- *Corresponding Author:
- S. D. Joshi
Department of Pharmaceutical Chemistry, S.E.T’s College of Pharmacy, S. R. Nagar, Dharwad-580 002, India
E-mail: shrinivasdj@rediffmail.com
| Date of Submission | 18 October 2012 |
| Date of Revision | 07 March 2013 |
| Date of Acceptance | 24 March 2013 |
| Indian J Pharm Sci,2013;75(3):310-323 |
Abstract
A series of 4-(2,5-dimethylpyrrol-1-yl)/4-pyrrol-1-yl benzoic acid hydrazide analogs, some derived triazoles, azetidinones, thiazolidinones, and pyrroles have been synthesized in good yields and structures of these compounds were established by IR, 1 H NMR, 13 C NMR, mass spectral, and elemental analysis. These compounds were evaluated for their preliminary in vitro antibacterial, antifungal, and antitubercular activity against Mycobacterium tuberculosis H 37 Rv strain by the broth dilution assay method. Twenty one of these compounds displayed good antimicrobial activity, with a MIC value of 1-4 μg/ml. Several compounds 4c, 8-10, 15b-15h, and 16b-16d exhibited good in vitro antitubercular activity with MIC value 1-2 μg/ml. Further, some title compounds were also assessed for their cytotoxic activity (IC 50 ) against mammalian Vero cell lines and A 549 (lung adenocarcinoma) cell lines using the MTT assay method. The results revealed that these compounds exhibit antitubercular activity at non-cytotoxic concentrations.
Keywords
Pyrroles, acid hydrazide derivatives, antibacterial activity, antitubercular activity, antifungal activity, broth dilution assay method, cytotoxicity
Introduction
Tuberculosis (TB) is a communicable disease caused by Mycobacterium tuberculosis and remains as a one of the most contagious and deadly diseases and is a major threat for public health. Despite decades of fighting TB, the disease is gaining ground as the mycobacteria’s resistance to drugs. Every year, India adds the largest number of people to the global TB population. WHO records show that of the 11 lakh deaths reported in 2010, more than 3 lakh were from India, the largest number recorded, followed by Bangladesh, Indonesia, and Pakistan. The increasing spread of TB together with the emergence of resistance against conventional drugs has put enormous pressure on public health systems to introduce new TB treatments.
Nowadays, double drug development and/ or multi-therapeutic strategies, which utilize new chemical entities with two (or more than two) different N-heterocyclic skeletons, are also valid and perspective to create new antitubercular drugs. These strategies have likely to overcome the drug resistant TB problem.
Pyrrole nucleus has been gaining prominence due to the fact that its derivatives have been found to possess wide spectrum of activities like antibacterial [1], antifungal [2], antitubercular [3], and anticancer [4]. However, we have also reported substituted pyrrole derivatives as potent antibacterial and antitubercular agents [5,6]. Moreover, thiazolidinones, azetidinones, and triazoles are other important pharmacodynamic heterocyclic nuclei which when incorporated in different heterocyclic templates have been reported to possess potent antitubercular activity.
The 2-azetidinone ring system known as β-lactam since 1907, a common structural feature of a number of broad spectrum β-lactam antibiotics, including penicillins, cephalosporins, monobactams, clavulanic acid, sulbactams and carbapenems, which have been extensively used as chemotherapeutic agents to treat microbial diseases and bacterial infections [7]. The 2-azetidinone derivatives have been reported to possess wide range of biological activities like antifungal [8], anticancer [9], antibacterial [10], and antitubercular [11].
Thiazolidinones are thiazolidine derivatives and have an atom of sulfur at position 1, an atom of nitrogen at position 3 and a carbonyl group at position 2, 4, or 5. However, its derivatives fit into the most commonly studied moieties and its presence in penicillin was the first recognition of its occurrence in nature. The 4-thiazolidinone scaffold is very versatile and has featured in a number of clinically used drugs. Thiazolidinones derivatives have exhibited various biological activities like anticancer [12], antitubercular [13], antibacterial [14,15], and antifungal [16].
1,2,3-Triazoles have occupied an important role not only in organic chemistry but also in medicinal chemistry due to their easy synthesis by click chemistry and attractive features. Triazole and its derivatives possess broad spectrum of biological activities which include antifungal [17], antibacterial [18], antitubercular [19], anticancer [20], and antiviral [21].
Lipophilicity is a key property that influences the ability of a drug to reach the target by transmembrane diffusion and to have a major effect on the biological activity. The azole antituberculars are regarded as emerging class and triazoles known as lipophilic analogs similar to imidazoles are expected to increase log P. Triazoles are utilized as pharmacophores because of their favorable metabolic profile and ability to engage in hydrogen bonding.
Keeping in view the above facts and continuing our program on the development of efficient methods to generate drug-like nitrogen-containing molecules [5,6,18], we were interested in a simple synthesis of pyrrole analogs containing the thiazolidin-4-one, azetidinon-4-one, triazole moiety or other different N-heterocyclic skeletons and study their antimicrobial, antitubercular activities followed by cytotoxic activity.
