- *Corresponding Author:
- Z. Yi
Department of Pharmacy, Affiliated Hospital of Nantong University, Yancheng Third People's Hospital, Yancheng, Jiangsu 224500, China
E-mail: j598shensong@163.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “219-225” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Peroxides and metals have been emerged as alternative antibacterial agents. However, the application of peroxides or metals alone for antibacterial treatment cannot yield satisfactory results. Combined therapy based on peroxides and metals is expected to generate synergistic effects. In this work, uniform zinc peroxide nanoparticles with an average size of 60 nm were synthesized as antimicrobial agent. Zinc peroxide nanoparticles were relatively stable in a neutral environment and showed quick release of hydrogen peroxide and zinc ion in acidic environment. After incubation with Escherichia coli and Staphylococcus aureus, large amount of reactive oxygen species was generated. Zinc peroxide nanoparticles illustrated outstanding antibacterial activity for Escherichia coli and Staphylococcus aureus, holding a great potential to be a highly efficient antibacterial agent.
Keywords
Metal peroxides, antibacterial, reactive oxygen species, zinc peroxide, nanoparticles, Escherichia coli and Staphylococcus aureus
Treatment of bacterial infection is still a challenge due to the emergence of bacterial resistance, which leads to the exploration of alternative antibacterial and treatments[1,2]. Therefore, it is extremely urgent to synthesize novel materials with broadspectrum antimicrobial activity to enhance or replace traditional antibiotics. These drugs play crucial role in surgeries, immunotherapy, and other medical procedures, significantly reducing human mortality and morbidity rates[3]. The mechanism of antibiotic resistance is complex and bacteria generally reduce the effectiveness of antibiotics through several different mechanisms[4]. These include reducing their permeability[5], actively pumping antibiotics out of the cell[6], altering or modifying the antibiotic's target affinity[7], rendering the antibiotic inactive, or modifying it in some way[8,9].
Peroxides and metals hold great promise as alternatives to antibiotics. Peroxides, such as Hydrogen peroxide (H2O2) and Sodium hypochlorite (NaClO), can cause oxidation of bacteria and has been widely used as bactericide[10,11]. These compounds generate Reactive Oxygen Species (ROS), which can disrupt essential cellular functions and damage bacterial cells by oxidizing cellular components[12,13]. Metals kill bacteria by different mechanisms; when metals come in contact with bacterial cells, they disrupt the outer membranes and penetrate the cells. Inside the cells, these metal ions interfere with various cellular processes, such as depleting antioxidants, disrupting the normal functioning of proteins and enzymes, damaging the cellular membranes and interfering with the electron transport, thereby causing genotoxicity[14,15]. Considering different antibacterial mechanisms, it is reasonable to deduce that the combination of the peroxides and metals is a potential method to enhance the antimicrobial activity. Among different metal oxides, Zinc Oxide (ZnO) is a widely used antimicrobial and/ or antifungal agent due to its advantages such as lower cytotoxicity, better selectivity and higher stability[16,17]. Previous studies have shown that Zinc peroxide (ZnO2) Nanoparticles (NPs) can release Oxygen (O2) triggered by temperature or pH in dispersing media[18]. It can be proved that the nanoparticles continuously release O2 for about 3 d at a pH<7.5 in an aqueous dispersion. The amount of O2 released depends on the different samples and is controlled by the different compositions and selected pH values of the samples.
