- Corresponding Author:
- Nguyen D. Thanh
Department of Chemistry, College of Science, Hanoi National University, 19 Le Thanh Tong, Ha Noi 10000, Viet Nam
E-mail: nguyendinhthanh@hus.edu.vns
| Date of Submission | 01 February 2011 |
| Date of Revision | 24 January 2012 |
| Date of Acceptance | 13 February 2012 |
| Indian J Pharm Sci, 2012, 74 (1): 54-62 |
Abstract
Some new substituted benzaldehyde (2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl) thiosemicarbazones were synthesised by reaction of 2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl thiosemicarbazide and different substituted benzaldehydes. The reaction was performed using conventional and microwave-assisted heating methods. The structures of thiosemicarbazones were confirmed by spectroscopic (IR, 1H NMR, 13C NMR and MS) method. The antioxidant activity of these thiosemicarbazones was evaluated, in vitro and in vivo, and it’s shown that some of these compounds had significant antioxidant activity.
Keywords
Antioxidant activity, D-galactose, microwave-assisted, thiosemicarbazones
Introduction
Thiosemicarbazones, which have NH-C(=S) NHN=C bond, are a class of compounds that have been evaluated over the last 50 years as antivirals and as anticancer therapeutics, as well as for their parasiticidal action against Plasmodium falciparum and Trypanasoma cruzi which are the causative agents of malaria and Chagas’s disease, respectively [1]. The chemistry of thiosemicarbazide derivatives of saccharides is interested [2,3]. These compounds arouse interest as versatile intermediates for preparing various (e.g., heterocyclic) derivatives as well. Thiosemicarbazones can be used for making complex formation of metallic ions [4-13]. Thiosemicarbazones exhibit various biological activities such as antituberculosis [14,15], antimicrobial [9,16-18], antiinflammatory [19], anticonvulsant [9,20], antihypertensive [21], local anesthetic [22], anticancer [10,23], hypoglycemic [24], and cytotoxic activities [9], also antioxidant agents [11,25]. A number of galactosyl thiosemicarbazide derivatives showed significant in vivo antimicrobial and in vitro antioxidant activity, which could be used as leads for the development of effective antiatherosclerotic agents [2,20,26,27]. On the other hand these molecules can also serve as phosphane-free multidentate ligands for transition-metal catalysis, and they are efficient ligands for palladium-catalyzed coupling reactions in air [25].
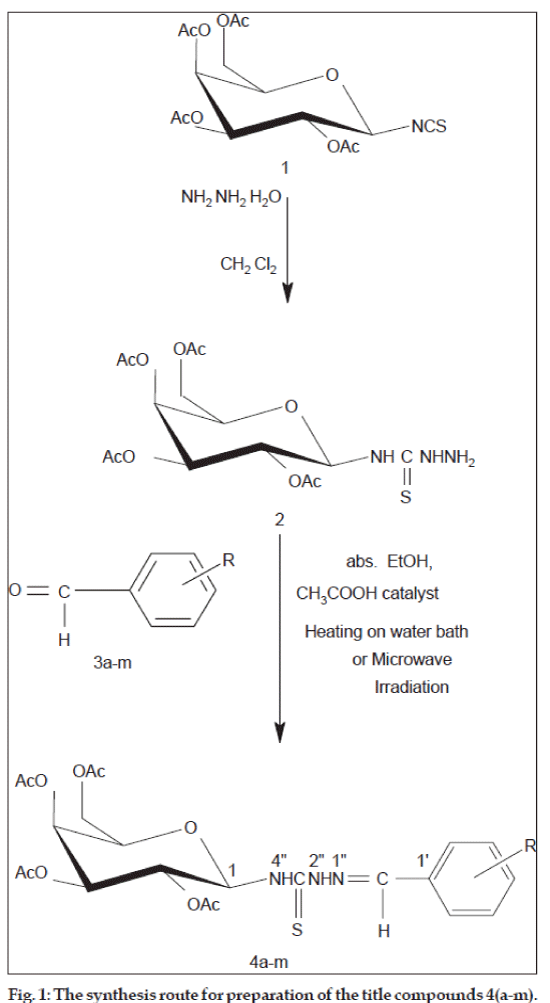
In the past some papers have been published for the synthesis of aldehyde/ketone (per-O-acetylated glycopyranosyl)thiosemicarbazones [2,3,18,25,28-30]. The main synthetic step for the synthesis of these molecules is being the reaction of (per- O-acetylglycosyl)thiosemicarbazide with the coresponding carbonyl compounds. Continuing our studied on the synthesis and the reactivity of (per-O-acetatylglycopyranosyl)isothiocyanate and (per-O-acetatylglycopyranosyl) thiosemicarbazides [29,30], we report herein a systematic study for the synthesis and spectral characterization of a series of aromatic aldehyde 4-(b-D-galactopyranosyl)thiosemicarbazones using microwave-assisted method [31].
Materials and Methods
All melting points were determined by open capillary method on Stuart SMP3 instrument (Bibby Sterilin Ltd, UK) and are uncorrected. IR spectra (KBr disc) were recorded on a Impact 410 FT-IR Spectrometer (Nicolet, USA). 1H and 13C NMR spectra were recorded on Bruker Avance Spectrometer AV500 (Bruker, Germany) at 500.13 MHz and 125.77 MHz, respectively, using DMSO-d6 as solvent and TMS as an internal standard. All the starting materials and reagents were purchased from commercial suppliers and used after further purification. (2,3,4,6-Tetra-Oacetyl- b-D-galactopyranosyl)isothiocyanate (1) was prepared by the reaction of (tetra-O-acetylated-b-Dgalactopyranosyl) bromide (prepared from D-galactose, using the procedure for D-glucose) [32] with lead thiocyanate in dried toluene [18]. (2,3,4,6-Tetra-Oacetyl- β-D-galactopyranosyl)thiosemicarbazide (2) was prepared from corresponding isothiocyanate compound by modifying our method [30].
General procedure for synthesis of substituted benzaldehyde (2,3,4,6-tetra-O-acetyl-β-Dgalactopyranosyl) thiosemicarbazones (4a-m)
Conventional Method (for compounds 4a, 4b, 4d and 4m): A suspension mixture of (2,3,4,6-tetra- O-acetyl-β-D-glucopyranosyl)thiosemicarbazide (1) (4.21 g, 1 mmol) and corresponding substituted benzaldehyde 3(a-m) (1 mmol) and glacial acetic acid (1 ml) in methanol (20 ml) was refluxed for 90 min. The solvent was removed under reduced pressure and the residue was triturated with water, the precipitate was filtered by suction and recrystallized from 95% ethanol or 70% ethanol to afford the title compounds of benzaldehyde (2,3,4,6-tetra-O-acetyl-β- D-galactopyranosyl)thiosemicarbazones (4a-m).
Microwave-assisted Method (for all compounds): A suspension mixture of (2,3,4,6-tetra-O-acetyl- β-D-glucopyranosyl)thiosemicarbazide 1 (4.21 g, 1 mmol) and corresponding substituted benzaldehyde 3(a-m) (1 mmol) and glacial acetic acid (0.05 ml) in 99.5% ethanol (2–5 ml) was irradiated with reflux for 5-7 min in microwave oven. The suspension mixture became clear solution after irradiating in 3-4 min. After reaction the mixture was cooled to room temperature, the colourless crystals were filtered with suction. The crude product was recrystallized from 95% ethanol or 70% ethanol to afford the title compounds of benzaldehyde (2,3,4,6-tetra-O-acetyl-β- D-galactopyranosyl)thiosemicarbazones (4a-m). The physical and spectral (IR, 1H NMR, 13C NMR and MS) data are in good agreement with their structures.
