 being considered to indicate statistical significance.
| Genes | Forward (5′-3′) | Reverse (3′-5′) |
|---|---|---|
| GAPDH | AGAAGGCTGGGGCTCATTTG | AGGGGCCATCCACAGTCTTC |
| HO-1 | ACAGGGTGACAGAAGAGGCTAA | CTGTGAGGGACTCTGGTCTTTG |
| NQO1 | CAGCGGCTCCATGTACT | GACCTGGAAGCCACAGAAG |
Table 1: The List of Gene Primers Used in Qrt-Pcr Analysis
Results and Discussion
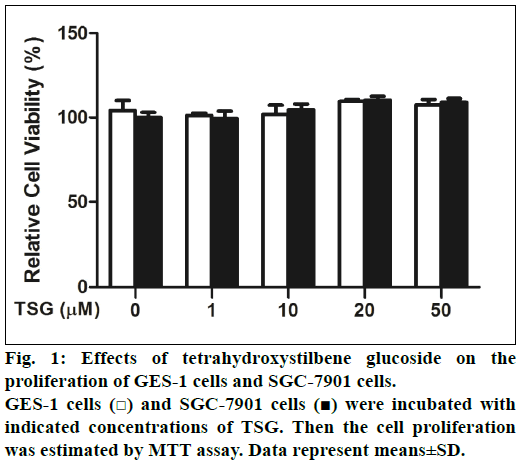
First of all, the effects of TSG on GES-1 cells and SGC-7901 cells proliferation were examined. Cells were treated with 0-50 μM TSG for 24 h and cell proliferation was analyzed using the MTT assay. Compared with untreated control cells, we found that TSG did not inhibit either the proliferation of GES- 1cells or SGC-7901 cells (fig. 1).
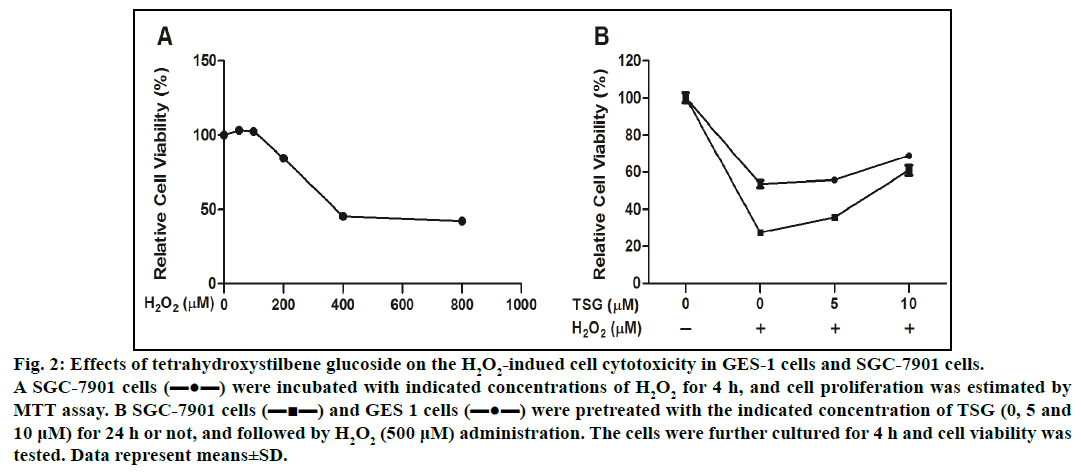
To examine the cytotoxic potential of H2O2-induced cell damage, we selected the tumor cells (SGC-7901 cells) with high reproduction and easy accessibility. SGC-7901 cells were treated with increasing concentration of H2O2 (0-800 μM) for 4 h, the cell viability was significantly decreased to 42.2±0.62%. The results shown in fig. 2A demonstrated that IC50 valued was 510±4.95 μM. Therefore we selected the concentration of 500 μM H2O2 for the following experiment. As shown in fig. 2B, pretreatment with the indicated concentration of TSG reduced H2O2-induced cell damage.
Fig. 2: Effects of tetrahydroxystilbene glucoside on the H2O2-indued cell cytotoxicity in GES-1 cells and SGC-7901 cells.
A SGC-7901 cells (▬●▬) were incubated with indicated concentrations of H2O2 for 4 h, and cell proliferation was estimated by
MTT assay. B SGC-7901 cells (▬■▬) and GES 1 cells (▬●▬) were pretreated with the indicated concentration of TSG (0, 5 and
10 μM) for 24 h or not, and followed by H2O2 (500 μM) administration. The cells were further cultured for 4 h and cell viability was
tested. Data represent means±SD.
