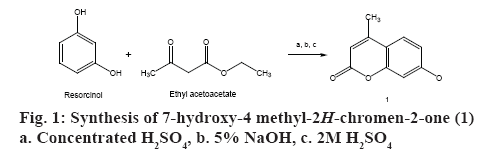- *Corresponding Author:
- A. A. Gawai
Department of Pharmaceutical Chemistry, Anuradha College of Pharmacy, Chikhli-443 201, India
E-mail: drashishgawai@gmail.com
| Date of Submission | 12 April 2017 |
| Date of Revision | 13 June 2018 |
| Date of Acceptance | 24 January 2019 |
| Indian J Pharm Sci 2019;81(2):241-248 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study focused on the synthesis, preliminary pharmacological screening and acute toxicity testing of novel series of 7-(2-(benzo[d]thiazol-2-ylamino)ethoxy)-4-methyl-2H-chromen-2-one derivatives (4a-4k). This novel series was designated and synthesized by refluxing 2-amino benzothiazoles substituted derivatives (3a-3k) with 7-(2-chloroethoxy)-4-methyl-2H-chromen-2-one (2) in dry pyridine. All the synthesized compounds were screened and evaluated for dopamine D2 and serotonin 5HT2 antagonistic activity as a measure of atypical antipsychotic property. Compounds 4b, 4c, 4e, 4g and 4i have shown good preliminary pharmacological activity and these compounds were subjected to acute toxicity (lethal dose) studies for determining effective dose range using probit log scale method. The therapeutic index of the selected compounds was determined to compare with other series of compounds to select better active compound(s). The structures of all compounds were confirmed using spectral studies. All the synthesized compounds showed good dopamine D2 and serotonin 5HT2A receptor antagonist activity, which could indicate atypical antipsychotic activity. Compounds (4e) and (4b) with electron withdrawing substituents showed better atypical antipsychotic activity.
Keywords
Antipsychotic, schizophrenia, acute toxicity, effective dose, chromen-2-one
Schizophrenia is a complex disorder whose etiology and pathogenesis is not completely understood. Schizophrenia is associated with increased activity at dopaminergic and serotoninergic receptor sites [1,2]. Dopamine D2 occupancy is most important and required for an antipsychotic response. However, it has been proven that, extrapyramidal side effects are also increased with the increase of dopamine receptor occupancy above 80 % [3]. Some antipsychotic drugs like clozapine, olanzapine (figure 1) have lower dopamine D2 occupancy with faster dissociation from the receptor [4-6]. The newer atypical antipsychotics compounds have interaction with multiple receptor targets. These drugs act through blockade of several receptors like dopamine, serotonin (5HT), adrenergic, muscarinic and histamine receptors [7,8]. In the atypical antipsychotic activity, 5HT receptor acts as an important target [9]. The atypical antipsychotic agents possess 5HT2A antagonistic activity along with D2 receptor antagonistic activity and these targets are the basis for the design of newer potential atypical antipsychotic agents. These potential atypical antipsychotic agents have fewer side effects [10-12]. These new potential antipsychotics will provide knowledge about the molecular mechanisms of action and is important for understanding of the pathophysiology of schizophrenia and for design of new drugs with improved efficacy and fewer side effects than the existing ones.
The aim of present work was to synthesize new molecule like chromen-2-one derivatives, which might act on D2 receptors as well as 5HT receptor and evaluate their pharmacological action for atypical antipsychotic activity.
Derivatives of benzothiazole attached with chromen- 2-one moiety by ethoxy polar side chain linkage were synthesized. Benzothiazole derivatives were reported to have 5HT antagonistic property where as chromen- 2-one moiety was reported to have D2 antagonistic activity [13,14]. The aim of the present work was to synthesize some novel chromen-2-one derivatives with minimum side effects and with increased pharmacological activity. The combination of these two antagonistic moieties could possibly lead to a new series of antipsychotic drugs with reduced central nervous system side-effects.
Materials and Methods
Melting points were determined by open capillary method on Campbell electronic apparatus and are uncorrected. All the chemicals used for the reaction were of AR grade (Sigma-Aldrich; Qualigen). All the reaction were routinely monitored and purity of the synthesized compounds was checked by thin layer chromatography by using Merck precoated silica G254 plates and visualized in iodine and UV light. The infra-red spectra were recorded by a Jasco-V-5300 Fourier-transform infrared spectroscopy (FTIR) using potassium bromide (KBr, transmittance, vmax in cm-1) disc method. 1H nuclear magnetic resonance (NMR) spectra was recorded in dimethyl sulfoxide (DMSO)-d6 on 300 MHz Jeol spectrophotometer using tetramethylsilane (TMS) as internal reference standard (chemical shifts in δ, ppm). In this study, the synthesis of the targeted compounds was carried out as a part of Ph. D. research work at Department of Pharmaceutical Sciences, Dibrugarh University, Dibrugarh-786004, Assam, India and Pharmacological study was carried out at TVES’s College of Pharmacy, Nehru Vidyanagar, Faizpur-425503, Jalgaon, Maharashtra, India.