4-Nitrobenzaldehyde (2,3,4,6-tetra-O-acetyl-β-Dgalactopyranosyl) thiosemicarbazone (4a)
Light yellow solid; mp 157-158°; IR (KBr, cm–1): 3337 (NH), 1744 (C=O), 1587 (CH=N), 1226, 1048 (C-O-C); 1H NMR (DMSO-d6, δ.ppm): 9.00 (d, 1H, J 9.0 Hz, H-4”), 12.17 (s, 1H, 1H, H-2”), 8.20 (s, 1H, H imine), 5.93 (t, 1H, J 9.0 Hz, H-1), 5.35 (m, 1H, H-2), 5.40 (dd, 1H, J 10.0, 3.5 Hz, H-3), 5.35 (m, 1H, H-4), 4.33 (t, 1H, J 6.5 Hz, H-5), 4.07 (d, 1H, J 6.5 Hz, H-6), 8.14 (d, 1H, J 9.0 Hz, H-2’),8.27 (d, 1H, J 9.0 Hz, H-3’), 8.27 (d, 1H, 1H, J 9.0 Hz, H-5’), 8.14 (d, 1H, J 9.0 Hz, H-6’), 1.96- 2.16 (s, 1H, 12H, CH3CO); 13C NMR (DMSO-d6, δ ppm): 178.84 (C=S), 81.94 (C-1), 68.67 (C-2), 70.61 (C-3), 67.53 (C-4), 71.71 (C-5), 61.28 (C-6), 140.21 (C-1’), 123.77 (C-2’), 128.53 (C-3’), 141.23 (C-4’), 128.53 (C-5’), 123.77 (C-6’), 147.90 (C-imine), 20.32-20.51 (CH3CO), 169.36-170.01 (CH3CO); MS m/z: 555 (M+ + H, 72%), 577 (M+ + Na, 100%) for C22H26N4O11S.
3-Nitrobenzaldehyde (2,3,4,6-tetra-O-acetyl-β-Dgalactopyranosyl) thiosemicarbazone (4b)
Light yellow solid; mp 169-170°; IR (KBr, cm–1): 3338 (NH), 1745 (C=O), 1625 (CH=N), 1228, 1054 (C-O-C); 1H NMR (DMSO-d6, δ ppm): 8.96 (d, 1H, J 9.0 Hz, H-4”), 12.13 (s, 1H, H-2”), 8.22 (s, 1H, H imine), 5.91 (t, 1H, J 9.0 Hz, H-1), 5.34 (m, 1H, 1H, H-2), 5.41 (dd, 1H, J 9.5, 3.5 Hz, H-3), 5.34 (m, 1H, H-4), 4.34 (t, 1H, J 6.5 Hz, H-5), 4.06 (m, 1H, H-6), 8.22 (s, 1H, H-2’), 8.36 (d, 1H, J 8.0 Hz, H-4’), 7.74 (t, 1H, J 8.0 Hz, H-5’), 8.26 (dd, 1H, J 8.0, 1.0 Hz, H-6’), 1.96-2.00 (s, 1H, CH3CO); 13C NMR (DMSO-d6, δ ppm): 178.69 (C=S), 81.89 (C-1), 68.62 (C-2), 70.50 (C-3), 67.50 (C-4), 71.64 (C-5), 61.23 (C-6), 130.15 (C-1’), 135.71 (C-2’), 141.58 (C-3’), 133.44 (C-4’), 124.40 (C-5’), 122.06 (C-6’), 148.33 (C-imine), 20.32- 20.52 (CH3CO), 169.33-169.99 (CH3CO); MS m/z: 554 (M+ 100%) for C22H26N4O11S.
4-Fluorobenzaldehyde (2,3,4,6-tetra-O-acetyl-β-Dgalactopyranosyl) thiosemicarbazone (4c)
White solid; mp 113-114°; IR (KBr, cm–1): 3341 (NH), 1606 (CH=N), 1750 (C=O), 1261, 1045 (C-O-C); 1H NMR (DMSO-d6, δ.ppm): 8.75 (d, 1H, J 9.0 Hz, H-4”), 11.93 (s, 1H, H-2”), 8.11 (s, 1H, H imine), 5.90 (t, 1H, J 9.0 Hz, H-1), 5.32 (m, 1H, H-2), 5.40 (dd, 1H, J 10.0, 3.5 Hz, H-3), 5.32 (m, 1H, H-4), 4.33 (t, 1H, J 6.0 Hz, H-5), 4.06 (m, 1H, H-6), 7.28 (t, 1H, J 9.0 Hz, H-2’), 7.92 (dd, 1H, J 9.0, 6.0 Hz, H-3’), 7.92 (dd, J 9.0, 6.0 Hz, H-5’), 7.28 (t, 1H, J 9.0 Hz, H-6’), 2.02-2.15 (s, 12H, CH3CO); 13C NMR (DMSO-d6, δ ppm): 178.35 (C=S), 81.76 (C-1), 68.61 (C-2), 70.55 (C- 3), 67.51 (C-4), 71.56 (C-5), 61.24 (C-6), 130.37 (C-1’), 129.84 (C-2’), 115.73 (C-3’), 163.25 (C-4’), 115.73 (C-5’), 129.84 (C-6’), 142.67 (C-imine), 20.29-20.48 (CH3CO), 169.31-169.98 (CH3CO); MS m/z: 528 (M+ + H, 66%), 550 (M+ + Na, 100%) for C22H26FN3O9S.
4-Chlorobenzaldehyde (2,3,4,6-tetra-O-acetyl-β-Dgalactopyranosyl) thiosemicarbazone (4d)
White solid, mp 173-174°; IR (KBr, cm–1): 3325 (NH), 1754 (C=O), 1600 (CH=N), 1245, 1054 (C-O-C); 1H NMR (DMSO-d6, δ ppm): 8.78 (d, 1H, J 9.0 Hz, H-4”), 11.95 (s, 1H, H-2”), 8.08 (s, 1H, H imine), 5.88 (t, 1H, J 9.0 Hz, H-1), 5.30 (t, 1H, J 9.5 Hz, H-2), 5.37 (dd, 1H, J 10, 3.5 Hz, H-3), 5.32 (d, 1H, J 4.0 Hz, H-4), 4.30 (t, 1H, J 6.5 Hz, H-5), 4.04 (d, 1H, J 6.5 Hz, H-6), 7.48 (d, 1H, J 8.5 Hz, H-2’), 7.86 (d, 1H, J 8.5 Hz, H-3’), 7.86 (d, 1H, J 8.5 Hz, H-5’), 7.48 (d, 1H, 8.5 Hz, H-6’), 2.02-2.15 (s, 12H, CH3CO); 13C NMR (DMSO-d6, δ ppm): 178.53 (C=S), 81.92 (C-1), 68.73 (C-2), 70.68 (C-3), 67.62 (C-4), 71.72 (C-5), 61.37 (C-6), 134.86 (C-1’), 128.88 (C-2’), 129.36 (C-3’), 132.81 (C-4’), 129.36 (C-5’), 128.88 (C-6’), 142.70 (C-imine), 20.41-20.61 (CH3CO), 169.51-170.17 (CH3CO); MS m/z: 544/546 (M+ + H, 100%/34%), 566/568 (M+ + Na, 98%/39%) for C22H26 35ClN3O9S/C22H26 37ClN3O9S.