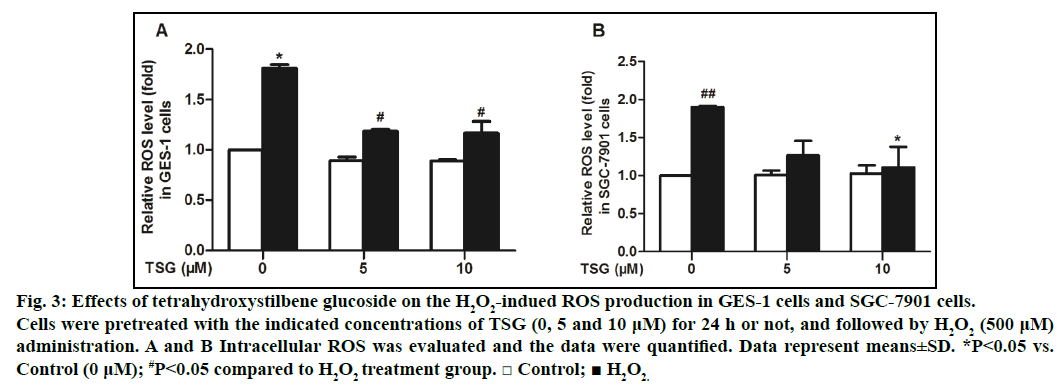
After that, we examined the effect of TSG on ROS generation in GES-1 cells and SGC-7901 cells. Generation of ROS in cells was measured using fluorescence assay with DCFH-DA probe by flow cytometry. The results demonstrated that TSG could dose-dependently attenuate H2O2-induced ROS production compared with the H2O2 treatment alone (figs. 3A and 3B).
Fig. 3: Effects of tetrahydroxystilbene glucoside on the H2O2-indued ROS production in GES-1 cells and SGC-7901 cells.
Cells were pretreated with the indicated concentrations of TSG (0, 5 and 10 μM) for 24 h or not, and followed by H2O2 (500 μM)
administration. A and B Intracellular ROS was evaluated and the data were quantified. Data represent means±SD. *P<0.05 vs.
Control (0 μM); #P<0.05 compared to H2O2 treatment group. □ Control; ■ H2O2.
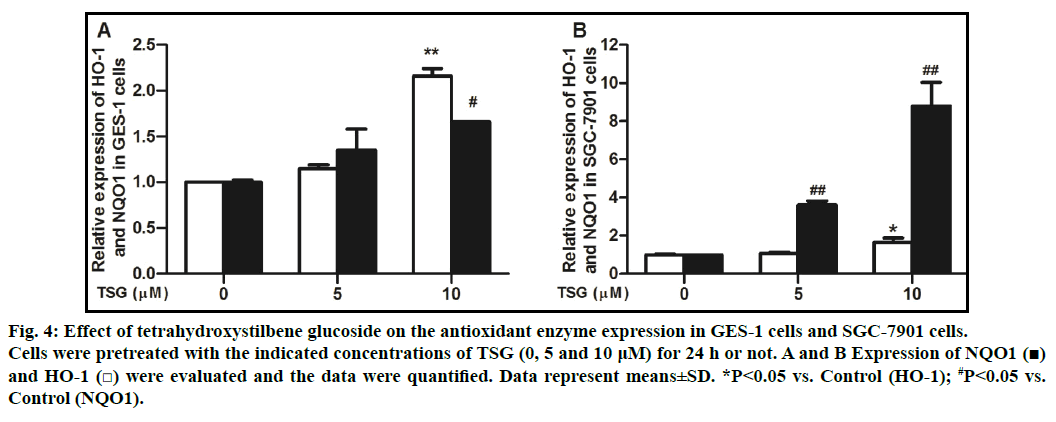
To explore whether TSG leads to the expression of antioxidant enzymes, we examined the gene levels of HO-1 and NQO1. qRT-PCR analysis was conducted to confirm the induction of antioxidant gene. Treatment of cells with TSG (0, 5 and 10 μM) for 24 h showed that the compound induced the expression of NQO1 and HO-1 genes in a concentration-dependent manner (figs. 4A and 4B).
Fig. 4: Effect of tetrahydroxystilbene glucoside on the antioxidant enzyme expression in GES-1 cells and SGC-7901 cells.
Cells were pretreated with the indicated concentrations of TSG (0, 5 and 10 μM) for 24 h or not. A and B Expression of NQO1 (■)
and HO-1 (□) were evaluated and the data were quantified. Data represent means±SD. *P<0.05 vs. Control (HO-1); #P<0.05 vs.
Control (NQO1).