Synthesis of 7-hydroxy-4 methyl-2H-chromen-2- one (1):
Compound (1) was prepared according to the method described by Pechmann and Duisberg [15]. One hundred millilitres of concentrated sulphuric acid (sp.gr. 1.84) was kept in an ice-bath. When temperature fell below 10°, a solution of resorcinol (10 g, 0.091 mol) and ethylacetoacetate (13 ml, 0.103 mol) was added with continuous stirring for 2 h. The temperature was maintained below 10° throughout the addition. The reaction mixture was kept at room temperature for 18 h after which it was poured with vigorous stirring into a mixture of 200 g of crushed ice and 300 ml of distilled water. Precipitate was collected by vacuum filtration and washed with cold water (325 ml). The solid was dissolved in 150 ml of 5 % sodium hydroxide, filtered, and 2 M sulphuric acid (55 ml) was added to it with vigorous stirring until the solution was acidic. The crude product was collected by filtration at the pump, washed with cold water and dried. The product was recrystallized from ethanol to give 7-hydroxy- 4 methyl-2H-chromen-2-one (1) [15]. The schematic representation of the synthesis is shown in figure 1.
Synthesis of 7-(2-chloroethoxy)-4-methyl-2Hchromen- 2-one (2):
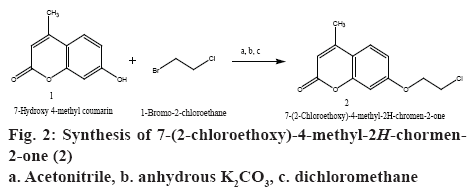
The compound 7-hydroxy-4-methyl-2H-chromen-2- one (1) (0.01 mol) was dissolved in 10 ml of acetonitrile with anhydrous K2CO3 (0.01 mol) was added to the solution. 1-Bromo-2-chloroethane, 0.01 mol was added drop-wise to the mixture in the round bottom flask over a period of 15 min. The reaction was refluxed for 18 h. The filtrate was removed under vacuum using molecular distiller to afford dry solid. The solid obtained was dissolved in dichloromethane; the organic layer was washed with water and dried over anhydrous sodium sulphate. The organic layer was separated and evaporated to dryness to afford crude product, which was then recrystallized using ethanol to get 7-(2-chloroethoxy)-4-methyl-2H-chromen-2-one (2). The schematic representation of the synthesis was shown in figure 2.
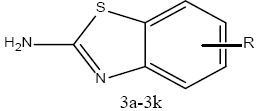
Synthesis of 2-amino benzothiazoles substituted derivatives (3a-3k):
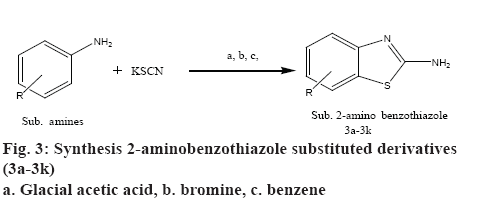
To 20 ml of glacial acetic acid precooled to 5°, 8 g (0.08 mol) of potassium thiocyanate and 1.45 g (0.01 mol) of substituted aniline were added. The mixture was placed in a freezing mixture of ice and salt and mechanically stirred while 1.6 ml of bromine in 6 ml of glacial acetic acid was added from a dropping funnel at such a rate that the temperature doesn’t rise beyond 0°. After addition of bromine for 105 min, the solution was stirred for an additional 2 h at 0° and at room temperature for 10 h. It was then allowed to stand overnight during which period an orange precipitate was settled at the bottom to which 6 ml of water was added quickly and the slurry formed was heated at 85° on a steam bath and filtered in hot condition. The orange residue was placed in a reaction flask and treated with 10 ml of glacial acetic acid, heated again to 85° and filtered in hot state. The combined filtrate was cooled and neutralized with concentrated ammonia to pH 6 when a dark yellow precipitate was collected and recrystallized from benzene to get 2-amino substituted benzothiazole derivatives (3a-3k). The physicochemical characterization of these contents are shown in Table 1. The schematic representation of the synthesis is shown in figure 3.