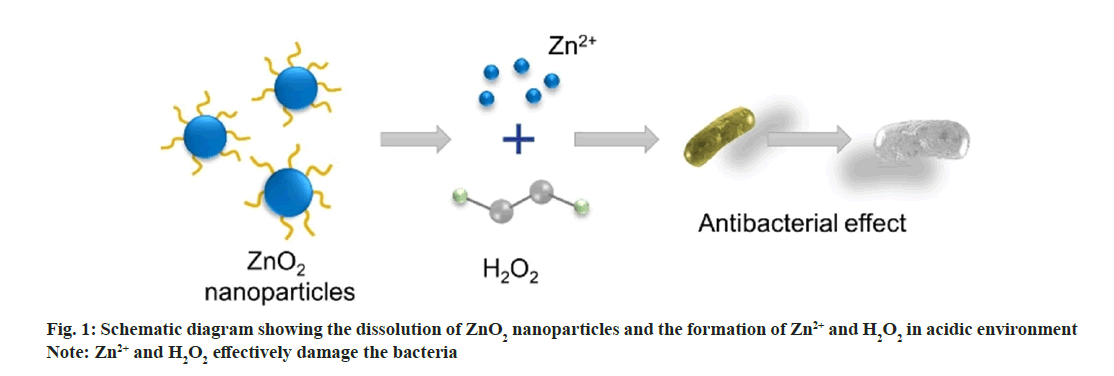
In this work, we synthesized uniform ZnO2 NPs to further improve the antimicrobial activity (fig. 1). ZnO2 NPs were synthesized by oxidizing zinc acetate with H2O2 using Polyvinylpyrrolidone (PVP) as stabilizer[19]. We evaluated the reaction of ZnO2 NPs in Phosphate Buffered Saline (PBS) at pH 5.5 and 7.4 and demonstrated that the Zinc (Zn2+) ions and hydrogen peroxide release rates are greatly accelerated in acidic environments. Simultaneously, we found that in the presence of ZnO2 NPs, ROS can be produced in both Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus), laying the foundation for the antibacterial effect of ZnO2 NPs. The toxicity experiments were conducted to explore the antibacterial effect of ZnO2 NPs, which opened up a new path for the future application of ZnO2.
Materials and Methods
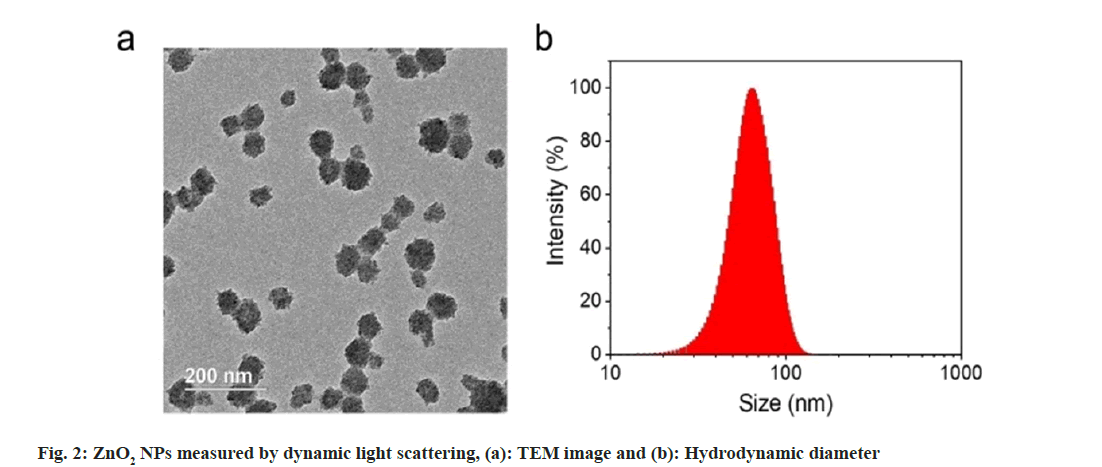
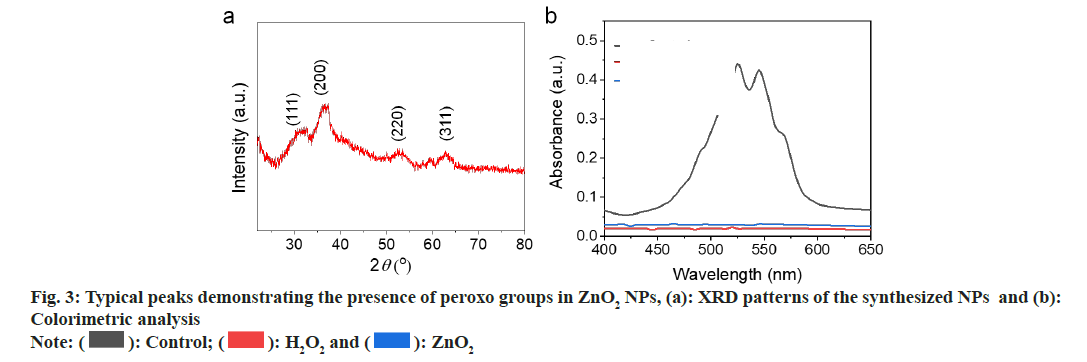
We used Transmission Electron Microscopy (TEM) for characterization of the obtained ZnO2 NPs. As shown in fig. 2a, the ZnO2 NPs were uniform in spherical morphology with an average diameter of 60 nm; Dynamic Light Scattering (DLS) was used to determine the hydrated particle size by. The average size increased to 70 nm due to the inclusion of solvation shell (fig. 2b). X-Ray Diffraction (XRD) was utilized to detect the crystal structure of the ZnO2 NPs. As shown in fig. 3a, the pattern of ZnO2 with typical peaks at 31°, 37°, 53° and 63° which was supported by the Joint Committee on Powder Diffraction Standards (JCPDS) (having number: 00-076-1364), attributing to cubic zinc blende structure[20,21]. We have noticed that the diffraction peaks of ZnO2 NPs were wide and weak, indicating relatively small size of the nanocrystals. Small sized crystals implied the capability of PVP in inhibiting the formation and growth of crystals.