Materials and Methods
Chemicals used in the synthesis of the titled compounds were purchased from Sigma–Aldrich, St. Loise, MO, USA, S. D. Fine-Chem Limited, Mumbai, and Spectrochem Pvt. Ltd. Mumbai, India. The melting points of synthesized compounds were determined on melting point apparatus from Shital Scientific Industries, Mumbai and were uncorrected; infra-red spectra were recorded on a Bruker spectrophotometer by using KBr pellets. 1H and 13C-NMR spectra were recorded on Bruker Avance III 500 MHz instruments using DMSO-d6 as solvent and TMS as internal standard, chemical shifts are expressed as δ values (ppm). Mass spectra (MS) were taken in Jeol GCmate II GC-Mass spectrometer and Schimadzu QP 20105 GC-Mass spectrometer. Microanalyses of compounds were also performed on Leco Tru Spec CHNS Analyzer for the determination of percentage of C, H, and N. All the new compounds exhibited spectral data consistent with the proposed structures and values of microanalysis are within ±0.4% of the theoretical values. Analytical thin-layer chromatography (TLC) was performed on precoated TLC sheets of silica gel 60 F254 (Merck, Darmstadt, Germany), visualizing by long- and short-wavelength ultraviolet (UV) lamps.
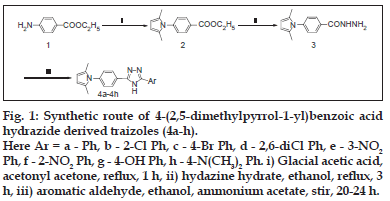
The synthetic strategies adopted to obtain the target compounds are depicted in figs. 1-3. The compound 4-(2,5-dimethylpyrrol-1-yl) benzoic acid hydrazide (3) was chosen as starting compound to design several derived compounds and traizoles. Ethyl 4-aminobenzoate (1) was prepared by the reaction of 4-aminobenzoic acid and absolute ethanol in the presence of HCl gas. Paal-Knorr condensation reaction between ethyl 4-aminobenzoate and acetonyl acetone in glacial acetic acid furnished ethyl 4-(2,5-dimethylpyrrol-1-yl) benzoate (2). Nucleophillic reaction of hydrazine hydrate with the ester (2) in ethanol medium produced 4-(2,5-dimethylpyrrol-1-yl) benzoic acid hydrazide (3). Reaction of compound (3) with aromatic aldehydes in the presence of ammonium acetate yielded 1,2,4-traizoles (4a-h).
Fig.1: Synthetic route of 4-(2,5-dimethylpyrrol-1-yl)benzoic acid hydrazide derived traizoles (4a-h). Here Ar = a - Ph, b - 2-Cl Ph, c - 4-Br Ph, d - 2,6-diCl Ph, e - 3-NO2 Ph, f - 2-NO2 Ph, g - 4-OH Ph, h - 4-N(CH3)2 Ph. i) Glacial acetic acid, acetonyl acetone, reflux, 1 h, ii) hydazine hydrate, ethanol, reflux, 3 h, iii) aromatic aldehyde, ethanol, ammonium acetate, stir, 20-24 h.
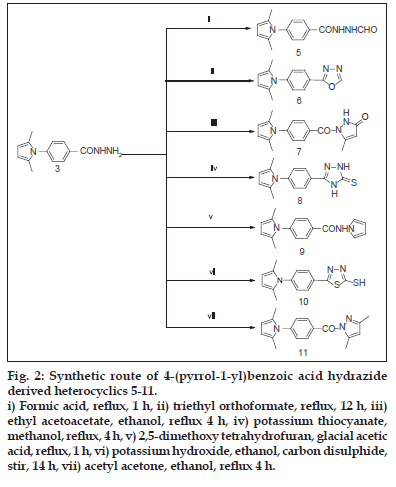
Fig. 2: Synthetic route of 4-(pyrrol-1-yl)benzoic acid hydrazide derived heterocyclics 5-11. i) Formic acid, reflux, 1 h, ii) triethyl orthoformate, reflux, 12 h, iii) ethyl acetoacetate, ethanol, reflux 4 h, iv) potassium thiocyanate, methanol, reflux, 4 h, v) 2,5-dimethoxy tetrahydrofuran, glacial acetic acid, reflux, 1 h, vi) potassium hydroxide, ethanol, carbon disulphide, stir, 14 h, vii) acetyl acetone, ethanol, reflux 4 h.
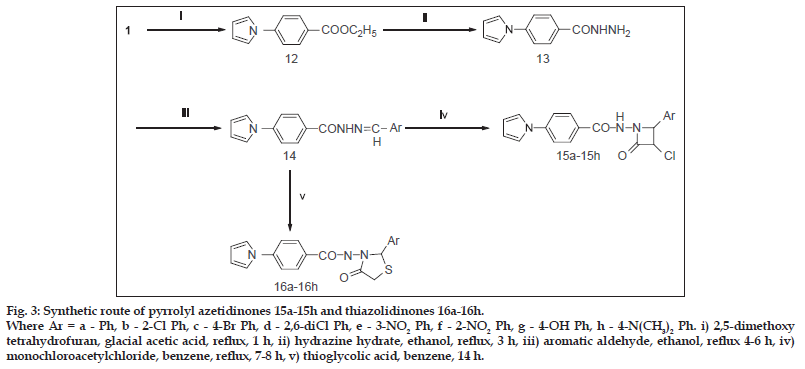
Fig. 3: Synthetic route of pyrrolyl azetidinones 15a-15h and thiazolidinones 16a-16h. Where Ar = a - Ph, b - 2-Cl Ph, c - 4-Br Ph, d - 2,6-diCl Ph, e - 3-NO2 Ph, f - 2-NO2 Ph, g - 4-OH Ph, h - 4-N(CH3)2 Ph. i) 2,5-dimethoxy tetrahydrofuran, glacial acetic acid, reflux, 1 h, ii) hydrazine hydrate, ethanol, reflux, 3 h, iii) aromatic aldehyde, ethanol, reflux 4-6 h, iv) monochloroacetylchloride, benzene, reflux, 7-8 h, v) thioglycolic acid, benzene, 14 h.