 |
|||||
|---|---|---|---|---|---|
| Compound | Molecular formula | R- Substitution | Yield (%) | mp (°) ♦ | Rf* |
| 3a | C7H6N2S | H | 59 | 157-158 | 0.65 |
| 3b | C7H5ClN2S | 6-Cl | 57 | 169-170 | 0.35 |
| 3c | C7H8N2OS | 6-OCH3 | 38 | 148-149 | 0.58 |
| 3d | C7H5N3O2S | 6-NO2 | 20 | 198-199 | 0.62 |
| 3e | C7H5N2ClS | 4-Cl | 43 | 130-131 | 0.39 |
| 3f | C7H5FN2S | 6-F | 35 | 145-146 | 0.58 |
| 3g | C7H4ClFN2S | 6-F, 5-Cl | 63 | 205-206 | 0.65 |
| 3h | C7H8N2OS | 4-CH3 | 48 | 183-184 | 0.52 |
| 3i | C7H8N2S | 6-CH3 | 59 | 171-172 | 0.60 |
| 3j | C7H5ClN2S | 5-Cl | 45 | 167-168 | 0.43 |
| 3k | C7H5Br N2S | 6-Br | 36 | 138-139 | 0.68 |
♦ Melting points (mp) were uncorrected. *Mobile phase for (3a-3k) was benzene:ethyl acetate (4:1)
Table 1: Physicochemical Data of Synthesized Intermediate Compounds (3A-3K)
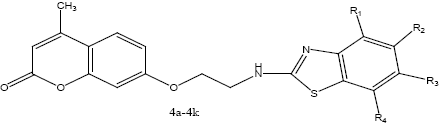
Synthesis of 7-(2-(benzo[d]thiazol-2-ylamino) ethoxy)-4-methyl-2H-chromen-2-one derivatives (4a-4k):
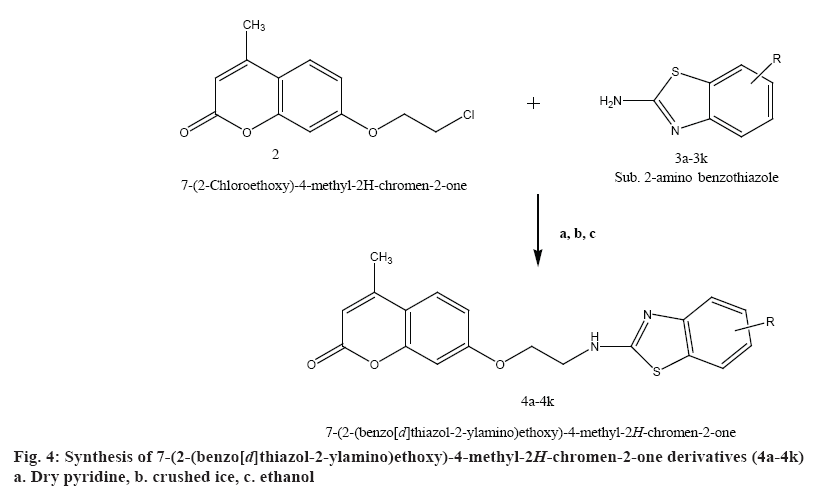
A mixture of 7-(2-chloroethoxy)-4-methyl-2Hchromen- 2-one (2) with compounds 3a-3k 0.01 mol was added to the reaction flask and refluxed in dry pyridine for 24 h. The solvent was distilled off. The mixture was collected and poured on to crushed ice. The solid product was filtered and recrystallized from ethanol to obtain the final compounds (4a-4k). Physiochemical characterization of these contents are shown in the Table 2. The schematic representation of the synthesis is shown in figure 4.
 |
||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | Molecular formula | R1 | R2 | R3 | R4 | Yield (%) | mp (°)♦ | Rf* |
| 4a | C19H16N2O3S | - | - | - | - | 8080 | 180-18284 | 0.66 |
| 4b | C19H15ClN2O3S | - | - | Cl | - | 53 | 130 | 0.35 |
| 4c | C20H18N2O4S | - | - | OCH3 | - | 69 | 170 | 0.5 |
| 4d | C19H15N3O5S | - | - | NO2 | - | 73 | 160-161 | 0.63 |
| 4e | C19H15ClN2O3S | Cl | - | - | - | 75 | 152-153 | 0.59 |
| 4f | C19H15FN2O3S | - | - | F | - | 63 | 174 | 0.32 |
| 4g | C19H14ClFN2O3S | - | Cl | F | - | 68 | 130-131 | 0.38 |
| 4h | C20H18N2O3S | CH3 | - | - | - | 55 | 168-169 | 0.44 |
| 4i | C20H18N2O3S | - | - | CH3 | - | 68 | 154-155 | 0.60 |
| 4j | C19H15ClN2O3S | - | Cl | - | - | 18 | 149 | 0.49 |
| 4k | C19H15BrN2O3S | Br | - | - | - | 13 | 134 | 0.55 |
♦ Melting points (mp) were uncorrected. *Mobile phase for (4a-4k) was benzene:ethyl acetate (4:1)
Table 2: Physicochemical Data of Synthesized Compounds (4A-4K)
Pharmacological evaluation of atypical antipsychotic activity:
The pharmacological evaluation of all synthesized compounds was done for atypical antipsychotic activity using apomorphine-induced climbing behaviour and 5-hydroxytryptophan (5HTP)-induced head twitches for D2 receptor and 5HT receptor inhibitory action, respectively. After preliminary pharmacological screening of atypical antipsychotic activity, some active compounds were selected for acute toxicity (LD50) studies in female Wistar rats. LD50 was studied using Organisation for Economic Co-operation and Development (OECD) guidelines TG 425 (up and down method) and the effective dose (ED50) was determined using probit log scale method of statistical analysis for 5HTP-induced head twitches by oral route of administration in female Wistar rats.
Experimental animals:
Albino Swiss male mice, 20-25 g, were used for preliminary screening of all synthesized compounds.