PVP molecules play an essential role in the formation of the nanocrystals during the formation of ZnO2 NPs. PVP can chelate with metal ions and then assist the ions to form ZnO2 nanocrystals in the presence of oxidants. From the TEM image of ZnO2 NPs, we found that the ZnO2 NPs were composed of many small nanocrystals. The formation of the larger nanocrystals aggregations was because of the tendency to reduce the high surface energy. Meanwhile, PVP can also prevent the sudden and random aggregation of the nanocrystals to help the formation of uniform ZnO2 NPs owing to the steric hindrance effect of the PVP.
Results and Discussion
In neutral and acidic environment, ZnO2 NPs could react with Water (H2O) and Hydrogen ions (H+) to produce H2O2 by the reactions (1) and (2), respectively.
ZnO2+2H+→Zn2++H2O2 (1)
ZnO2+2H+→Zn2++H2O2 (2)
In acidic environment, ZnO2 NPs reacted with H+ to generate H2O2 as per reaction (1). Thus generated H2O2 was determined by Potassium Permanganate (KMnO4)-based colorimetric method.
3H++2KMnO4+5H2O2→2K++2Mn2++5O2+ 8H2O (3)
Purple colored Permanganate (MnO4 -) ion was reduced by peroxo groups to form colourless Manganese (Mn2+) ion. As shown in fig. 3b, the purple color of MnO4 - in acidic solution disappeared after addition of H2O2 or ZnO2 NPs, indicating the formation of H2O2 in the acidic medium.
Most metal oxides are unstable and will hydrolyze in H2O. However, the hydrolysis rate is quite different. Calcium peroxide (CaO2) NPs hydrolyze quickly in aqueous solution, while the hydrolysis of Copper peroxide (CuO2) was much slower[22,23]. We have evaluated the reaction of ZnO2 NPs at pH 5.5 and 7.4 Phosphate Buffer Saline (PBS) and determined the release rates of Zn2+ and H2O2. As shown in fig. 4a and fig. 4b, both Zn2+ and H2O2 were gradually released from the ZnO2 NPs at pH 5.5, showing a cumulative release >60 % at 4 h. In contrast, the release rates were slower at pH 7.4 PBS that only 5 % was released after 6 h. These results indicate that the ZnO2 NPs are relatively stable in neutral environment and will decompose quickly in acidic medium. The bacteria acidic microenvironment also facilitates the release of zinc and hydrogen peroxide.