4-Bromobenzaldehyde (2,3,4,6-tetra-O-acetyl-β-Dgalactopyranosyl) thiosemicarbazone (4e)
White solid, mp 159-160°; IR (KBr, cm–1): 3331 (NH), 1748 (C=O), 1595 (CH=N), 1227, 1052 (C-O-C); 1H NMR (DMSO-d6, δ ppm): 8.77 (d, 1H, J 9.0 Hz, H-4”), 11.95 (s, 1H, H-2”), 8.06 (s, 1H, H imine), 5.88 (t, 1H, J 9.0 Hz, H-1), 5.30 (t, 1H, J 10.0 Hz, H-2), 5.37 (dd, 1H, J 10.0, 4.0 Hz, H-3), 5.31 (d, 1H, 4.5, H-4), 4.30 (t, 1H, J 6.5 Hz, H-5), 4.03 (d, 1H, J 6.5 Hz, H-6), 7.79 (d, 1H, J 8.5 Hz, H-2’), 7.61 (d, 1H, J 8.5 Hz, H-3’), 7.61 (d, 1H, J 8.5 Hz, H-5’), 7.79 (d, 1H, J 8.5 Hz, H-6’), 1.93-2.13 (s, 12H, CH3CO); 13C NMR (DMSO-d6, δ ppm): 178.41 (C=S), 81.77 (C-1), 68.59 (C-2), 70.54 (C-3), 67.48 (C-4), 71.56 (C-5), 61.21 (C-6), 133.05 (C-1’), 131.62 (C-2’), 129.43 (C-3’), 123.50 (C-4’), 129.43 (C-5’), 131.62 (C-6’), 142.56 (C-imine), 20.28-20.47 (CH3CO), 169.27-169.94 (CH3CO); MS m/z: 588/590 (M+ + H, 89%/78%), 610/612 (M+ + Na, 100%/97%) for C22H26 79BrN3O9S/C22H26 81BrN3O9S.
4-Methybenzaldehyde (2,3,4,6-tetra-O-acetyl-β-Dgalactopyranosyl) thiosemicarbazone (4f)
White solid, mp 180-181°; IR (KBr, cm–1): 3334 (NH), 1747 (C=), 1609 (CH=N), 1233, 1054 (C-O-C); 1H NMR (DMSO-d6, δ ppm): 8.62 (d, 1H, J 9.0 Hz, H-4”), 11.85 (s, 1H, H-2”), 8.06 (s, 1H, H imine), 5.85 (t, 1H, J 9.5 Hz, H-1), 5.27 (t, 1H, J 10.0 Hz, H-2), 5.36 (dd, 1H, J 9.5, 4.0 Hz, H-3), 5.31 (d, 1H, J 3.5 Hz, H-4), 4.29 (t, 1H, J 6.5 Hz, H-5),4.03 (d, 1H, J 6.5 Hz, H-6), 7.69 (d, 1H, J 8.0 Hz, H-2’), 7.23 (d, 1H, J 8.0 Hz, H-3’), 7.23 (d, 1H, J 8.0 Hz, H-5’), 7.69 (d, 1H, J 8.0 Hz, H-6’), 1.93-2.13 (s, 12H, CH3CO); 13C NMR (DMSO-d6, δ ppm): 178.22 (C=S), 81.75 (C-1), 68.63 (C-2), 70.57 (C-3), 67.57 (C-4), 71.59 (C-5), 61.29 (C-6), 131.03 (C-1’), 129.40 (C-2’), 127.62 (C-3’), 140.32 (C-4’), 127.62 (C-5’), 129.40 (C-6’), 144.11 (C-imine), 20.35-21.00 (CH3CO), 169.41-170.13 (CH3CO), 18.53 (4’-CH3); MS m/z: 524 (M+ + H, 100%), 546 (M+ + Na, 84%) for C23H29N3O9S.
4-Isopropylbenzaldehyde (2,3,4,6-tetra-O-acetyl-β- D-galactopyranosyl)thiosemicarbazone (4g)
White solid, mp 172-173°; IR (KBr, cm–1): 3355 (NH), 1748 (C=O), 1608 (CH=N), 1223, 1054 (C-O-C); 1H NMR (DMSO-d6, δ ppm): 8.63 (d, 1H, J 9.5 Hz, H-4”), 11.92 (s, 1H, H-2”), 8.10 (s, 1H, H imine), 5.87 (t, 1H, J 9.5 Hz, H-1), 5.30 (t, 1H, J 10.0 Hz, H-2), 5.41 (dd, 1H, J 10.0, 3.5 Hz, H-3), 5.35 (d, 1H, J 3.5 Hz, H-4), 4.33 (t, 1H, J 6.5 Hz, H-5), 4.06 (d, 1H, J 6.5 Hz, H-6), 7.32 (d, 1H, J 8.0 Hz, H-2’), 7.50 (d, 1H, J 8.0 Hz, H-3’), 7.50 (d, 1H, J 8.0 Hz, H-5’), 7.32 (d, 1H, J 8.0 Hz, H-6’), 1.96-2.16 (s, 1H, CH3CO); 13C NMR (DMSO-d6, δ ppm): 178.17 (C=S), 81.61 (C-1), 68.53 (C-2), 70.46 (C-3), 67.48 (C-4), 71.48 (C-5), 61.18 (C-6), 131.37 C-1’), 126.64 (C-2’), 127.62 (C-3’), 150.95 (C-4’), 127.62 (C-5’), 126.64 (C-6’), 143.87 (C-imine), 20.26-20.45 (CH3CO), 169.25-170.02 (CH3CO), 33.34 [4’-CH(CH3)2], 23.56 [4’-CH(CH3)2]; MS m/z: 552 (M+ + H, 88%), 574 (M+ + Na, 100%) for C25H33N3O9S.
4-Hydroxybenzaldehyde (2,3,4,6-tetra-O-acetyl-β-Dgalactopyranosyl) thiosemicarbazone (4h)
White solid, mp 234-235°; IR (KBr, cm–1): 3354 (NH), 1752 (C=O), 1608 (CH=N), 1216, 1039 (C-O-C); 1H NMR (DMSO-d6, δ ppm): 8.53 (d, 1H, J 9.0 Hz, H-4”), 11.76 (s, 1H, H-2”), 8.01 (s, 1H, H imine), 5.86 (t, 1H, J 9.0 Hz, H-1), 5.23 (t, 1H, J 9.5 Hz, H-2), 5.38 (dd, J 10.0, 4.0 Hz, H-3), 5.33 (d, 1H, J 3.5 Hz, H-4), 4.30 (t, 1H, J 6.0 Hz, H-5), 4.04 (d, 1H, J 7.0 Hz, H-6), 6.82 (d, 1H, J 8.5 Hz, H-2’), 7.65 (d, 1H, J 8.5 Hz, H-3’), 7.65 (d, 1H, J 8.5 Hz, H-5’), 6.82 (d, 1H, J 8.5 Hz, H-6’), 1.94- 2.14 (s, 1H, CH3CO); 13C NMR (DMSO-d6, δ ppm): 177.78 (C=S), 81.64 (C-1), 68.61 (C-2), 70.53 (C-3), 67.53 (C-4), 71.51 (C-5), 61.25 (C-6), 144.31 (C-1’), 129.41 (C-2’), 115.66 (C-3’), 124.68 (C-4’), 115.66 (C-5’), 129.41 (C-6’), 159.70 (C-imine),20.31-20.51 (CH3CO), 169.35-170.09 (CH3CO); MS m/z: 526 (M+ + H, 81%), 548 (M+ + Na, 100%) for C22H27N3O10S.