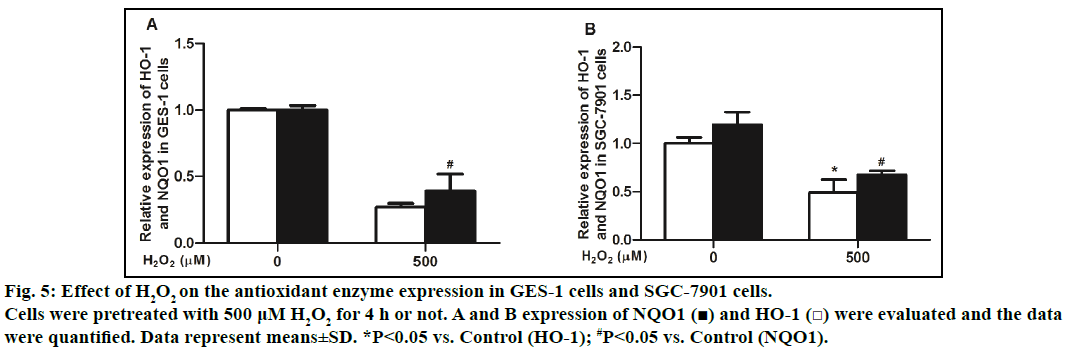
We examined the gene levels of HO-1 and NQO1 after the negative control H2O2 treatment. The cells treated with 500 μM H2O2 for 4 h showed that the expression of NQO1 and HO-1 genes were reduced. The elevated ROS is an essential factor in the onset and development of gastric epithelial injury and various gastric disorders. Cells possess a plenty of cytoprotective enzymes, which protect living cells from oxidative stressinduced damage. However, exogenous administration of antioxidants is also an important strategy to protect cells against oxidative damage. TSG has been reported to counteract oxidative stress in different cell lines [9,15,16]. In this study, we first demonstrated that TSG could protect gastric epithelial GES-1 cells from oxidative damage. It is further found that TSG protected oxidative damages through inhibition of oxidative stress and up-regulation the expression of antioxidant enzymes HO-1 and NQO1.
Different from endogenous H2O2, exogenously added H2O2 is less effective at leading to a signaling response [17], whereas it can rapidly diffuse across membranes and immediately increase ROS lever, resulting in cell damage that ultimately decreases cellular function [18,19]. Our results demonstrated that GES-1 cells showed a dose-dependent viability loss after exposed to H2O2 and ROS levers elevated immediately. Meanwhile, pretreated with different concentration of TSG significantly decreased cell viability loss, reduced the ROS generation and activating antioxidant enzymes of HO-1 and NQO1. As shown in fig. 4, pretreated TSG activated antioxidant enzymes of HO-1 and NQO1 in GES-1 cells and SGC-7901 cells. In GES-1cells, HO-1 was increased about 2.16 folds compared with that of control group and NQO1 was increased about 1.66 folds. In SGC-7901 cells, HO-1 was increased about 1.66 folds compared with that of control group and NQO1 was increased about 8.79 folds. These results indicated that HO-1 might be, at least in part, mainly involved in this process in GES-1 cells, and NQO1 might play a leading role in SGC-7901 cells, though further investigation is required. These results suggested that TSG might exert gastro protective effect through an antioxidant pathway, which was in agreement with other reports [4,20].
We also found that TSG exerted cytoprotective effect against H2O2-induced cell death in GES-1 cells, and also played a protective role in gastric cancer cells (SGC-7901), although the protection on SGC-7901 cells was not strong (fig. 5). These results indicated that TSG protected gastric epithelial cells from oxidative damage and at the same time, might reduce the effects of other antitumor drugs.
Fig. 5: Effect of H2O2 on the antioxidant enzyme expression in GES-1 cells and SGC-7901 cells.
Cells were pretreated with 500 μM H2O2 for 4 h or not. A and B expression of NQO1 (■) and HO-1 (□) were evaluated and the data
were quantified. Data represent means±SD. *P<0.05 vs. Control (HO-1); #P<0.05 vs. Control (NQO1).
In summary, TSG presents cytoprotective effect against oxidative damage and may be further development as a therapeutic agent against gastric diseases. It should be mentioned that the detail molecular mechanisms for antioxidant effect of TSG are important aspects to study in the future. Furthermore, this study aimed to make available information on the prevention of TSG against hydrogen peroxide-induced cell death. It would be interest to test the treatment of TSG. We considered performing these experiments in our future studies.
Acknowledgments
This work was financially supported by Zhejiang Provincial Natural Science Fund (LZ15H310001) and Zhejiang Province Chinese Medicine Scientific Research Fund Project (2013ZA083) and Zhejiang Provincial Department of Education Research Fund Project (751214A00401). We extremely thank Dr. Si- Yue Lou, Dr. Cheng-Hua Lou and Dr. Lun-Ming Liu from Zhejiang Chinese Medical University for their technical help.