The synthetic routes for the preparation of pyrrolyl derivatives 5-11 are summarized in fig. 2 4-(2,5-dimethyl-1H-pyrrol-1-yl)-N’- formylbenzohydrazide (5) was synthesized by the reaction of hydrazide (3) with formic acid. Further on reaction with triethyl orthoformate compound 3 yielded 2-(4-(2,5-dimethyl-1H-pyrrol-1-yl) phenyl)- 1,3,4-oxadiazole (6). Hydrazide 3 on reaction with ethyl acetoacetate yielded 1-(4-(2,5-dimethyl-1H-pyrrol-1-yl) benzoyl)-5-methyl-1H-pyrazol-3 (2H)- one (7). Treatment of compound 3 with potassium thiocyanate afforded 3-(4-(2,5-dimethyl-1H-pyrrol- 1-yl) phenyl)-1H-1,2,4-triazole-5 (4H)-thione (8). 4-(2,5-dimethyl-1H-pyrrol-1-yl)-N-(1H-pyrrol-1-yl) benzamide (9) has been synthesized by refluxing compound 3 with 2,5-dimethoxy tetrahydrofuran in the presence of glacial acetic acid. Treatment of 3 with carbon disulphide and potassium hydroxide afforded 5-(4-(2,5-dimethyl-1H-pyrrol-1-yl) phenyl)- 1,3,4-thiadiazole-2-thiol (10). Further 3 reacted with acetyl acetone to afford 3,5-dimethyl-1Hpyrazol- 1-yl-(4-(2,5-dimethyl-1H-pyrrol-1-yl) phenyl) methanone (11).
As shown in fig. 3, compound (13) on reaction with aromatic aldehyde in ethanol gave 4-pyrrol-1-yl benzoic acid (arylidene) hydrazides (14a-h). The Staudinger reaction of hydrazones (14a-h) with chloroacetylchloride and tirethylamine in the presence of dry benzene afforded N-(2-substituted-3-chloro- 4-oxoazetidin-1-yl)-4-(1H-pyrrol-1-yl) benzamides (15a-h). The reaction of hydrazones (14a-h) with thioglycolic acid in the presence of anhydrous zinc chloride afforded N-(4-oxo-2-substitutedthiazolidin-3- yl)-4-(1H-pyrrol-1-yl) benzamides (16a-h).
The structures of newly synthesized compounds were assigned on the basis of their spectral and analytical data. The physical data, FT-IR, NMR, and mass spectral data for all the synthesized compounds are reported in experimental protocols.
Synthesis of ethyl 4-(2,5-dimethylpyrrol-1-yl) benzoate (2)
A mixture of acetonyl acetone (0.12 moles 13.69 g) and ethyl 4-aminobenzoate (2) (0.1 moles, 16.5 g) in glacial acetic acid (100 ml) was refluxed for 1 h. The solvent was removed under reduced pressure, residue thus obtained was collected by filtration, washed with water, dried and recrystallized from ethanol (yield 65%) mp 87-88° [6].
Synthesis of 4-(2,5-dimethylpyrrol-1-yl) benzoic acid hydrazide (3)
Compound 2 was synthesized by refluxing a mixture of ethyl 4-(2,5-dimethylpyrrol-1-yl) benzoate (1) (0.015 moles, 3.64 g) with hydrazine hydrate (10 ml) in absolute ethanol (10 ml) for 3 h (monitored by TLC). The cooled mixture was poured gradually onto crushed ice cubes with stirring. The mixture was allowed to stand and solid was separated. It was filtered, washed thoroughly with cold water, dried and recrystallized from ethanol (yield 80%) mp 170-172° [6].
Synthesis of 3-(4-(2,5-dimethyl-1H-pyrrol-1-yl) phenyl)-5-substituted-4H-1,2,4-traizoles (4a-h)
Equimolar quantity of 4-(2,5-dimethylpyrrol-1-yl) benzoic acid hydrazide (2) and different aromatic aldehydes was stirred in ethanol for 20-24 h in the presence of ammonium acetate. The solution was neutralized with liquid ammonia solution and the product obtained was filtered, washed with water, and dried. The crude solid was recrystallized from dioxane-ethanol to give the products.
3-(4-(2,5-Dimethyl-1H-pyrrol-1-yl) phenyl)- 5-phenyl-4H-1,2,4-traizole (4a): Yield 63% mp 244-246°; IR (KBr cm−1): 3245 (NH); 1H NMR (500 MHz, DMSO-d6) δ ppm: 1.96 (s, 6H, 2CH3), 5.79 (s, 2H, pyrrole-C3 and C4-H), 7.55-7.95 (m, 9H, aromatic protons), 11.01 (s, 1H, NH, disappeared on D2O exchange); 13C NMR (500 MHz, DMSO-d6) δ ppm: 162.40 (triazole-C5), 148.20 (triazole-C3), 141.48 (bridging phenyl-C4), 134.50 (phenyl-C1 at C5 of triazole), 132.12 (pyrrole-C2 and C5), 130.18 (phenyl-C3 and C5 at C5 of triazole), 129.20 (phenyl-C4 at C5 of triazole), 128.22 (bridging phenyl-C1), 127.92 (bridging phenyl-C2 and C6), 127.26 (phenyl-C2 and C6 at C5 of triazole), 120.81 (bridging phenyl-C3 and C5), 111.82 (pyrrole-C3 and C4), 10.24 (CH3); Mass (m/z): 314.38.