3-Methoxybenzaldehyde (2,3,4,6-tetra-O-acetyl-β-Dgalactopyranosyl) thiosemicarbazone (4i)
White solid, mp 223-224°; IR (KBr, cm–1): 3348 (NH), 1745 (C=O), 1582 (CH=N), 1220, 1055 (C-O-C); 1H NMR (DMSO-d6, δ ppm): 8.67 (d, 1H, J 8.5 Hz, H-4”), 11.97 (s, 1H, H-2”), 8.08 (s, 1H, H imine), 5.82 (t, 1H, J 9.0 Hz, H-1), 5.29 (t, 1H, J 10.0 Hz, H-2), 5.40 (dd, 1H, J 10.0, 4.0 Hz, H-3), 5.33 (d, 1H, J 3.5 Hz, H-4), 4.31 (t, 1H, J 6.5 Hz, H-5), 4.05 (m, 1H, H-6), 7.46 (d, 1H, J 1.0 Hz, H-2’), 7.34 (m, 1H, H-4’), 7.34 (m, 1H, H-5’), 7.01 (ddd, 1H, J 8.0, 1.4, 1.0 Hz, H-6’), 1.95-2.14 (s, 1H, CH3CO); 13C NMR (DMSO-d6, δ ppm): 178.42 (C=S), 81.64 (C-1), 68.45 (C-2), 70.41 (C-3), 67.51 (C-4), 71.48 (C-5), 61.16 (C-6), 135.11 (C-1’), 129.78 (C-2’), 159.58 (C-3’), 120.77 (C-4’), 111.38 (C-5’), 116.57 (C-6’), 143.65 (C-imine), 20.32-20.50 (CH3CO), 169.31-170.25 (CH3CO), 55.26 (s, 3H, 3’-OCH3); MS m/z: 540 (M+ + H, 100%), 562 (M+ + Na, 83%) for C23H29N3O10S.
3-Hydroxy-4-methoxybenzaldehyde (2,3,4,6-tetra- O-acetyl-β-D-galactopyranosyl) thiosemicarbazone (4j)
White solid, mp 181-182°; IR (KBr, cm–1): 3313 (NH), 1744 (C=O), 1600 (CH=N), 1243, 1040 (C-O-C); 1H NMR (DMSO-d6, δ ppm): 8.51 (d, 1H, J 9.0 Hz, H-4”), 11.78 (s, 1H, H-2”), 7.98 (s, 1H, H imine), 5.89 (t, 1H, J 9.0 Hz, H-1), 5.26 (t, 1H, J 9.5 Hz, H-2), 5.39 (dd, 1H, J 10.0, 4.0 Hz, H-3), 5.32 (d, 1H, J 3.5 Hz, H-4), 4.31 (t, 1H, J 6.5 Hz, H-5), 4.04 (d, 1H, J 6.5 Hz, H-6), 7.31 (d, 1H, J 2.0 Hz, H-2’), 6.96 (d, 1H, J 8.5 Hz, H-5’), 7.14 (dd, 1H, J 8.5, 2.0 Hz, H-6’), 1.93-2.15 (s, 1H, CH3CO); 13C NMR (DMSO-d6, δ ppm): 177.79 (C=S), 81.65 (C-1), 68.63 (C-2), 70.53 (C-3), 67.54 (C-4), 71.55 (C-5), 61.29 (C-6), 126.51 (C-1’), 120.70 (C-2’), 146.74 (C-3’), 150.03 (C-4’), 113.31 (C-5’), 111.78 (C-6’), 144.51 (C-imine), 20.33-20.53 (CH3CO), 169.34-170.04 (CH3CO), 55.69 (4’-OCH3); MS m/z: 556 (M+ + H, 36%), 578 (M+ + Na, 100%) for C23H29N3O11S.
3-Methoxy-4-hydroxybenzaldehyde (2,3,4,6-tetra- O-acetyl-β-D-galactopyranosyl) thiosemicarbazone (4k)
White solid, mp 246-247°; IR (KBr, cm–1): 3352 (NH), 1744 (C=O), 1601 (CH=N), 1223, 1055; 1H NMR (DMSO-d6, δ ppm): 8.51 (d, 1H, J 8.5 Hz, H-4”), 11.85 (s, 1H, H-2”), 8.01 (s, 1H, H imine), 5.77 (t, 1H, J 9.0, H-1), 5.26 (t, 1H, J 9.5 Hz, H-2), 5.42 (dd, 1H, J 10.0, 3.5, H-3), 5.33 (d, 1H, J 3.5 Hz, H-4), 4.31 (t, 1H, J 6.5 Hz, H-5), 4.05 (m, 1H, H-6), 7.48 (d, 1H, J 1.5 Hz, H-2’), 6.83 (d, 1H, J 8.0 Hz, H-5’), 7.12 (dd, J 8.0, 4.0 Hz, H-6’), 1.96-2.14 (s, 1H, CH3CO); 13C NMR (DMSO-d6, δ ppm): 177.90 (C=S), 81.54 (C-1), 68.38 (C-2), 70.31 (C-3), 67.55 (C-4), 71.41 (C-5), 61.10 (C-6), 125.07 (C-1’), 109.58 (C-2’), 148.13 (C-3’), 149.23 (C-4’), 119.26 (C-5’), 122.63 (C-6’), 144.28 (C-imine), 20.32-20.49 (CH3CO), 169.30-170.53 (CH3CO), 55.73 (3’-OCH3); MS m/z: 556 (M+ + H, 65%), 578 (M+ + Na, 100%) for C23H29N3O11S.
3-Ethoxy-4-hydroxybenzaldehyde (2,3,4,6-tetra-Oacetyl-β-D-galactopyranosyl) thiosemicarbazone (4l)
White solid, mp 204-205°; IR (KBr, cm–1): 3345 (NH), 1747 (C=O), 1600 (CH=N), 1223, 1051 (C-O-C); 1H NMR (DMSO-d6, δ ppm): 8.49 (d, 1H, J 9.0 Hz, H-4”), 11.84 (s, 1H, H-2”), 8.01 (s, 1H, H imine), 5.79 (t, 1H, J 9.5 Hz, H-1), 5.26 (t, 1H, J 10.0, H-2), 5.42 (d, 1H, d, J 10, 4.0 Hz, H-3), 5.35 (d, 1H, J 3.5 Hz, H-4), 4.32 (t, 1H, J 6.5 Hz, H-5), 4.04 (m, 1H, H-6), 7.43 (d, 1H, J 1.5 Hz, H-2’), 6.85 (d, 1H, J 8.0 Hz, H-5’), 7.15 (dd, 1H, J 8.0, 1.5 Hz, H-6’), 1.97-2.15 (s, 1H, CH3CO); 13C NMR (DMSO-d6, δ ppm): 177.86 (C=S), 81.56 (C-1), 68.39 (C-2), 70.34 (C-3), 67.56 (C-4), 71.44 (C-5), 61.11 (C-6), 125.03 (C-1’), 122.45 (C-2’), 147.16 (C-3’), 149.56 (C-4’), 115.48 (C-5’), 111.11 (C-6’), 144.44 (C-imine), 20.32-20.48 (CH3CO), 169.30-170.48 (CH3CO), 63.93 [3’-OCH2CH3], 14.68 [3’-OCH2CH3]; MS m/z: 570 (M+ + H, 100%), 592 (M+ + Na, 87%) for C24H31N3O11S.