It is reported that bacterial infection will form weak acidic microenvironment. Hence, H2O2 is expected to generate after incubation of the bacteria with ZnO2 NPs. The intracellular ROS was detected using 2,7-Dichloro-dihydro-Fluorescein Diacetate (DCFH-DA) as an indicator, which is nonfluorescent and can be oxidized by ROS to form fluorescent Dichlorodihydrofluorescein (DCF).
As shown in fig. 5a and fig. 5b, barely visible fluorescence was observed in E. coli and S. aureus without the treatment of ZnO2 NPs. In contrast, bacteria treated with ZnO2 NPs illustrated bright green fluorescence, indicating the generation of ROS in bacteria. We also noticed that the strength of the fluorescence was time dependent. The fluorescence intensities of E. coli and S. aureus at 2 h were 1.58 and 2.15 times to those of 0.5 h, respectively (fig. 6a and fig. 6b). It is necessary to search appropriate incubation time to enhance the antibacterial effects of ZnO2 NPs. The generated ROS will cause oxidative stress to bacterial cells and result in the damage to protein, membrane lipids and Deoxyribonucleic Acid (DNA).
In addition to the evaluation of size, morphology, crystallinity, generation of ROS and the O2 release performance of ZnO2 NPs were also studied. Previous studies have indicated that O2 release from ZnO2 NPs was related to temperature or pH values. We investigated its O2 release in aqueous solutions with different pH values. ZnO2 NPs dissociates into Zn2+ ions and H2O2 in acidic aqueous media, while H2O2 can be catalyzed by the metal on the NPs surface to produce into H2O and O2. We measured the time-dependent release of O2 from ZnO2 NPs in different pH media using an O2 meter.
The determinations were performed in degassed PBS in nitrogen atmosphere to influence of atmospheric O2 on quantification. PBS solution without ZnO2 nanoparticles determined in identical conditions was used as control. The experiment was performed at room temperature (T=25°) in neutral and weakly acidic PBS with pH values of 7.4 and 6.5, respectively. The results indicated that there is a constant O2 release for at least 96 h and then reached a plateau resulting in a saturation curve. Since PBS solution was degassed and placed under a nitrogen atmosphere, there was negligible O2 present during the determination. After dispersing the NPs in PBS, O2 was constantly released, causing the increase of O2 concentration in H2O. When the reaction of NPs with H2O was completed, O2 concentration reached the plateau. Considering the high solubility of O2 in H2O (8.7- 8.4 mg/l at 25°)[24,25], the plateau was because of the nanoparticle deactivation. The determinations of the ZnO2 nanoparticles in different pH value i.e., at 6.5 revealed that the amount of released O2 was dependent on the pH. As shown in fig. 7, the O2 release increased as the decrease of pH value. The amount of released O2 increased from 0.73 to 2.1 mg/l with changing pH value from 7.4 and 6.5. These results were in line with the expectations because the acidic environment accelerates the O2 release by accelerating the decomposition of the ZnO2 NPs.