All compounds were suspended in 0.5 % carboxy methyl cellulose (CMC) with water for injection and given by intraperitoneal (ip) route of administration. For acute toxicity and effective dose determination, healthy Wistar female rats weighing of 150-180 g were used and doses were given orally. Acute oral toxicity of selected compounds were determined by suspending the test compounds in 0.5 % CMC and administered by gavage at a fixed volume and studies were conducted according to the OECD guidelines TG 425. Mortality of the treated rats was recorded after 14 h. All the animals were housed in clean plastic cages in the laboratory animal room (23±2°) on a standard pellet diet and tap water given ad libitum, with 12 h dark/ light cycle. Mice and rats were allowed to acclimate to laboratory conditions for at least one week before treatment.
Apomorphine-induced climbing behaviour:
The effect of test compounds on climbing behaviour was determined using apomorphine-induced climbing behaviour in mice. Animals were grouped randomly containing six animals in each group. The test groups received dose of test compounds (5 mg/kg). The control and standard group received 0.5 % CMC (5 ml/kg) and olanzapine (1 mg/kg), respectively. Climbing behaviour was assessed in the animals by placing them individually in cylindrical wire mesh cage (height 18 cm, diameter 14 cm) 5 min after administration of apomorphine (1.0 mg/kg). The animals were kept in the cage, and observed at the interval of 10, 20, 30 min after the administration of apomorphine. The following score was assigned to an individual animal: 0, when all four paws on the floor; 1, when two paws on the mesh; and 2, when all the four paws on the mesh. The score was summed up for each animal. Data were expressed as percentage of blockage of climbing relative to apomorphine-treated control mice [16,17]. The results are summarized in Table 3.
Compounds |
% Inhibition | Selected potent compounds | Final selection | ||
|---|---|---|---|---|---|
| Climbinga | Head twitchesb | Acute toxicityc (LD50) | Effective dosed (ED50) |
Therapeutic index (TI) | |
| Stde | 85 | 85 | - | - | - |
| 4ad | 65 | 36 | - | - | - |
| 4b | 60 | 72 | 1200 | 94 | 12.73 |
| 4c | 65 | 62 | 1700 | 159.9 | 10.63 |
| 4d | 70 | 57 | - | - | - |
| 4e | 80 | 77 | 797 | 54.7 | 14.57 |
| 4f | 78 | 45 | - | - | - |
| 4g | 63 | 55 | 2400 | 257 | 9.34 |
| 4h | 65 | 57 | - | - | - |
| 4i | 64 | 52 | 2100 | 198.2 | 10.60 |
| 4j | 62 | 60 | - | - | - |
| 4k | 72 | 68 | 1000 | 79 | 12.66 |
aApomorphine-induced climbing behaviour in mice, *n=6, p<0.05, apomorphine 1 mg/kg. b5HTP-induced head twitches in mice, *n=6, p<0.05, cAcute toxicity studies by OECD guidelines using up and down method on rats. dEffective dose determination by probit log scale method of statistical analysis on rats. Oral route by gavages with 0.5 % carboxy methyl cellulose, estandard dose of olanzapine was 1 mg/kg, ip route with 0.5 % carboxy methyl cellulose
Table 3: Pharmacological Evaluation of the Synthesized Compounds
5HTP-induced head twitches:
The head twitches were determined in mice using 5HTP induced head twitches method. Mice were grouped and administered test compounds (5 mg/kg) and control similarly but the standard group received olanzapine (1 mg/kg). The head twitches in mice were counted after 20 min of 5HTP (100 mg/kg) administration at an interval of 5 min and for a period of 1 h [18]. The results are summarized in Table 3.
Acute oral toxicity study (up and down method, OECD guideline 425):
The LD50 was calculated using the method of maximum likelihood. This test consists of a single ordered dose progression in which animals are dosed, one at a time, at a minimum of 48-h intervals. The first animal receives a dose a step below the level of the best estimate of the LD50. If the animal survives, the dose for the next animal is increased by (a factor of) 3.2 times of the original dose; if it dies, the dose for the next animal is decreased by a similar dose progression (note: 3.2 is the default factor corresponding to a dose progression of one half log unit). Each animal should be observed carefully for up to 48 h before making a decision on whether and how much to dose the next animal. That decision is based on the 48 h survival pattern of all the animals up to that time. The testing will be completed with only 4 animals after initial reversal in animal outcome [19,20]. The results are summarized in Table 3.
Effective dose (ED50) determination:
ED50 of all selected compounds was calculated by using probit log scale method of statistical analysis [21]. The ED50 was determined by using 5HTP-induced head twitches activity. Overnight fasted rats were used and each dose group consisted of 10 rats. After administration of the drugs, animals were observed for 2 h for maximum activity in particular group. The calculation was made by using Miller and Tainter method of statistical analysis [22]. Probit values were plotted against log doses and ED50 value as the dose that corresponds to porbit 5. The results are summarized in Table 3.
Results and Discussion
The results of all synthesized compounds with respect to physicochemical data, spectral analysis were summarized as follows, 7-hydroxy-4 methyl-2Hchromen- 2-one (1), yield: 51 %, melting point (mp): 181° TLC (benzene:ethyl acetate, 4:1, Rf 0.45) FTIR (KBr, Vmax in cm-1): 3500 (O-H str), 3057 (Ar-C-H str), 1680 (C=O, str), 1601, 1566 and 1452 (Ar-C=C, str), 1336 and 1159 (-C-CO-O, str), 1215(-C-O phenol), 746 (C-H out of plane) 1H NMR (DMSO-d6, δ, ppm): 10.5 (bs, 1H, -OH), 7.51-7.53 (d, 1H, C5-H), 6.6-6.9 (m, 2H, C6-H), 6.06 (s, 1H, C3-H), 2.29 (s, 3H, C4-CH3).