4-Dimethylaminobenzaldehyde (2,3,4,6-tetra-Oacetyl- β-D-galactopyranosyl) thiosemicarbazone (4m)
White solid, mp 217-218°; IR (KBr, cm–1): 3343 (NH), 1744 (C=O), 1600 (CH=N), 1223, 1055 (C-O-C); 1H NMR (DMSO-d6, δ ppm): 8.43 (d, 1H, J 9.0 Hz, H-4”), 11.71 (s, 1H, H-2”), 7.99 (s, 1H, H imine), 5.85 (t, 1H, J 9.5 Hz, H-1), 5.26 (t, 1H, J 10.0 Hz, H-2), 5.40 (dd, J 10.0, 3.5 Hz, H-3), 5.34 (d, 1H, J 3.5 Hz, H-4), 4.31 (t, 1H, J 6.5 Hz, H-5), 4.05 (d, 1H, 6.5 Hz, H-6), 6.73 (d, 1H, J 9.0 Hz, H-2’), 7.61 (d, 1H, J 9.0 Hz, H-3’), 7.61 (d, 1H, J 9.0 Hz, H-5’), 6.73 (d, 1H, J 9.0 Hz, H-6’), 1.95- 2.15 (s, 1H, CH3CO); 13C NMR (DMSO-d6, δ ppm): 177.25 (C=S), 81.50 (C-1), 68.50 (C-2), 70.42 (C-3), 67.48 (C-4), 71.38 (C-5), 61.16 (C-6), 120.77 (C-1’), 111.62 (C-2’), 128.86 (C-3’), 151.65 (C-4’), 128.86 (C-5’), 111.62 (C-6’), 144.80 (C-imine), 20.26- 20.45 (CH3CO), 169.24-170.05 (CH3CO), 20.37 [4’- N(CH3)2]; MS m/z: 553 (M+ + H, 100%), 575 (M+ + Na, 64%) for C24H32N4O9S.
Screening for Antioxidant activity
Chrysin, dicyclohexylcarbodiimide (DCC) and diethylphosphoryl cyanide (DEPC) were purchased from Sigma Chemical Co. Other derivatizing reagents were obtained from Aldrich Chemical Co. Sodium azide, ethylenediamine tetraacetic acid (EDTA), b-nicotinamide adenine dinucleotide phosphate, reduced form (NADPH), cumene hydroperoxide, glutathione reductase, DL-α-tocopherol acetate, carbon tetrachloride (CCl4), xanthine, potassium cyanide (KCN), sodium dodecylsulfate, trichloroacetic acid (TCA), cytochrome C, thiobarbituric acid, n-butanol and pyridine were purchased from Sigma Chem. Co. All other chemicals and reagents were analytical grade.
Screening for Antioxidant activity by DPPH method
All the synthesised compounds were evaluated for antioxidant activity and comprared with standard drug (resveratrol). The activity was evaluated using the DPPH method [33-35]. The 150mM solution of DPPH (195 ml) was added to standard solution (resveratrol) and tested sample solutions (5 ml each) of different concentrations (0.5, 1.0, 2.0, 4.0, 8.0 and 12.0 mM) on 96-hole ELISA plates and allow to react at temperature 25° in incubator. After 30 min the absorbance values were measured at 518 nm and converted into the percentage antioxidant activity (AA) using formula, AA% = [(AbsDPPH – Abssample)/ (AbsDPPH – Absethanol)].100%, where AbsDPPH was the absorbance of DPPH solution which was used as a negative prepared by adding 5 μl ethanol to 195 μl of 150 mM solution of DPPH in ethanol, Abssample was the absorbance of sample solution, Absethanol was the absorbance of ethanol, which was used as a blank. The positive controls were those using the standard solution containing resveratrol. All tests and analyses were undertaken on three replicates and the results averaged. The IC50 values were calculated by linear regression plots, where the abscissa represented the concentration of tested compound solution (0.5, 1.0,2.0, 4.0, 8.0 and 12.0 mM) and the ordinate the average percent of antioxidant activity from three separate tests. The results are tabulated in Table 1
| Conc. | Scavenging effect for DPPH (%) | IC50 | ||||||
|---|---|---|---|---|---|---|---|---|
| Compd. | 12.5 | 25 | 50 | 100 | 200 | 300 | (µM) | |
| 4a | 6.11 | 11.32 | 18.47 | 29.08 | 53.30 | 64.46 | 210 | |
| 4b | 7.05 | 13.74 | 19.63 | 26.29 | 38.31 | 51.24 | 283 | |
| 4c | 8.51 | 13.32 | 17.08 | 34.34 | 55.63 | 67.19 | 197 | |
| 4d | 7.15 | 10.09 | 17.61 | 19.82 | 38.37 | 55.42 | 270 | |
| 4e | 5.38 | 9.04 | 17.46 | 23.51 | 35.42 | 44.31 | >300 | |
| 4f | 7.21 | 12.76 | 18.06 | 32.84 | 53.27 | 65.03 | 206 | |
| 4g | 2.17 | 5.32 | 9.65 | 15.09 | 18.13 | 24.48 | >300 | |
| 4h | 11.45 | 22.61 | 33.27 | 49.18 | 68.74 | 75.08 | 108 | |
| 4i | 7.34 | 11.46 | 15.63 | 27.17 | 34.02 | 55.07 | 276 | |
| 4j | 8.16 | 17.43 | 28.21 | 40.09 | 56.80 | 69.61 | 182 | |
| 4k | 9.45 | 27.11 | 45.64 | 60.30 | 71.23 | 74.05 | 75 | |
| 4l | 14.16 | 30.24 | 45.38 | 59.42 | 68.34 | 69.16 | 71 | |
| 4m | 14.32 | 30.86 | 48.94 | 68.17 | 74.54 | 78.47 | 56 | |
| Resveratrol | 9.13 | 22.56 | 33.84 | 54.03 | 70.44 | 75.62 | 94 | |
Table 1: antioxidant activity of synthesised Compounds by dpph method
Antioxidant assay in vivo
Albino rats of Wistar strain, weighing 100–150 g were used in all experiments. Animals were maintained on 12 h light/dark cycle at approximately 22° and allowed food and water ad libitum. Rats were injected i.p, with a mixture of CCl4 in olive oil (1: 1) at a dose of 0.6 ml/kg to induce hepatotoxicity. Control animals were given the vehicle alone. Rats were pretreated once with DL-a-tocopherol acetate (a dose of 400 mg/kg) and test samples were given i.p. at a dose of 100 mg/kg/day for seven consecutive days prior to the administration of CCl4. Animals were sacrified 24 h after CCl4 dosing and blood was collected by decapitation for the determination of serum transaminases.