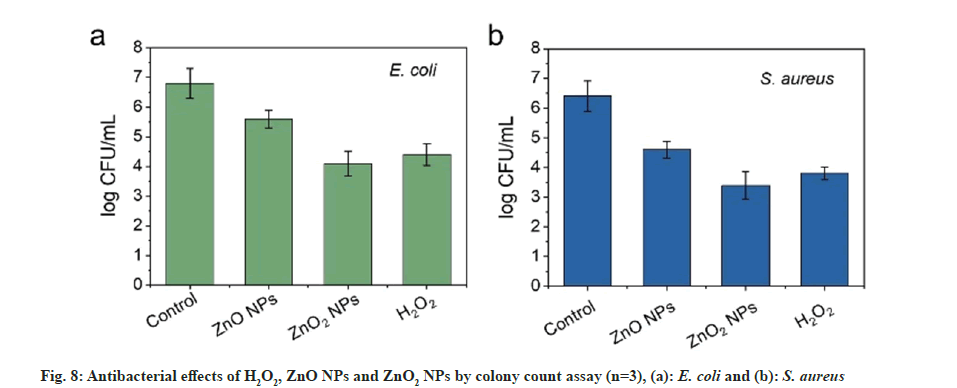
ZnO is a transition metal oxide and semiconductor with wide band gap (3.3 eV). Oxidizing character and oxidizing sites would be created once there is radiation with energy larger than the band gap of the ZnO. Thus, formed oxidizing sites are capable of oxidizing water molecules and hydroxide anions to form strong oxidizing species and generate antibacterial effect. For ZnO2 NPs, can react with H2O and H+ to release ROS and are expected to cause better antibacterial effect. The antibacterial activity of the ZnO2 NPs was then evaluated using E. coli and S. aureus as model bacteria by colony counting assay method. To comprehensively understand the antibacterial efficiency, ZnO NPs were used as control. As presented in fig. 8a and fig. 8b, ZnO2 NPs reduced the number of E. coli and S. aureus by about 40 % and 50 %, respectively. The toxicity of ZnO2 NPs towards the E. coli and S. aureus was much stronger than the ZnO NPs. The increased toxicity of ZnO2 NPs should be attributed to the synergistic effect of H2O2 and Zn2+. The effect of ZnO2 NPs was comparable to H2O2. Although ZnO NPs are considered as a bacteriostatic agent, their effect could be further improved by forming peroxides. For the antibacterial mechanism, it is reported that metal NPs will attach to the surfaces of bacteria by the adsorption and electrostatic interaction, resulting in damage to the cell membrane. Meanwhile, the released Zn2+ ions can chelate with teicoic and lipoteichoic acids, and then be carried by passive diffusion across membrane proteins[16].
The Minimum Inhibitory Concentration (MIC) values of the ZnO2 NPs were further studied by micro-broth dilution method; MICs against E. coli and S. aureus were 90 and 60 μg/ml, respectively. In contrast, MICs of ZnO NPs for E. coli and S. aureus were 350 and 200 μg/l, respectively. These results were in agreement with the results obtained by colony counting assay method, suggesting the enhancement of the bacterial viability inhibition effect.
In summary, ZnO2 NPs with uniform size were successfully synthesized with the help of PVP through a facile method. NPs could release H2O2 and Zn2+ quickly in acidic environment. ZnO2 NPs showed outstanding antibacterial activity, owing to the synergistic effect of released H2O2 and Zn2+. Although the ZnO2 NPs were relatively stable in H2O, it is difficult to store for long-time storage in aqueous medium. For clinic application, dispersing the ZnO2 NPs in non-aqueous media, such as glycerin, may be a feasible method to enhance the stability.
Acknowledgement:
This study was financially supported by the National Natural Science Foundation of China. (Grant No: 82272662 and 22074150).
Conflict of interests:
The authors declared no conflict of interests.
References
- Muteeb G, Rehman MT, Shahwan M, Aatif M. Origin of antibiotics and antibiotic resistance, and their impacts on drug development: A narrative review. Pharmaceuticals 2023;16(11):1-54.
[Crossref] [Google Scholar] [PubMed]
- Helmy YA, Taha-Abdelaziz K, Hawwas HA, Ghosh S, AlKafaas SS, Moawad MM, et al. Antimicrobial resistance and recent alternatives to antibiotics for the control of bacterial pathogens with an emphasis on foodborne pathogens. Antibiotics 2023;12(2):1-52.