7-(2-Chloroethoxy)-4-methyl-2H-chromen-2-one (2), yield: 59 %, mp: 120-121°, TLC (benzene:ethylacetate, 4:1, Rf 0.65) FTIR (KBr Vmax in cm-1): 2931.6 (Ar-C-H str) 1724 (C=O str), 1682 (Ar-C=C str), 1386- 1201 (-C-CO-O, str), 1240 (-C-O phenol), 644 (C-Cl str) and 746 (C-H out of plane) 1H NMR (DMSO-d6, δ, ppm): 7.51 (d, 1H, C5-H), 6.6-6.9 (m, 2H, C6-H and C8- H), 6 (s, 1H, C3-H), 2.15 (s, 3H, C4-CH3). 4.20 (t, 2H, O-CH2 linkage), 4.12 (t, 2H, Cl-CH2 linkage).
7-(2-(benzo[d]thiazol-2-ylamino) ethoxy)-4-methyl- 2H-chromen-2-one derivatives (4a-4k), 7-(2-(benzo[d] thiazol-2-ylamino)ethoxy)-4-methyl-2H-chromen-2- one (4a), FTIR (KBr Vmax in cm-1): 3359.5 (N-H str), 2990 (Ar-C-H str), 1688 (C=O str), 1587-1440 (C=C str), 1248 (C-N str), 748 (C-S str) 1H NMR (DMSO-d6, δ, ppm): 8.6 (t, 1H, N-H), 7.16 (d, 1H, C5-H in chromen- 2-one), 6.6-6.9 (d, 2H, C6-H and C8-H in chormen- 2-one), 5.90 (s, 1H, C3-H in chromen-2-one), 2.3 (s, 3H, C4-CH3 in chromen-2-one), 4.24 (t, 2H, O-CH2 linkage), 4.12 (t, 2H, N-CH2 linkage), 7.47 (m, 2H, C5’-H, and C6’-H in benzothiazole), 8.10 (d, 2H, C4’-H and C7’H in benzothiazole).
7-(2-(6-Chlorobenzo[d]thiazol-2-ylamino)ethoxy)-4- methyl-2H-chromen-2-one (4b), FTIR (KBr Vmax in cm-1): 3211 (N-H str), 2966 (C-H str), 1732 (C=O str), 1458-1365 (C=C str), 1277 (C-N str), 1171 (-CO-O-C str), 781 (C-S str) 1H NMR (DMSO-d6, δ, ppm): 9.11 (t, 1H, N-H), 7.23 (d, 1H, C5-H in chromen-2-one), 6.6-6.9 (d, 2H, C6-H and C8-H in chormen-2-one), 6.05 (s, 1H, C3-H in chromen-2-one), 2.0 (s, 3H, C4-CH3 in chromen-2-one). 4.5 (t, 2H, O-CH2 linkage), 4.60 (t, 2H, NH-CH2 linkage), 7.60 (d, 1H, C5’-H in benzothiazole), 8.15 (d, 2H, C4’-H and C7’-H in benzothiazole).
7-(2-(6-methoxybenzo[d]thiazol-2-ylamino)ethoxy)- 4-methyl-2H-chromen-2-one (4c), FTIR (KBr Vmax in cm-1): 3410 (N-H str), 2854 (C-H str), 1715 (C=O str), 1452 (C=C str), 1264 (C-N str), 1134 (-CO-O-C str), 747 (C-S ben) 1H NMR (DMSO-d6, δ, ppm): 8.1 (t, 1H, N-H), 7.4 (d, 1H, C5-H in chromen-2-one), 6.5-6.9 (d, 2H, C6-H and C8-H in chormen-2-one), 5.6 (s, 1H, C3-H in chromen-2-one), 2.1 (s, 3H, C4-CH3 in chromen-2-one), 4.12 (t, 2H, O-CH2 linkage), 4.0 (t, 2H, N-CH2 linkage), 7.6 (s, 1H, C7’-H in benzothiazole), 8.1 (d, 1H, C4’-H in benzothiazole), 7.1 (d, 1H, C5’-H in benzothiazole), 3.7 (s, 3H, OCH3 in benzothiazole).
7-(2-(6-nitrobenzo[d]thiazol-2-ylamino)ethoxy)-4- methyl-2H-chromen-2-one (4d), FTIR (KBr Vmax in cm-1): 3341 (N-H str), 1685 (C=O str), 1588 (C=C str), 1310 (C-O-O str), 1266 (C-N str), 754 (C-N ben) 1H NMR (DMSO-d6, δ, ppm): 8.5 (t, 1H, N-H), 7.5 (d, 1H, C5-H in chromen-2-one), 6.5-6.9 (d, 2H, C6-H and C8-H in chormen-2-one), 6.2 (s, 1H, C3-H in chromen- 2-one), 2.1 (s, 3H, C4-CH3 in chromen-2-one). 4.0 (t, 2H, O-CH2 linkage), 4.2 (t, 2H, N-CH2 linkage), 8.47 (m, 2H, C4’-H and C5’-H in benzothiazole), 8.56 (s, 1H, C7’-H in benzothiazole).