Hepatic tissues were carefully excised and homogenized in cold 1.15% KCl-10 mM phosphate buffer with EDTA (pH 7.4) and centrifuged at 12 000 rpm for 8 min. The supernatant was further centrifuged at 45 000 rpm for 50 min to obtain cytosolic extract for the measurement of liver cytosolic SOD, catalase and GSH-px activities. The protein content was measured by the method of Lowry et al. [36] with bovine serum albumin as a standard.
Determination of antioxidant enzyme activities
SOD was assayed by the method of McCord and Fridovich [37]. The reaction mixture was make from 300 ml of 0.5 mM solution of xanthine as substrate, 100 ml of 0.05 mM solution of KCN, 100 ml of solution of 1% sodium deoxycholate, 20 ml of solution of xanthine oxidase, 20 ml of solution of cytosolic extract and 300 ml of soltuion of 0.1 mM cytochrome C and placed in a 1 cm cuvette and the rate of increase in absorbance at 550 nm was recorded for 5 min. SOD activity was expressed as unit/mg protein.
Catalase was assayed by the method of Rigo and Rotilio [38,39]. The cytosolic extract of liver (40 ml) diluted 10 times was added with 0.13 mM phosphate buffer (pH 7.0, 500 ml), distilled by 660 ml of water and 1800 ml of 15 mM solution of H2O2 and thoroughly mixed. The rate of changes in the absorbance at 240 nm for 5 min was recorded. Catalase activity was expressed as unit/mg protein.
Statistical analysis
Results were subjected to one-way ANOVA and p<0.05 was considered significant. The post hoc analysis was carried out by Dunnet’s multiple comparison test [40].
Results and Discussion
Condensation reaction of tetra-O-acetyl-b- D-galactopyranosyl thiosemicarbazide 2 with a number of substituted benzaldehydes 3a-m lead to form a series of benzaldehyde (tetra-O-acetyl-b-Dgalactopyranosyl) thiosemicarbazones 4a-m (fig. 1 and Table 2). The reaction was performed by using microwave-assissted heating and conventional heating methods. The microwave-assisted synthetic pathway was carried out using minimum amount of solvent (ethanol) and deceased reaction time comparing conventional heating pathway (2-3 ml volume versus 20 ml, and 2-7 min versus 90 min, respectively). Reaction time was from 2 min to 7 min depending on substituent’s nature: withdrawing substituents need shorter reaction time than donating ones. In the first period of reaction when reaction was starting to irradiate about 1-3 min, the pasty mixture of reagents in methanol was dissolved and the reaction became homogenous. In the final period of reaction the solid product appeared and precipitated out. The products yields of microwawe-asisted method were fairly high from 60% to 98%, while ones of conventional heating methods were lower, from 32% to 64%. In some cases with benzaldehydes having 4-Cl, 4-NO2 and 4-Br groups the yields attained 98%. These compounds can dissolved in ethanol toluene, chloroform, DMF,… and have high melting points. The synthesised products were characterized by IR, 1H NMR and 13C NMR spectral data.
| Compd. | R | Microwave-assisted method | Conventional method | ||||
|---|---|---|---|---|---|---|---|
| Reaction | Ethanol | Yield, % | Reaction | Ethanol | Yield, % | ||
| time, min | solvent, ml | time, min | solvent, ml | ||||
| 4a | 4-NO2 | 5 | 3 | 97 | 90 | 20 | 48 |
| 4b | 3-NO2 | 5 | 3 | 70 | 90 | 20 | 60 |
| 4c | 4-F | 5 | 2 | 73 | |||
| 4d | 4-Cl | 5 | 2 | 98 | 90 | 20 | 32 |
| 4e | 4-Br | 5 | 2 | 98 | |||
| 4f | 4-Me | 5 | 2 | 60 | |||
| 4g | 4-iPr | 5 | 2 | 75 | |||
| 4h | 4-OH | 5 | 3 | 75 | |||
| 4i | 3-OMe | 5 | 2 | 85 | |||
| 4j | 3-OH-4-OMe | 7 | 3 | 75 | |||
| 4k | 3-OMe-4-OH | 7 | 3 | 70 | |||
| 4l | 3-OEt-4-OH | 7 | 3 | 80 | |||
| 4m | 4-NMe2 | 7 | 3 | 74 | 90 | 20 | 64 |
Table 2: Synthetic Conditions For Compounds 4a-M
The IR spectra of compounds 4a-m showed characteristic absorptions in the range of 3354- 3313 cm-1 (N-H bond), 1752-1744, 1261-1216 and 1055-1045 cm-1 (ester), 1370-1378 cm-1 (C=S), and 1625-1587 cm-1 (CH=N bond). The anomeric proton H-1 is represented as a triplet at δ = 5.90- 5.95 ppm due to the coupling with both H-4” and H-2 protons in the 1H NMR spectra of 4(a-m). The coupling constant values, JH-1,H-2 = 9.0-9.5 Hz, for the pyranose ring agreed with trans-axial H-H disposition and confirmed the b-anomeric configuration of compounds 4a-m. Signals of NH protons of the thiourea component in compounds 4a-m appeared at δ = 12.17-11.71 ppm (in singlet) for H-2” and δ = 9.00-8.43 ppm (in doublet, JNH,H-1 = 9.5-8.5 Hz) for H-4”. Proton of azomethine bond had chemical shift at δ = 8.22- 7.98 ppm in singlet. Other protons in pyranose ring had signals in region of 5.93-4.03 ppm. Protons in benzene ring appeared at 8.27-6.73 ppm. The 13C-NMR spectra showed the thiocarbonyl carbon atom with chemical shift at δ =178.84-177.25 ppm. Carbon atom of azomethine bond showed chemical shift at δ = 159.70-142.56 ppm. Carbon atoms of benzene and pyranose rings had signals at δ = 159.58-111.11 and δ = 81.94-61.10 ppm , respectively. Acetate ester in sugar component had signals at δ = 20.51-20.26 and δ = 170.53-169.24 ppm for carbon atoms in methyl and carbonyl groups, respectively. Protons in methyl group of acetate ester had chemical shifts at δ = 2.16-1.93 ppm.
The in vitro method of the scavenging of the stable DPPH radical is extensively used to evaluate antioxidant activities in less time than other methods. DPPH is a stable free radical molecule that can accept an electron or hydrogen radical and thus be converted into a stable, diamagnetic molecule. DPPH has an odd electron and so has a strong absorption band at 518 nm. When this electron becomes paired off, the absorption decreases stoichiometrically with respect to the number of electrons taken up. Such a change in the absorbance produced in this reaction has been widely applied to test the capacity of numerous molecules to act as free radical scavengers. The scavenging effect of the synthesized compounds 4a- m on the DPPH radical was evaluated according to the methods of Shimada et al. [33], Leong and Shui [34] and Braca et al [35].