[Crossref] [Google Scholar] [PubMed]
- Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol 2015;13(1):42-51.
[Crossref] [Google Scholar] [PubMed]
- Darby EM, Trampari E, Siasat P, Gaya MS, Alav I, Webber MA, et al. Molecular mechanisms of antibiotic resistance revisited. Nat Rev Microbiol 2023;21(5):280-95.
[Crossref] [Google Scholar] [PubMed]
- Mishra NN, Bayer AS, Tran TT, Shamoo Y, Mileykovskaya E, Dowhan W, et al. Daptomycin resistance in Enterococci is associated with distinct alterations of cell membrane phospholipid content. PLoS One 2012;7(8):1-10.
[Crossref] [Google Scholar] [PubMed]
- Nazarov PA. MDR pumps as crossroads of resistance: Antibiotics and bacteriophages. Antibiotics 2022;11(6):1-18.
[Crossref] [Google Scholar] [PubMed]
- Schaenzer AJ, Wright GD. Antibiotic resistance by enzymatic modification of antibiotic targets. Trends Mol Med 2020;26(8):768-82.
[Crossref] [Google Scholar] [PubMed]
- Szychowski J, Kondo J, Zahr O, Auclair K, Westhof E, Hanessian S, et al. Inhibition of aminoglycoside-deactivating enzymes APH (3′)-IIIa and AAC (6′)-Ii by amphiphilic paromomycin O2″-ether analogues. ChemMedChem 2011;6(11):1961-66.
[Crossref] [Google Scholar] [PubMed]
- Ramirez MS, Tolmasky ME. Aminoglycoside modifying enzymes. Drug Resist Updat 2010;13(6):151-71.
[Crossref] [Google Scholar] [PubMed]
- Parisay I, Talebi M, Asadi S, Nikbakht MH. Antimicrobial efficacy of 2.5 % sodium hypochlorite, 2 % chlorhexidine, and 1.5 % hydrogen peroxide on Enterococcus faecalis in pulpectomy of necrotic primary teeth. J Dent Mater Tech 2021;10(2):94-101.
- Afrasiabi S, Chiniforush N. An in vitro study on the efficacy of hydrogen peroxide mediated high-power photodynamic therapy affecting Enterococcus faecalis biofilm formation and dispersal. Photodiagnosis Photodyn Ther 2023;41:103310.
[Crossref] [Google Scholar] [PubMed]
- Li H, Zhou X, Huang Y, Liao B, Cheng L, Ren B. Reactive oxygen species in pathogen clearance: The killing mechanisms, the adaption response, and the side effects. Front Microbiol 2021;11:1-9.
[Crossref] [Google Scholar] [PubMed]
- Lam PL, Wong RM, Lam KH, Hung LK, Wong MM, Yung LH, et al. The role of reactive oxygen species in the biological activity of antimicrobial agents: An updated mini review. Chem Biol Interact 2020;320:1-45.
[Crossref] [Google Scholar] [PubMed]
- Zhang E, Zhao X, Hu J, Wang R, Fu S, Qin G. Antibacterial metals and alloys for potential biomedical implants. Bioact Mater 2021;6(8):2569-612.
[Crossref] [Google Scholar] [PubMed]
- Godoy-Gallardo M, Eckhard U, Delgado LM, de Roo Puente YJ, Hoyos-Nogués M, Gil FJ, et al. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact Mater 2021;6(12):4470-90.
[Crossref] [Google Scholar] [PubMed]
- da Silva BL, Caetano BL, Chiari-Andréo BG, Pietro RC, Chiavacci LA. Increased antibacterial activity of ZnO nanoparticles: Influence of size and surface modification. Colloids Surf B Biointerfaces 2019;177:440-7.
[Crossref] [Google Scholar] [PubMed]
- Sirelkhatim A, Mahmud S, Seeni A, Kaus NH, Ann LC, Bakhori SK, et al. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nanomicro Lett 2015;7:219-42.