3-(2-Chlorophenyl)-5-(4-(2,5-dimethyl-1H-pyrrol-1-yl) phenyl)-4H-1,2,4-triazole (4b): Yield 72% mp 254-256°; IR (KBr cm−1): 3233 (NH); 1H NMR (500 MHz, DMSO-d6) δ ppm: 1.92 (s, 6H, 2CH3), 5.81 (s, 2H, pyrrole-C3 and C4-H), 7.60-7.99 (m, 8H, aromatic protons), 10.88 (s, 1H, NH, disappeared on D2O exchange); 13C NMR (500 MHz, DMSO-d6) δ ppm: 164.12 (triazole-C5), 146.55 (triazole-C3), 140.98 (bridging phenyl-C4), 135.22 (phenyl-C1 at C3 of triazole),131.55 (pyrrole-C2 and C5), 129.88 (phenyl-C3 and C5 at C3 of triazole), 129.52 (phenyl-C4 at C3 of triazole), 129.22 (bridging phenyl-C1), 128.16 (phenyl-C2 at C3 of triazole), 127.60 (bridging phenyl-C2 and C6), 120.77 (bridging phenyl-C3 and C5), 110.12 (pyrrole-C3 and C4), 11.55 (CH3); Mass (m/z): 348.83.
3-(4-Bromophenyl)-5-(4-(2,5-dimethyl-1H-pyrrol-1-yl) phenyl)-4H-1,2,4-triazole (4c): Yield 77% mp 232-234°; IR (KBr cm−1): 3267 (NH); 1H NMR (500 MHz, DMSO-d6) δ ppm: 1.96 (s, 6H, 2CH3), 5.79 (s, 2H, pyrrole-C3 and C4-H), 7.78-8.10 (m, 8H, aromatic protons), 10.94 (s, 1H, NH, disappeared on D2O exchange); 13C NMR (500 MHz, DMSO-d6) δ ppm: 160.88 (triazole-C5), 145.23 (triazole-C3), 140.28 (bridging phenyl-C4), 134.52 (phenyl-C1 at C3 of triazole), 132.18 (phenyl-C3 and C5 at C3 of triazole), 131.26 (pyrrole-C2 and C5), 130.82 (bridging phenyl-C1), 128.92 (phenyl-C2 and C6 at C3 of triazole), 127.44 (bridging phenyl-C2 and C6), 123.75 (phenyl-C4 at C3 of triazole), 120.25 (bridging phenyl-C3 and C5), 110.66 (pyrrole-C3 and C4), 9.12 (CH3); Mass (m/z): 393.28.
3-(2,6-Dichlorophenyl)-5-(4-(2,5-dimethyl-1H-pyrrol- 1-yl) phenyl)-4H-1,2,4-triazole (4d): Yield 56% mp 278-280°; IR (KBr cm−1): 3250 (NH); 1H NMR (500 MHz, DMSO-d6) δ ppm: 1.99 (s, 6H, 2CH3), 5.75 (s, 2H, pyrrole-C3 and C4-H), 7.62-8.06 (m, 7H, aromatic protons), 10.74 (s, 1H, NH, disappeared on D2O exchange); 13C NMR (500 MHz, DMSO-d6) δ ppm: 161.23 (triazole-C5), 142.12 (triazole-C3), 140.56 (bridging phenyl-C4), 136.67 (phenyl-C1 at C3 of triazole), 133.77 (phenyl-C2 and C6 at C3 of triazole), 132.15 (bridging phenyl-C1), 132.08 (pyrrole-C2 and C5), 130.95 (phenyl-C4 at C3 of triazole), 127.50 (phenyl-C3 and C5 at C3 of triazole), 127.15 (bridging phenyl-C2 and C6), 120.45 (bridging phenyl-C3 and C5), 110.72 (pyrrole-C3 and C4), 8.42 (CH3); Mass (m/z): 383.27.
3-(4-(2,5-Dimethyl-1H-pyrrol-1-yl) phenyl)-5- (3-nitrophenyl)-4H-1,2,4-triazole (4e): Yield 50% mp 266-268°; IR (KBr cm−1): 3263 (NH), 1572 (NO2); 1H NMR (500 MHz, DMSO-d6) δ ppm: 1.90 (s, 6H, 2CH3), 5.80 (s, 2H, pyrrole-C3 and C4-H), 7.77-8.20 (m, 8H, aromatic protons), 10.66 (s, 1H, NH, disappeared on D2O exchange); 13C NMR (500 MHz, DMSO-d6)δ ppm: 163.33 (triazole-C3), 149.56 (phenyl-C5 at C5 of triazole), 144.76 (triazole-C5), 141.32 (bridging phenyl-C4), 137.43 (phenyl-C1 at C5 of triazole), 133.15 (phenyl-C2 at C5 of triazole), 133.23 (bridging phenyl-C1), 132.88 (pyrrole-C2 and C5), 129.22 (phenyl-C3 at C5 of triazole), 127.36 (bridging phenyl-C2 and C6), 126.84 (phenyl-C6 at C5 of triazole), 122.90 (phenyl-C4 at C5 of triazole), 120.42 (bridging phenyl-C3 and C5), 110.66 (pyrrole-C3 and C4), 8.88 (CH3); Mass (m/z): 359.38.
3-(4-(2,5-Dimethyl-1H-pyrrol-1-yl) phenyl)- 5-(2-nitrophenyl)-4H-1,2,4-triazole (4f): Yield 54% mp 276-278°; IR (KBr cm−1): 3288 (NH), 1588 (NO2); 1H NMR (500 MHz, DMSO-d6) δ ppm: 1.92 (s, 6H, 2CH3), 5.83 (s, 2H, pyrrole-C3 and C4-H), 7.59-8.10 (m, 8H, aromatic protons), 10.72 (s, 1H, NH, disappeared on D2O exchange); 13C NMR (500 MHz, DMSO-d6) δ ppm: 162.13 (triazole-C3), 147.23 (phenyl-C6 at C5 of triazole), 145.12 (triazole-C5), 140.12 (bridging phenyl-C4), 135.34 (phenyl-C3 at C5 of triazole), 133.82 (bridging phenyl-C1), 132.66 (phenyl-C1 at C5 of triazole), 131.45 (pyrrole-C2 and C5), 129.88 (phenyl-C4 at C5 of triazole), 128.10 (phenyl-C2 at C5 of triazole), 127.72 (bridging phenyl-C2 and C6), 125.22 (phenyl-C5 at C5 of triazole), 121.10 (bridging phenyl-C3 and C5), 111.06 (pyrrole-C3 and C4), 8.29 (CH3); Mass (m/z): 359.38.