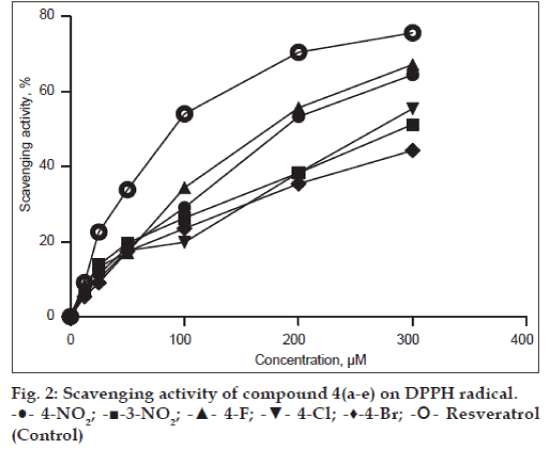
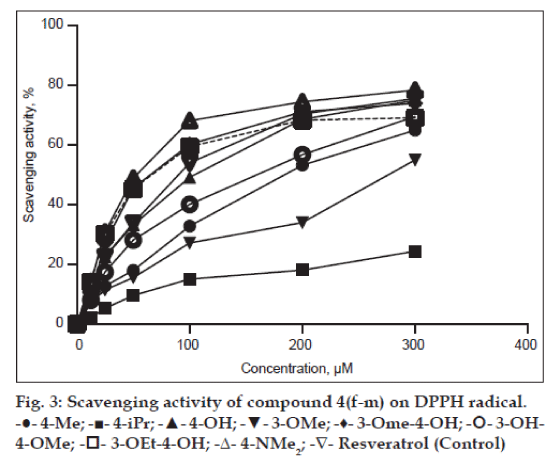
Amongst the compounds screened for antioxidant activity, 4h, 4k, 4l and 4m showed good antioxidant activity. The compounds with substituents such as 4-OH (4h), 3-OMe-4-OH (4k), 3-OEt-4-OH (4l) and 4-NMe2 (4m) showed very good antioxidant activity. Remained compounds do not show any antioxidant activity (Table 1, fig. 2 and 3).
Figure 3: Scavenging activity of compound 4(f-m) on DPPH radical.
Compounds 4a-m were tested in vivo for their anti-oxidant acitivities and the results are shown in Table 3. These compounds, when administered i.p, with a dry weight equivalent dosage of 100 mg/ kg/ day of total extract for seven consecutive days in the CCl4-intoxicated rats, was shown to cause a significant elevation of free radical scavenging enzyme activities such as SOD, catalase and GSH-px. As shown in Table 1, some of these compounds (4k, 4l and 4m) caused significant elevation of SOD activity. Similar results were obtained in case of the catalase and the GSH-px activities as shown in Table 3.
| Compd. | SOD (unit/ | GHS-px (unit/ | Catalase (unit/ |
|---|---|---|---|
| mg protein) | mg protein) | mg protein) | |
| 4a | 8.75±0.49 | 0.69±0.02 | 351.48±12.23 |
| 4b | 8.96±0.52 | 0.70±0.01 | 359.57±11.83 |
| 4c | 8.65±0.45 | 0.62±0.01 | 349.61±12.43 |
| 4d | 8.89±0.62 | 0.68±0.01 | 357.87±12.23 |
| 4e | 9.90±0.67 | 0.97±0.01 | 387.56±12.42 |
| 4f | 8.78±0.35 | 0.67±0.02 | 351.21±11.53 |
| 4g | 9.89±0.62 | 0.98±0.01 | 389.87±12.78 |
| 4h | 8.14±0.56 | 0.48±0.02 | 334.67±10.37 |
| 4i | 8.91±0.32 | 0.69±0.01 | 364.72±11.97 |
| 4j | 8.54±0.56 | 0.54±0.02 | 345.56±11.77 |
| 4k | 6.54±0.34 | 0.34±0.03 | 299.78±13.54 |
| 4l | 6.35±0.45 | 0.65±0.02 | 316.56±12.45 |
| 4m | 5.76±0.54 | 0.67±0.02 | 306.34±10.32 |
| Resveratrol | 7.43±0.50 | 0.32±0.02 | 294.22±10.23 |
| Control | 5.39±0.23 | 0.26±0.01 | 216.12±11.34 |
Table 3: effect of compounds 4(a-m) on the Liver cytosolic sod, the liver cytosolic gshpx, The liver cytosolic catalase activities and The hepatic mda production
In conclusion, a series of substituted benzaldehyde (2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl) thiosemicarbazones have been synthesised from 2,3,4,6-tetra-O-acetyl-β-D-galctopyranosyl thiosemicarbazide and substituted benzaldehydes using conventional heating and microwave-assisted heating method. The antioxidant activity of these thiosemicarbazones was evaluated, in vitro and in vivo, and it’s shown that some of these compounds had significant antioxidant activity.
Acknowledgements
The authors thank Vietnam’s National Foundation for Science and Technology Development (NAFOSTED) for providing the financial support.
References
- Greenbaum DC, Mackey Z, Hansell E, Doyle P, Gut J, Caffrey CR,et al. Synthesis and structure-activity relationships of parasiticidalthiosemicarbazone cysteine protease inhibitors against Plasmodiumfalciparum, Trypanosomabrucei, and Trypanosomacruzi. J Med Chem2004;47:3212-9.
- Yang B, Zhang SS, and Li HX. Synthesis and characterization ofnovel thiosemicarbazones bearing sugar moieties. Chem Res Chin Univ2006;22:738-41.
- Alho MA, D’Accorso NB. Behavior of free sugar thiosemicarbazonestoward heterocyclization reactions. Carbohydr Res 2000;328:481-8.
- Ganjali MR, Hosseini M, Salavati-Niasari M, Poursaberi T,Shamsipur M, Javanbakht M, et al. Nickel ion-selective coated graphitePVC-membrane electrode based on benzylbis(thiosemicarbazone).Electroanalysis 2002;14:526-31.
- Naik AD, Reddy PA, Nethaji M, Chakravarty AR. Ternary copper(II) complexes of thiosemicarbazones and heterocyclic bases showingN3OS coordination as models for the type-2 centers of coppermonooxygenases. InorgChimActa 2003;349:149-58.
- El-Metwally NM, Gabr IM, Shallaby AM, El-Asmy AA. Synthesis andspectroscopic characterization of new mono- and binuclear complexesof some NH(1) thiosemicarbazides. J CoordChem 2005;58:1145-9.
- Sharma S, Athar F, Maurya MR, Azam A. Copper (II) complexeswith substituted thiosemicarbazones of thiophene-2-carboxaldehyde:Synthesis, characterization and antiamoebic activity againstE. histolytica. Eur J Med Chem 2005;40:1414-9.
- Sarma LS, Kumar JR, Reddy KJ, Reddy AV. Development of anextractive spectrophotometric method for the determination ofcopper(II) in leafy vegetable and pharmaceutical samples usingpyridoxal-4-phenyl-3-thiosemicarbazone (PPT). J Agric Food Chem2005;53:5492-8.
- Aly MM, Mohamed YA, El-Bayouki KA, Basyouni WM, Abbas SY.Synthesis of some new 4(3H)-quinazolinone-2-carboxaldehydethiosemicarbazones and their metal complexes and a study on theiranticonvulsant, analgesic, cytotoxic and antimicrobial activities.Part-1.Eur J Med Chem 2010;45:3365-73.
- Stanojkovic TP, Kovala-Demertzi D, Primikyri A, Garcia-Santos I,Castineiras A, Juranic Z, et al. Zinc(II) complexes of 2-acetylpyridine 1-(4-fluorophenyl)-piperazinylthiosemicarbazone:Synthesis,spectroscopic study and crystal structures - Potential anticancer drugs.J InorgBiochem 2010;104:467-76.
- Liu ZC, Wang BD, Yang ZY, Li Y, Qin DD, Li TR. Synthesis, crystalstructure, DNA interaction and antioxidant activities of two novelwater-soluble Cu(2+) complexes derivated from 2-oxo-quinoline-3-carbaldehyde Schiff-bases. Eur J Med Chem 2009;44:4477-84.