7-(2-(4-chlorobenzo[d]thiazol-2-ylamino)ethoxy)-4- methyl-2H-chromen-2-one (4e), FTIR (KBr Vmax in cm-1): 3310 (N-H str), 2920 (C-H str), 1734 (C=O str), 1504 (C=C str), 1140 (C-O str), 582 (C-Cl ben), 781 (C-S ben) 1H NMR (DMSO-d6, δ, ppm): 9.1 (t, 1H, N-H), 7.20 (d, 1H, C5-H in chromen-2-one), 6.5-6.9 (d, 2H, C6-H and C8-H in chormen-2-one), 6.1 (s, 1H, C3-H in chromen-2-one), 2.4 (s, 3H, C4-CH3 in chromen-2-one). 4.2 (t, 2H, O-CH2 linkage), 4.62 (t, 2H, N-CH2 linkage), 7.4-7.6 (m, 2H, C5’-H and C6’-H in benzothiazole), 8.1 (d, 1H, C7’-H in benzothiazole).
7-(2-(6-fluorobenzo[d]thiazol-2-ylamino)ethoxy)-4- methyl-2H-chromen-2-one (4f), FTIR (KBr Vmax in cm-1): 3327 (N-H str), 2922 (C-H str), 1703 (C=O str), 1596-1334 (C=C str), 1135 (C-O str), 929 (C-F ben), 751 (C-S ben) 1H NMR (DMSO-d6, δ, ppm): 9.3 (t, 1H, N-H), 7.2 (d, 1H, C5-H in chromen-2-one), 6.4- 6.9 (d, 2H, C6-H and C8-H in chormen-2-one), 6.20 (s, 1H, C3-H in chromen-2-one), 2.32 (s, 3H, C4-CH3 in chromen-2-one). 4.11 (t, 2H, O-CH2 linkage), 4.32 (t, 2H, N-CH2 linkage), 7.2-7.8 (m, 2H, C5’-H and C7’-H in benzothiazole), 8.01 (d, 2H, C4’-H in benzothiazole).
7-(2-(5-chloro-6-fluorobenzo[d]thiazol-2-ylamino) ethoxy)-4-methyl-2H-chromen-2-one (4g), FTIR (KBr Vmax in cm-1): 3360 (N-H str), 2920 (C-H str), 1720 (C=O str), 1589-1365 (C=C str), 667 (C-Cl ben), 931 (C-F ben), 1153 (CO-O-C str), 1253.9 (C-O-O str) 1H NMR (DMSO-d6, δ, ppm): 9.6 (t, 1H, N-H), 7.26 (d, 1H, C5-H in chromen-2-one), 6.5-6.9 (d, 2H, C6-H and C8-H in chormen-2-one), 6.13 (s, 1H, C3-H in chromen-2-one), 2.17 (s, 3H, C4-CH3 in chromen-2- one), 4.34 (t, 2H, O-CH2 linkage), 4.48 (t, 2H, N-CH2 linkage), 7.89 (s, 1H, C7’-H in benzothiazole), 8.30 (s, 1H, C4’-H in benzothiazole).
7-(2-(4-methylbenzo[d]thiazol-2-ylamino)ethoxy)- 4-methyl-2H-chromen-2-one (4h), FTIR (KBr Vmax in cm-1): 3342 (N-H str), 2920 (C-H str), 1693 (C=O str), 1565 (C=C str), 1259 (C-N str), 841 (C-H out of plane), 1174 (CO-O-C str), 743 (C-S Str) 1H NMR (DMSO-d6, δ, ppm): 8.3 (t, 1H, N-H), 7.10 (d, 1H, C5-H in chromen-2-one), 6.6-6.9 (d, 2H, C6-H and C8-H in chormen-2-one), 5.90 (s, 1H, C3-H in chromen-2-one), 1.9 (s, 3H, C4-CH3 in chromen-2-one) 4.3 (t, 2H, O-CH2 linkage), 4.10 (t, 2H, N-CH2 linkage), 7.3-7.4 (m, 2H, C5’-H and C6’-H in benzothiazole), 8.0 (d, 1H, C7’-H in benzothiazole), 2.35 (s, 3H, -CH3 of benzothiazole).
7-(2-(6-methylbenzo[d]thiazol-2-ylamino)ethoxy)-4- methyl-2H-chromen-2-one (4i), FTIR (KBr Vmax in cm-1): 3095 (N-H str), 2991 (C-H str), 1611 (C=O str), 1428 (C=C str), 1282 (C-N str), 1184 (CO-O-C str), 851 (C-H str), 732 (C-S str) 1H NMR (DMSO-d6, δ, ppm): 8.1 (t, 1H, N-H), 6.96 (d, 1H, C5-H in chromen-2-one), 6.5-6.6 (d, 2H, C6-H and C8-H in chormen-2-one), 6.0 (s, 1H, C3-H in chromen-2-one), 2.23 (s, 3H, C4-CH3 in chromen-2-one). 4.34 (t, 2H, O-CH2 linkage), 4.17 (t, 2H, N-CH2 linkage), 7.9-8.1 (m, 2H, C4’-H and C7’-H in benzothiazole), 7.4 (d, 1H, C5’-H in benzothiazole).