4-(5-(4-(2,5-Dimethyl-1H-pyrrol-1-yl) phenyl)- 4H-1,2,4-triazol-3-yl) phenol (4g): Yield 66% mp 250-252°; IR (KBr cm−1): 3420 (OH), 3288 (NH); 1H NMR (500 MHz, DMSO-d6) δ ppm: 1.93 (s, 6H, 2CH3), 5.85 (s, 2H, pyrrole-C3 and C4-H), 7.89-8.22 (m, 8H, aromatic protons), 10.46 (s, 1H, NH, disappeared on D2O exchange), 11.56 (s, 1H, OH); 13C NMR (500 MHz, DMSO-d6) δ ppm: 160.22 (triazole-C5), 156.23 (phenyl-C4 at C3 of triazole), 144.92 (triazole-C3), 140.42 (bridging phenyl-C4), 116.32 (phenyl-C3 and C5 at C3 of triazole), 133.26 (bridging phenyl-C1), 129.59 (phenyl-C1 at C3 of triazole), 132.44 (pyrrole-C2 and C5), 128.45 (phenyl-C2 and C6 at C3 of triazole), 127.29 (bridging phenyl-C2 and C6), 120.82 (bridging phenyl-C3 and C5), 110.29 (pyrrole-C3 and C4), 8.32 (CH3); Mass (m/z): 330.38.
4-(5-(4-(2,5-Dimethyl-1H-pyrrol-1-yl) phenyl)-4H- 1,2,4-triazol-3-yl)-N, N-dimethylaniline (4h): Yield 45% mp 283-285°; IR (KBr cm−1): 3258 (NH); 1H NMR (500 MHz, DMSO-d6) δ ppm: 1.95 (s, 6H, 2CH3), 2.96 (s, 6H, 2CH3), 5.80 (s, 2H, pyrrole-C3 and C4-H), 7.80-8.18 (m, 8H, aromatic protons), 10.55 (s, 1H, NH, disappeared on D2O exchange); Mass (m/z): 357.45.
Synthesis of 4-(2,5-dimethyl-1H-pyrrol-1-yl)-N’- formylbenzohydrazide (5)
A solution of 4-(2,5-dimethylpyrrol-1-yl) benzoic acid hydrazide (3) (0.003 moles, 0.687 g) in formic acid (5 ml) was refluxed for 1 h and the reaction mixture was left to stand at room temperature. The deposited pale yellow solid was filtered, washed, and recrystallized from ethanol.
Yield 55% mp 230-232°; IR (KBr cm−1): 3268 (NH), 1718 (CHO), 1678 (amide C=O); 1H NMR (500 MHz, DMSO-d6) δ ppm: 1.98 (s, 6H, 2CH3), 5.86 (s, 2H, pyrrole-C3 and C4-H), 7.92 (d, J=8.5 Hz, 2H, bridging phenyl-C3 and C5-H), 8.12 (d, J=8.5 Hz, 2H, bridging phenyl-C2 and C6-H), 8.34 (s, 1H, CHO), 9.85 (s, 1H, NH, disappeared on D2O exchange), 10.42 (s, 1H, NH, disappeared on D2O exchange); 13CNMR (500 MHz, DMSO-d6) δ ppm: 167.12 (CONH), 161.62 (NHCHO), 142.75 (bridging phenyl-C4), 132.85 (pyrrole-C2 and C5), 130.45 (bridging phenyl-C1), 127.55 (bridging phenyl-C2 and C6), 121.56 (bridging phenyl-C3 and C5), 110.70 (pyrrole-C3 and C4), 7.42 (CH3); Mass (m/z): 257.29.
Synthesis of 2-(4-(2,5-dimethyl-1H-pyrrol-1-yl) phenyl)-1,3,4-oxadiazole (6)
Triethyl orthoformate (10 ml) was added to hydrazide (3) (0.003 moles, 0.687 g) and refluxed for 12 h. The excess of triethyl orthoformate was removed under reduced pressure and the residue was triturated with petroleum ether (40-60°). The resulting solid was filtered, washed with petroleum ether, and recrystallized from DMF.
Yield 58% mp 242-245°; IR (KBr cm−1): 1559 (C=N); 1H NMR (500 MHz, DMSO-d6) δ ppm: 1.94 (s, 6H, 2CH3), 5.80 (s, 2H, pyrrole-C3 and C4-H), 7.77-8.36 (m, 5H, oxadiazole-C5-H, bridging phenyl- C2, C3, C5 and C6-H); 13C NMR (500 MHz, DMSO-d6) δ ppm: 169.24 (oxadiazole-C5), 159.23 (oxadiazole-C2), 141.12 (bridging phenyl-C4), 133.23 (bridging phenyl-C1), 131.56 (pyrrole-C2 and C5), 127.63 (bridging phenyl-C2 and C6),120.72 (bridging phenyl-C3 and C5), 111.20 (pyrrole-C3 and C4), 7.86 (CH3); Mass (m/z): 239.27.