Financial support and sponsorship
Nil.
Conflict of interest
The authors have declared that there is no conflict of interest.
References
- Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiological reviews 2014;94:329-54.
- Gal R, Halmosi R. The role of oxidative stress in heart failure. Orvosi hetilap 2015;156:1916-20.
- Halliwell B, Clement MV, Ramalingam J, Long LH. Hydrogen peroxide. Ubiquitous in cell culture and in vivo? IUBMB life 2000;50:251-57.
- Hu XT, Ding C, Zhou N, Xu C. Quercetin protects gastric epithelial cell from oxidative damage in vitro and in vivo. Eur J Pharmacol 2015;754:115-24.
- Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol 2000;279:L1029-37.
- Cui X, Li L, Yan G, Meng K, Lin Z, Nan Y, et al. High expression of NQO1 is associated with poor prognosis in serous ovarian carcinoma. BMC Cancer 2015;15:244.
- Rougee LR, Riches Z, Berman JM, Collier AC. The ontogeny and population variability of human hepatic NADPH dehydrogenase quinone oxido-reductase-1 (NQO1). Drug Metab Dispos 2016;44:967-74.
- Lin L, Ni B, Lin H, Zhang M, Li X, Yin X, et al. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: A review. J Ethnopharmacol 2015;159:158-83.
- Zhang JK, Yang L, Meng GL, Fan J, Chen JZ, He QZ, et al. Protective effect of tetrahydroxystilbene glucoside against hydrogen peroxide-induced dysfunction and oxidative stress in osteoblastic MC3T3-E1 cells. Eur J Pharmacol 2012;689:31-7.
- Yao W, Fan W, Huang C, Zhong H, Chen X, Zhang W. Proteomic analysis for antiatherosclerotic effect of tetrahydroxystilbene glucoside in rats. Biomede Pharmacother 2013;67:140-5.
- Huang C, Wang Y, Wang J, Yao W, Chen X, Zhang W. TSG (2,3,4',5-tetrahydroxystilbene 2-O-beta-D-glucoside) suppresses induction of pro-inflammatory factors by attenuating the binding activity of nuclear factor-κB in microglia. J Neuroinflammation 2013;10:129.
- Lv G, Lou Z, Chen S, Gu H, Shan L. Pharmacokinetlcs and tissue distribution of 2,3,5,4 tetrahydroxystilbene-2-O-β-D-glucoside from traditional Chinese medicine Polygonum multiflorum following oral administration to rats. J Ethnopharmacol 2011;137:449-56.
- Sun JH, Yuan ZF, Wang CY, Xu HJ, Zhang LT. Pharmacokinetics of stilbene glycoside from Polygonum multiflorum in rats in vivo. Chinese Traditional and Herbal Drugs 2005;36:405-8.
- Wang CY, Yuan ZF, Wang Q, Xie FL, Yang W, Zhang LT. HPLC simultaneous determination of stilbene glycoside and its metabolite in rat bile liquid. Chin J Pharm Anal 2009;29:53-57.
- Zhang W, Chen XF, Huang YJ, Chen QQ, Bao YJ, Zhu W. 2,3,4',5-Tetrahydroxystilbene-2-O-β-D-glucoside inhibits angiotensin II-induced cardiac fibroblast proliferation via suppression of the reactive oxygen species-extracellular signal-regulated kinase 1/2 pathway. Clin Exp Pharmacol Physiol 2012;39:429-37.
- Tao L, Li X, Zhang L, Tian J, Li X, Sun X, et al. Protective effect of tetrahydroxystilbene glucoside on 6-OHDA-induced apoptosis in PC12 cells through the ROS-NO pathway. PLoS one 2011;6:e26055.
- Choi MH, Lee IK, Kim GW, Kim BU, Han YH, Yu DY, et al. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature 2005;435:347-53.
- Ferreira IL, Resende R, Ferreiro E, Rego AC, Pereira CF. Multiple defects in energy metabolism in Alzheimer's disease. Curr Drug Targets 2010;11:1193-206.
- Jarrett SG, Lewin AS, Boulton ME. The importance of mitochondria in age-related and inherited eye disorders. Ophthalmic Res 2010;44:179-90.
- Harada S, Nakagawa T, Yokoe S, Edogawa S, Takeuchi T, Inoue T, et al. Autophagy deficiency diminishes indomethacin-induced intestinal epithelial cell damage through activation of the ERK/Nrf2/HO-1 Pathway. J Pharmacol Exp Ther 2015;355:353-61.