7-(2-(5-chlorobenzo[d]thiazol-2-ylamino)ethoxy)-4- methyl-2H-chromen-2-one (4j), FTIR (KBr Vmax in cm-1): 3089 (N-H str), 2972 (C-H str), 1708 (C=O str), 1555 (C=C str), 1246 (C-N str), 1180 (CO-O-C str), 816 (C-H str) 1H NMR (DMSO-d6, δ, ppm): 8.6 (t, 1H, N-H), 7.16 (d, 1H, C5-H in chromen-2-one), 6.6-6.9 (d, 2H, C6-H and C8-H in chormen-2-one), 5.90 (s, 1H, C3-H in chromen-2-one), 2.3 (s, 3H, C4-CH3 in chromen- 2-one), 4.24 (t, 2H, O-CH2 linkage), 4.12 (t, 2H, N-CH2 linkage), 7.56 (m, 1H, C6’-H in benzothiazole), 8-8.2 (d, 2H, C4’-H and C7’-H in benzothiazole).
7-(2-(4-bromobenzo[d]thiazol-2-ylamino)ethoxy)-4- methyl-2H-chromen-2-one (4k), FTIR (KBr Vmax in cm-1): 3267 (N-H str), 2891 (C-H str), 1732 (C=O str), 1492 (C=C str), 1226 (C-N str), 1056 (CO-O-C str), 701 (C-S str), 631 (C-Cl str) 1H NMR (DMSO-d6, δ, ppm): 8.4 (t, 1H, N-H), 7.20 (d, 1H, C5-H in chromen- 2-one), 6.6-6.9 (d, 2H, C6-H and C8-H in chormen- 2-one), 6.10 (s, 1H, C3-H in chromen-2-one), 2.1 (s, 3H, C4-CH3 in chromen-2-one). 4.11 (t, 2H, O-CH2 linkage), 4.18 (t, 2H, N-CH2 linkage), 7.72 (m, 1H, C5’-H in benzothiazole), 8.1-8.2 (d, 2H, C4’-H and C7’-H in benzothiazole).
Literature search revealed that D2 receptor blocking activity was the basis of development of typical antipsychotic agents with more side effects initially, but are highly effective to control positive symptoms of psychoses. Currently, the pharmacological basis of screening antipsychotics has extended to serotonergic receptor activity for development of atypical agents with less side effects as well as ability to control negative symptoms of psychoses.
Present study revealed that the synthesized compounds in series 4a-4k had a variety of antipsychotic activity. Compound 4a, which was not substituted with any group on benzothiazole ring gave very low % inhibition of 5HTP-induced head twitches and hence 4a was not selected for acute toxicity studies. Compounds 4b, 4e, 4j, which were substituted with a strong electron withdrawing group Cl, exhibited good activity. In compound 4j, where Cl was m-substituted, lowered the serotonergic and dopaminergic activity. Compounds 4f and 4k were substituted by F and Br groups, respectively and both the groups were highly electron withdrawing but bromo-substituted compound had demonstrated better serotonergic activity compared to fluoro-substituted compound and these were selected for toxicity studies. Compound 4g was substituted with Cl and F but did not show better activity than monosubstituted Cl or F compound. Compound 4d was substituted with electron withdrawing NO2 group, but did exhibit better serotonergic activity. Compound 4c was substituted with OCH3 group and compounds 4h and 4i were substituted with CH3 group. Compared to CH3 substituted compounds, OCH3 group was more electron withdrawing and as expected results of preliminary activity and toxicity supported this assumption.
Present study suggested that all synthesized compounds provided a chemical class of compounds that showed significant D2 antagonist activity in a pharmacological model predictive of antipsychotic activity and also had significant antagonistic activity at 5-HT receptor, an index of hypothesized atypical antipsychotic profile.
The compounds, which were substituted with chloro, bromo at ortho position and nitro, methyl and chloro at meta position showed significant D2 receptor antagonism and such compounds were selected for acute toxicity test. The compounds, which were substituted with chloro, bromo at ortho position and chloro, fluoro and methoxy at meta position showed significant 5HT receptor antagonistic activity and only such compounds were selected for acute toxicity testing. The selected compounds for toxicity studies were 4b, 4c, 4e, 4g, 4i and 4k. The effective dose (ED50) was calculated for only those compounds, which were selected for acute toxicity studies. The therapeutic index (TI) was determined using LD50 and ED50 values. From this data it was found that compounds (4b), (4e) and (4k) have maximum TI. From overall data it is possible to conclude that compounds, 7-(2-(6-chlorobenzo[d] thiazol-2-ylamino)ethoxy)-4-methyl-2H-chromen-2- one (4b) and 7-(2-(4-chlorobenzo[d]thiazol-2-ylamino) ethoxy)-4-methyl-2H-chromen-2-one (4e) have better atypical antipsychotic profile. Detailed chronic toxicity study as well as ligand binding studies are required for characterization of the compounds for understanding molecular mechanism, to predict therapeutic utility.