Synthesis of 1-(4-(2,5-dimethyl-1H-pyrrol-1-yl) benzoyl)-5-methyl-1H-pyrazol-3 (2H)-one (7)
A mixture of compound (3) (0.003 moles, 0.687 g) in ethanol (20 ml) and ethyl acetoacetate (0.003 moles, 0.390 g) was refluxed for 4 h. Glacial acetic acid (1 ml) was then added and the reaction mixture was refluxed for 1 h. The solvent was removed under reduced pressure; the residue was poured onto cold water. The separated solid was filtered, washed with water, and dried. The crude solid was recrystallized from aqueous alcohol.
Yield 72% mp 152-154°; IR (KBr cm−1): 3312 (NH), 1678 (amide C=O), 1690 (pyrazole C=O); 1H NMR (500 MHz, DMSO-d6) δ ppm: 1.93 (s, 6H, 2CH3), 2.15 (s, 3H, CH3), 4.50 (s, 1H, pyrazoline-H), 5.75 (s, 2H, pyrrole-C3 and C4-H), 7.80 (d, J=8.5 Hz, 2H, C3 and C5-H), 7.99 (d, J=8.5 Hz, 2H, C2 and C6-H), 10.68 (s, 1H, NH, disappeared on D2O exchange); 13C NMR (500 MHz, DMSO-d6) δ ppm: 169.52 (CONH), 165.20 (pyrazole-C3), 146.28 (pyrazole-C5), 142.33 (bridging phenyl-C4), 132.12 (bridging phenyl-C1), 129.45 (pyrrole-C2 and C5), 128.87 (bridging phenyl-C2 and C6), 127.82 (bridging phenyl-C3 and C5), 109.45 (pyrrole-C3 and C4), 102.64 (pyrazole-C4), 21.32 (pyrazole-CH3), 11.25 (CH3); Mass (m/z): 295.34.
Synthesis of 3-(4-(2,5-dimethyl-1H-pyrrol-1-yl) phenyl)-1H-1,2,4-triazole-5 (4H)-thione (8)
To a solution of (3) (0.003 moles, 0.687 g) in methanol (25 ml) potassium thiocyanate (2 moles, 0.19 g) and Conc. HCl (3 ml) were added and the reaction mixture was refluxed for 4 h. The solvent was removed under reduced pressure; the residue was triturated with water and neutralized to pH 6 using aqueous ammonia. The separated product was filtered, washed with water, dried and recrystallized from ethanol.
A suspension of thiosemicarbazide in aqueous solution of sodium hydroxide (10%, 5 ml) was gently refluxed for 4 h. The reaction mixture was cooled and slowly quenched onto crushed ice with stirring and acidified with diluted HCl. The solid which separated was filtered, washed with cold water, dried and recrystallized from mixture of 1,4-dioxane and ethanol.
Yield 59% mp 258-260°; IR (KBr cm−1): 3256 (NH); 1H NMR (500 MHz, DMSO-d6) δ ppm: 1.97 (s, 6H, 2CH3), 5.81 (s, 2H, pyrrole-C3 and C4-H), 7.95 (d, J=8.5 Hz, 2H, bridging phenyl -C3 and C5-H), 7.85 (d, J=8.5 Hz, 2H, bridging phenyl-C2 and C6-H), 9.80 (s, 1H, NH, disappeared on D2O exchange), 10.42 (s, 1H, NH, disappeared on D2O exchange); 13C NMR (500 MHz, DMSO-d6) δ ppm: 182.24 (triazole-C5), 148.87 (triazole-C3), 140.14 (bridging phenyl-C4), 130.52 (bridging phenyl-C1), 130.22 (pyrrole-C2 and C5), 129.42 (bridging phenyl-C3 and C5), 126.22 (bridging phenyl-C2 and C6), 108.23 (pyrrole-C3 and C4), 12.87 (CH3); Mass (m/z): 270.35.
Synthesis of 4-(2,5-dimethyl-1H-pyrrol-1-yl)-N-(1Hpyrrol- 1-yl) benzamide (9)
To a suspension of 4-(2,5-dimethylpyrrol-1-yl) benzoic acid hydrazide (3) (0.003 moles, 0.687 g) in glacial acetic acid (10 ml) was added 2,5-dimethoxy tetrahydrofuran (0.006 moles, 0.781 g) and the reaction mixture was refluxed for 1 h. The reaction mixture was concentrated to half of the original volume and poured into crushed ice (50 g). The separated solid was filtered, washed with water, dried and recrystallized from ethanol.
Yield 66% mp 260-262°; IR (KBr cm−1): 3252 (NH), 1682 (amide C=O); 1H NMR (500 MHz, DMSO-d6) δ ppm: 1.93 (s, 6H, 2CH3), 5.12 (s, 2H, amide pyrrole-C3 and C4-H), 5.62 (s, 2H, amide pyrrole-C2 and C5-H), 5.84 (s, 2H, pyrrole-C3 and C4-H), 8.25 (d, J=8.5 Hz, 2H, bridging phenyl -C3 and C5-H), 7.95 (d, J=8.5 Hz, 2H, bridging phenyl-C2 and C6-H), 10.73 (s, 1H, NH, disappeared on D2O exchange); 13C NMR (500 MHz, DMSO-d6) δ ppm: 170.63 (CONH), 141.26 (bridging phenyl-C4), 131.32 (bridging phenyl-C1), 131.29 (pyrrole-C2 and C5), 128.34 (bridging phenyl-C2 and C6), 121.87 (bridging phenyl-C3 and C5), 119.63 (pyrrole-C2 and C5 at amide), 110.96 (pyrrole-C3 and C4), 108.78 (pyrrole-C3 and C4 at amide), 8.39 (CH3); Mass (m/z): 279.34.