- Kovala-Demertzi D, Yadav PN, Wiecek J, Skoulika S, VaradinovaT,Demertzis MA. Zinc(II) complexes derived from pyridine-2-carbaldehydethiosemicarbazone and (1E)-1-pyridin-2-ylethan-1-onethiosemicarbazone. Synthesis, crystal structures and antiproliferativeactivity of zinc(II) complexes. J InorgBiochem 2006;100:1558-67.
- Kostas ID, Heropoulos GA, Kovala-Demertzi D, Yadav PN,Jasinski JP, Demertzis MA, et al. Microwave-promoted Suzuki-Miyaura cross-coupling of aryl halides with phenylboronic acid underaerobic conditions catalyzed by a new palladium complex with athiosemicarbazone ligand. Tetrahedron Lett 2006;47:4403-7.
- Sriram D, Yogeeswari P, Thirumurugan R, Pavana RK. Discoveryof new antitubercularoxazolylthiosemicarbazones. J Med Chem2006;49:3448-50.
- Desai NC, Shucla HK, Parekh BR, Thaker KA. Some new 2-aryl-3-isonicotamido-4-thiozolidinones and their 5 carboxymethyl homologuesas antitubercular and antibacterial agents. J Indian ChemSoc1984;61:455-7.
- Liesen AP, Aquino TM, Carvalho CS, Lima VT, Araújo JM, Lima JG,et al. Synthesis and evaluation of anti-Toxoplasma gondiiandantimicrobial activities of thiosemicarbazides, 4-thiazolidinones and1,3,4-thiadiazoles. Eur J Med Chem 2010;45:3685-91.
- Mamolo MG, Vio L, Banfi E. Synthesis and antimicrobial activity ofsome 2,5-disubstituted 1,3,4-thiadiazole derivatives. Farmaco 1996;51:71-4.
- Garnaik BK, Behera RK. Synthesis, antimicrobial and antifungalactivities of some 2-arylimino-4-tetra-O-acetyl-b-D-glucopyranosyl-4-thiazolidinoes. Indian J Chem 1988;27B:1157-8.
- Labanauskas L, Kalcas V, Udrenaite E, Gaidelis P, Brukstus A,Dauksas V. Synthesis of 3-(3,4-dimethoxyphenyl)-1H-1,2,4-triazole-5-thiol and 2-amino-5-(3,4-dimethoxyphenyl)-1,3,4-thiadiazol derivativesexhibiting antiinflammatory activity. Pharmazie 2001;56:617-9.
- Somogyi L. Structure and reactions of aldose semicarbazone andthiosemicarbazone derivatives under acetylating conditions. CarbohydrRes 1979;75:325-30.
- Turner S, Myers M, Gadie B, Hale SA, Horsley A, Nelson AJ, et al.Antihypertensive thiadiazoles. 2. Vasodilator activity of some 2-aryl-5-guanidino-1,3,4-thiadiazoles. J Med Chem 1988;31:906-13.
- Mazzone G, Pignatello R, Mazzone S, Panico A, Pennisi G, Castana R,et al. Synthesis and local anesthetic activity of alkylaminoacylderivatives of 2-amino-1,3,4-thiadiazole.Il Farmaco 1993;48:1207-24.
- Chou JY, Lai SY, Pan SL, Jow GM, Chern JW, Guh JH. Investigationof anticancer mechanism of thiadiazole-based compound in humannon-small cell lung cancer A549 cells original. BiochemPharmacol2003;66:115-24.
- Hanna M, Girges M, Rasala D, Gawineck R. Synthesis andpharmacological evaluation of some novel 5-thiadiazole and oxadiazolederivatives as potential hypoglycemic agents. ArzneimForsch-Drug Res1995;45:1074-8.
- Ghosh S, Misra AK, Bhatia G, Khan MM, Khanna AK. Synthesesand evaluation of glucosyl aryl thiosemicarbazide and glucosylthiosemicarbazone derivatives as antioxidant and antidyslipidemicagents. Bioorg Med ChemLett 2009;19:386-9.
- Rodriguez EC, Marcaurelle LA, Bertozzi CR. Aminooxy-, hydrazide-,and thiosemicarbazide-functionalized saccharides: versatile reagents forglycoconjugate synthesis. J Org Chem 1998;63:7134-5.
- Zelenin KN, Alekseyev VV, Terentiev PB, Kumetsova OB, Lashin VV,Ovcharenko VV, et al. Ring-ring tautomerism of aldohexosethiocarbohydrazones. Mendeleev Commun 1993;3:168-9.
- TenchiuDeleanu AC, Kostas ID, Kovala-Demertzi D, Terzis A.Synthesis and characterization of new aromatic aldehyde/ketone 4-(a-Dglucopyranosyl)thiosemicarbazones. Carbohydr Res 2009;344:1352-64.
- Thanh ND, Mai NT. Synthesis of N-tetra-O-acetyl-a-D-glucopyranosyl-N’-(4’,6’-diarylpyrimidin-2’-yl)thioureas. Carbohydr Res2009;344:2399- 405.
- Thanh ND, Kim Giang NK, Hoai LT. Microwave-Assisted Synthesis ofAcetophenone (per-O-acetylated-a-D-glucopyranosyl)thiosemicarbazones.Eur J Chem 2010;7:899-907.
- Loupy A. Microwave in organic synthesis. 2nd ed. Vol. 1. Weinheim:Wiley-VCH Verlag GmbH and Co KGaA; 2006. p. 579-94.
- Lemieux RL. Tetra-O-acetyl-a-D-Glucopyranosyl Bromide. In:Whistler RL, Wolfrom ML editors. Methods in Carbohydrate Chemistry.Vol. 2. New York: Academic Press; 1963. p. 221-2.
- Shimada K, Fujikawa K, Yahara K, Nakamura TJ. Antioxidativeproperties of xanthan on the autoxidation of soybean oil in cyclodextrinemulsion. Agric Food Chem 1992;40:945-8.
- Leong LP, Shui G. An investigation of antioxidant capacity of fruits inSingapore markets. Food Chem 2002;76:69-75.
- Braca A, Sortino C, Politi M, Morelli I, Mendez J. Antioxidantactivity of flavonoids from Licanialicaniaeflora. J Ethnopharmacol2002;79:379-81.
- Lowry OH, Posenbrough NN, Farr AL, Randall RJ. Protein measurementwith the folin phenol reagent. J BiolChem 1951;193:265-7 5.
- McCord JM, Fridovich I. Superoxide dismutase: An enzymatic functionfor erythrocuprein (hemocoprein). J BiolChem 1969;244:6049-55.
- Rigo A, Rotilio G. Simultaneous determination of superoxide dismutaseand catalase in biological materials by polarography. Anal Biochem1977;81:157-66.
- Karatas F, Koca M, Kara H, Servi S. Synthesis and oxidant propertiesof novel (5-bromobenzofuran-2-yl)(3-methyl-3-mesitylcyclobutyl)ketonethiosemicarbazone. Eur J Med Chem 2006;41:664-9.
- Marxen K, Vanselow KH, Lippemeier S, Hintze R, Ruser A,Hansen UP. Determination of DPPH radical oxidation caused bymethanolic extracts of some microalgal species by linear regressionanalysis of spectrophotometric measurements. Sensors 2007;7:2080-95.