Acknowledgements
The authors gratefully acknowledged and thanks TVES’s College of Pharmacy, Faizpur, for providing animal studies on albino mice for biological evaluation of the compounds. Also thanks are due towards University of Pune for providing 1H NMR spectroscopy data.
Conflict of interest
Authors declared that there is no conflict of interest.
References
- Kaczor AA, Silva AG, Loza MI, Kolb P, Castro M, Poso A. Structure based virtual screening for dopamine D2 receptor ligand as potential antipsychotics. Chem Med Chem 2016;11(7):718-29.
- Younkin J, Gaitonde SA, Ellaithy A, Vekariya R, Baki L, Moreno JL, et al. Reformulation a pharmacophore for 5HT2A serotonin receptor antagonist. ACS Chem Neurosci 2016;7(9):1292-9.
- Reeves S, Eggleston K, Cort E, McLachlan E, Brownings S, Nair A, et al. Therapeutic D2/3 receptor occupancies and response with low amisulpride blood concentration in very late-onset schizophrenia-like psychosis. Int J Geriatr Psychiatry 2018;33(2):396-404.
- Tauscher J, Hussain T, Agid O, Verhoeff NP, Wilson AA, Houle S, et al. Equivalent occupancy of dopamine D1 and D2 receptors with clozapine: Differentiation from other atypical antipsychotics. Am J Psychiatry 2004;161(9):1620-5.
- Seeman P. Dopamine D2 receptors as treatment targets in schizophrenia. Clin Schizophr Relat Psychoses 2010;4(1):56-73.
- Murray R, Correll CU, Reynolds GP, Taylor D. Atypical antipsychotics: recent research findings and applications to clinical practice. Ther Adv Psychopharmacol 2017;7(1 Suppl):1-14.
- Frederick CN, Marina M, Brain JL, Akira Sawa. Clozapine as a model for antipsychotic development. Neurotherapeutics 2017;14(3):750-61.
- Choi YK, Gardner MP, Tarazi FI. Developmental effects of antipsychotic drugs on serotonin receptor subtypes. Synapse 2017;71(10):e21988.
- Oerther S, Ahlenius S. Atypical antipsychotics and dopamine D1 receptor agonism: An in vivo experimental study using core temperature measurements in the rat. J Pharmacol Exp Ther 2000;292(2):731-6.
- Christopher JS, John HK, Albert AC. MDL 100,907: A selective 5-HT2A receptor antagonist for the treatment of schizophrenia. CNS Drug Rev 1997;3(1):49-67.
- Kharadi DG, Shah AR, Ganguly B. Comparison of adverse effects of newer atypical antipsychotics: An evidence based review. J Young Pharm 2017;9(2):140-4.
- Kapur S, Seeman P. Antipsychotic agents differ in how fast they come off the dopamine D2 receptors: Implications for atypical antipsychotic action. J Psychiatry Neurosci 2000;25(2):161-6.
- Lin F, Li F, Wang C, Wang J, Yang Y, Yang L, et al. Mechanism exploration of arylpiperazine derivatives targeting the 5-HT2A receptor by in silico methods. Molecule 2017;22(7):1064-86
- Kesten SR, Heffner TG, Johnson SJ, Pugsley TA, Wright JL, Wise LD. Design, synthesis, and evaluation of chromen-2-ones as potent and selective human dopamine D4 antagonists. J Med Chem 1999;42(18):3718-25.
- Pechmann HV, Cohen JB. Uber die Verbindungen der Phenole mit Acetessigather. Eur J Inorg Chem 1883;16:2119-28.
- Sreenivasa GM, Jayachandran E, Shivakumar B, Jayaraj KK, Vijaykumar MM. Synthesis of bioactive molecule fluoro-benzothiazole comprising potent heterocyclic moieties for anthelmentic activity. Arch Pharm Sci Res 2009;1(2):150-7.
- Davis AS, Jenner P, Marsden CD. A comparison of motor behaviours in groups of rats distinguished by their climbing response to apomorphine. Br J Pharmacol 1986;87(1):129-37.
- Chung IW, Moore NA, Oh WK, O'Neill MF, Ahn JS, Park JB, et al. Behavioural pharmacology of polygalasaponins indicates potential antipsychotic efficacy. Pharmacol Biochem Behav 2002;71:191-5.
- Bruce RD. An up and down procedure for acute toxicity testing. Fundam Appl Toxicol 1985;5(1):151-7.
- Gad SC, Smith AC, Cramp AL, Gavigan FA, Derelanko MJ. Innovative Designs and practices for acute systemic toxicity studies. Drug Chem Toxicol 1984;7(5):423-34.
- Kulkarni SK. Handbook of experimental pharmacology. 1st ed. Delhi, India: Vallabh Prakashan; 2002. p. 168-70.
- Miller LC, Trainter ML. Estimation of the ED50 and its error by means of logarithmic-Probit graph paper. Proc Soc Exp Biol Med 1944;1:57-261.