Synthesis of 5-(4-(2,5-dimethyl-1H-pyrrol-1-yl) phenyl)-1,3,4-thiadiazole-2-thiol (10)
To a solution of potassium hydroxide (0.0067 moles, 0.37 g) in absolute ethanol (30 ml) acid hydrazide (3) (0.003 moles, 0.687 g), carbon disulphide (0.006 moles, 0.45 ml) were added and the reaction mixture was agitated for 14 h. The solvent was removed under reduced pressure; the residue was triturated with water and neutralized using diluted HCl. The separated product was filtered, washed with water, dried and recrystallized from ethanol.
Yield 71% mp 252-254°; IR (KBr cm−1): 2759 (SH); 1H NMR (500 MHz, DMSO-d6) δ ppm: 1.89 (s, 6H, 2CH3), 3.43 (SH merged in HOD peak of DMSO-d6), 5.78 (s, 2H, pyrrole-C3 and C4-H), 7.90 (d, J=8.5 Hz, 2H, bridging phenyl-C3 and C5-H), 7.88 (d, J=8.5 Hz, 2H, bridging phenyl-C2 and C6-H); 13C NMR (300 MHz, DMSO-d6) δ ppm: 172.36 (thiadiazole-C2), 156.24 (thiadiazole-C5), 141.23 (bridging phenyl-C4), 133.92 (bridging phenyl-C1), 131.87 (pyrrole-C2 and C5), 128.56 (bridging phenyl-C2 and C6), 120.36 (bridging phenyl-C3 and C5), 110.98 (pyrrole-C3 and C4), 8.71 (CH3); Mass (m/z): 287.40.
Synthesis of (3,5-dimethyl-1H-pyrazol-1-yl) (4-(2,5-dimethyl-1H-pyrrol-1-yl) phenyl) methanone (11)
A mixture of hydrazide (3) (0.003 moles, 0.687 g) and acetyl acetone (0.003 moles, 0.300 g) in ethanol (20 ml) was refluxed for 4 h. To the resulting solution glacial acetic acid (1 ml) was added and refluxed for 1 h. The solvent was evaporated and the product was poured onto cold water, filtered and dried. The crude solid was recrystallized from aqueous alcohol.
Yield 66% mp 290-292°; IR (KBr cm−1): 1678 (C=O); 1H NMR (500 MHz, DMSO-d6) δ ppm: 1.92 (s, 6H, 2CH3), 2.30 (s, 3H, CH3), 2.46 (s, 3H, CH3), 5.82 (s, 2H, pyrrole-C3 and C4-H), 6.10 (s, 1H, pyrazole-CH), 7.98 (d, J=8.5 Hz, 2H, bridging phenyl -C3 and C5-H), 7.86 (d, J=8.5 Hz, 2H, bridging phenyl-C2 and C6-H); 13C NMR (500 MHz, DMSO-d6) δ ppm: 188.23 (CONH), 147.53 (pyrazole-C3 and C5), 145.75 (bridging phenyl-C4), 133.96 (bridging phenyl-C1), 131.75 (pyrrole-C2 and C5), 129.56 (bridging phenyl-C2 and C6), 121.43 (bridging phenyl-C3 and C5), 110.52 (pyrrole-C3 and C4), 106.47 (Pyrazole-C4), 14.82 (pyrazole-C5-CH3), 8.50 (pyrazole-C3-CH3), 7.82 (pyrrole-CH3); Mass (m/z): 293.36.
Synthesis of 4-pyrrol-1-yl benzoic acid hydrazide (13)
Ethyl 4-pyrrol-1-yl benzoate (12) (0.015 moles, 3.22 g) was refluxed with hydrazine hydrate (10 ml) in absolute ethanol (10 ml) for 3 h. The reaction mixture was cooled and the crystalline mass obtained was recrystallized from ethanol (yield 74%) mp 180- 182° [5].
Synthesis of 4-pyrrol-1-yl benzoic acid (arylidene) hydrazides (14a-h)
Equimolar quantity of 4-pyrrol-1-yl-benzoic acid hydrazide (13) and different aromatic aldehydes was refluxed in alcohol for 4-6 h in the presence of few drops of glacial acetic acid. The solvent was evaporated and the product poured onto cold water, filtered and dried. The crude solid was recrystallized from aqueous DMF to give the products [5].
Synthesis of N-(2-substituted-3-chloro-4-oxoazetidin- 1-yl)-4-(1H-pyrrol-1-yl) benzamides (15a-h)
To a solution of compound 14 (0.01 moles) and triethylamine (5-6 drops) in dry benzene (50 ml) was added in monochloroacetylchloride (0.015 moles) at 50°. The reaction mixture was stirred for 40 min at room temperature and refluxed for 7-8 h. The reaction mixture was filtered to remove triethylamine hydrogen chloride and the resultant solution was poured onto crushed ice with constant stirring. The solid thus obtained was recrystallized from methanol to give the products.