[Crossref] [Google Scholar] [PubMed]
- Bergs C, Brück L, Rosencrantz RR, Conrads G, Elling L, Pich A. Biofunctionalized Zinc peroxide (ZnO2) nanoparticles as active oxygen sources and antibacterial agents. RSC Adv 2017;7(62):38998-9010.
- Lin LS, Wang JF, Song J, Liu Y, Zhu G, Dai Y, et al. Cooperation of endogenous and exogenous reactive oxygen species induced by zinc peroxide nanoparticles to enhance oxidative stress-based cancer therapy. Theranostics 2019;9(24):380-400.
[Crossref] [Google Scholar] [PubMed]
- Morales-Mendoza JE, Paraguay-Delgado F, Moller JD, Herrera-Pérez G, Pariona N. Structure and optical properties of ZnO and ZnO2 nanoparticles. J Nano Res 2019;56:49-62.
- Chen W, Lu YH, Wang M, Kroner L, Paul H, Fecht HJ, et al. Synthesis, thermal stability and properties of ZnO2 nanoparticles. J Phys Chem C 2009;113(4):1320-4.
- Shen S, Mamat M, Zhang S, Cao J, Hood ZD, Figueroa‐Cosme L, et al. Synthesis of CaO2 nanocrystals and their spherical aggregates with uniform sizes for use as a biodegradable bacteriostatic agent. Small 2019;15(36):1-7.
[Crossref] [Google Scholar] [PubMed]
- Zhang R, Jiang G, Gao Q, Wang X, Wang Y, Xu X, et al. Sprayed copper peroxide nanodots for accelerating wound healing in a multidrug-resistant bacteria infected diabetic ulcer. Nanoscale 2021;13(37):15937-51.
[Crossref] [Google Scholar] [PubMed]
- Weiss RF. The solubility of nitrogen, oxygen and argon in water and seawater. Deep-Sea Res Oceanogr Abstr 1970;17(4):721-35.
- Mendes CR, Dilarri G, Forsan CF, Sapata VD, Lopes PR, de Moraes PB, et al. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci Rep 2022;12(1):1-10.
[Crossref] [Google Scholar] [PubMed]
 ): Control; (
): Control; ( ): H2O2 and (
): H2O2 and ( ): ZnO2
): ZnO2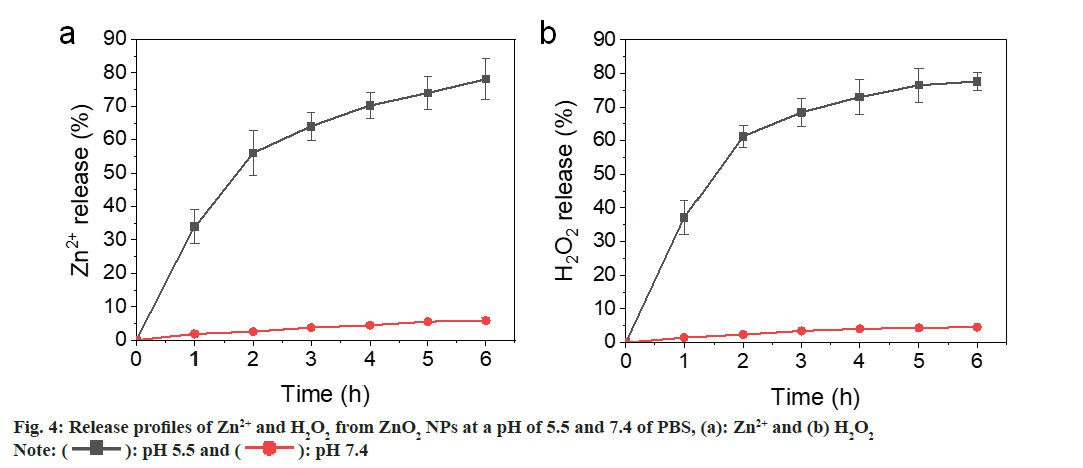
 ): pH 5.5 and (
): pH 5.5 and ( ): pH 7.4
): pH 7.4 
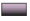 ): Control and (
): Control and ( ): ZnO2
): ZnO2 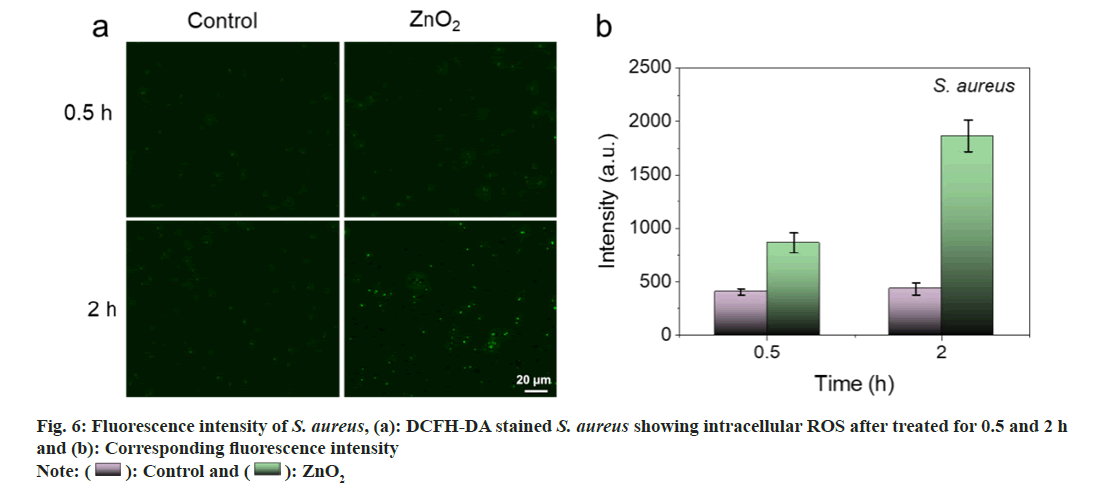
 ): Control and (
): Control and (  ): ZnO2
): ZnO2 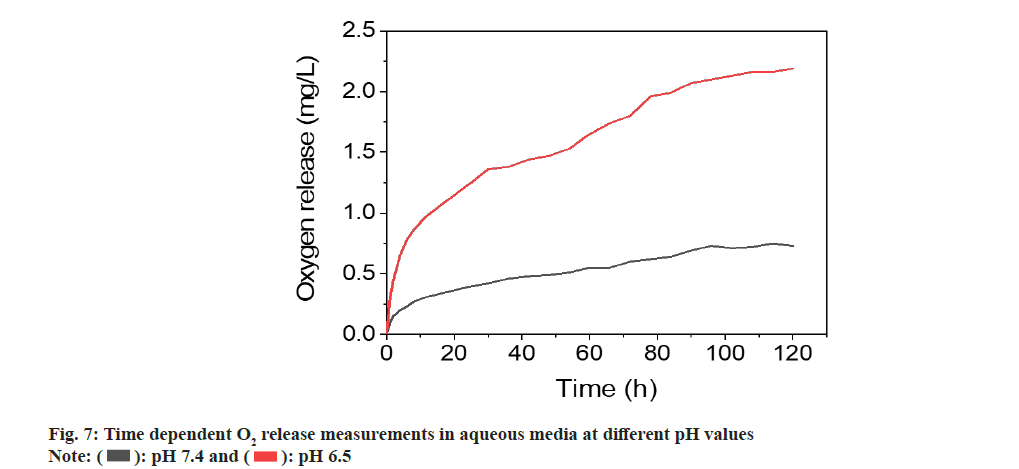
 ): pH 7.4 and (
): pH 7.4 and ( ): pH 6.5
): pH 6.5