N-(3-Chloro-2-oxo-4-phenylazetidin-1-yl)-4-(1Hpyrrol- 1-yl) benzamide (15a): Yield 84% mp 242- 244°; IR (KBr cm−1): 3225 (NH), 1745 (C=O of β lactam), 1680 (amide C=O); 1H NMR (300 MHz, DMSO-d6) δ ppm: 4.60 (d, 1H, CH-Cl), 5.96 (s, 1H, N-CH-Ar), 6.32 (s, 2H, pyrrole-C3 and C4-H), 7.40 (s, 2H, pyrrole-C2 and C5-H), 7.80-8.48 (m, 9H, Ar-H), 9.42 (s, 1H, CONH, disappeared on D2O exchange); 13C NMR (500 MHz, DMSO-d6) δ ppm: 167.64 (CONH), 163.29 (azetidinone-C2), 143.56 (bridging phenyl-C4), 142.67 (phenyl-C1 at C4 of azetidinone), 131.87 (bridging phenyl-C1), 128.38 (bridging phenyl-C2 and C6), 128.24 (phenyl-C3 and C5 at C4 of azetidinone), 127.16 (phenyl-C2 and C6 at C4 of azetidinone), 126.85 (phenyl-C4 at C4 of azetidinone), 119.65 (pyrrole-C2 and C5), 121.93 (bridging phenyl-C3 and C5), 110.15 (pyrrole-C3 and C4), 65.32 (azetidinone-C3), 63.58 (azetidinone-C4); Mass (m/z): 365.81.
N-(3-Chloro-2-(2-chlorophenyl)-4-oxoazetidin-1-yl)- 4-(1H-pyrrol-1-yl) benzamide (15b): Yield 74% mp 220-222°; IR (KBr cm−1): 3233 (NH), 1741 (C=O of β lactam), 1688 (amide C=O); 1H NMR (300 MHz, DMSO-d6) δ ppm: 4.62 (d, 1H, CH-Cl), 5.94 (s, 1H, N-CH-Ar), 6.30 (s, 2H, pyrrole-C3 and C4-H), 7.42 (s, 2H, pyrrole-C2 and C5-H), 7.86-8.52 (m, 8H, Ar-H), 9.39 (s, 1H, CONH, disappeared on D2O exchange); 13C NMR (500 MHz, DMSO-d6) δ ppm: 167.26 (CONH), 164.54 (azetidinone-C4), 143.82 (bridging phenyl-C4), 142.86 (phenyl-C1 at C2 of azetidinone), 132.45 (phenyl-C2 at C2 of azetidinone), 131.22 (bridging phenyl-C1), 128.69 (phenyl-C6 at C2 of azetidinone), 127.98 (bridging phenyl-C2 and C6), 128.26 (phenyl-C3 at C2 of azetidinone), 127.98 (phenyl-C4 at C2 of azetidinone), 126.59 (phenyl-C5 at C2 of azetidinone), 119.69 (pyrrole-C2 and C5), 120.99 (bridging phenyl-C3 and C5), 110.39 (pyrrole-C3 and C4), 64.56 (azetidinone-C3), 54.48 (azetidinone-C2); Mass (m/z): 400.26.
N-(2-(4-Bromophenyl)-3-chloro-4-oxoazetidin-1-yl)- 4-(1H-pyrrol-1-yl) benzamide (15c): Yield 69% mp 232-234°; IR (KBr cm−1): 3242 (NH), 1745 (C=O of β lactam), 1690 (amide C=O); 1H NMR (300 MHz, DMSO-d6) δ ppm: 4.59 (d, 1H, CH-Cl), 5.88 (s, 1H, N-CH-Ar), 6.28 (s, 2H, pyrrole-C3 and C4-H), 7.39 (s, 2H, pyrrole-C2 and C5-H), 7.78-8.42 (m, 8H, Ar-H), 9.32 (s, 1H, CONH, disappeared on D2O exchange); 13C NMR (500 MHz, DMSO-d6) δ ppm: 167.88 (CONH), 163.65 (azetidinone-C4), 143.78 (bridging phenyl-C4), 142.12 (phenyl-C1 at C2 of azetidinone), 131.58 (phenyl-C3 and C5 at C2 of azetidinone), 130.53 (bridging phenyl-C1), 129.75 (phenyl-C2 and C6 at C2 of azetidinone), 127.52 (bridging phenyl-C2 and C6), 122.26 (phenyl-C4 at C2 of azetidinone), 120.53 (bridging phenyl-C3 and C5), 119.53 (pyrrole-C2 and C5), 110.63 (pyrrole-C3 and C4), 64.49 (azetidinone-C3), 62.39 (azetidinone-C2); Mass (m/z): 444.71.
N-(3-Chloro-2-(2,6-dichlorophenyl)-4-oxoazetidin- 1-yl)-4-(1H-pyrrol-1-yl) benzamide (15d): Yield 59% mp 215-217°; IR (KBr cm−1): 3250 (NH), 1740 (C=O of β lactam), 1692 (amide C=O; 1H NMR (300 MHz, DMSO-d6) δ ppm: 4.63 (d, 1H, CH-Cl), 5.96 (s, 1H, N-CH-Ar), 6.34 (s, 2H, pyrrole-C3 and C4-H), 7.40 (s, 2H, pyrrole-C2 and C5-H), 7.82-8.52 (m, 7H, Ar-H), 9.33 (s, 1H, CONH, disappeared on D2O exchange); 13C NMR (500 MHz, DMSO-d6) δ ppm: 167.33 (CONH), 163.23 (azetidinone-C4), 142.26 (bridging phenyl-C4), 142.05 (phenyl-C1 at C2 of azetidinone), 133.26 (phenyl-C2 and C6 at C2 of azetidinone), 131.85 (bridging phenyl-C1), 129.34 (phenyl-C4 at C2 of azetidinone), 127.33 (bridging phenyl-C2 and C6), 126.23 (phenyl-C3 and C5 at C2 of azetidinone), 120.03 (bridging phenyl-C3 and C5), 119.23 (pyrrole-C2 and C5),110.13 (pyrrole-C3 and C4), 63.36 (azetidinone-C3), 45.29 (azetidinone-C2); Mass (m/z): 434.70.
N-(3-Chloro-2-(3-nitrophenyl)-4-oxoazetidin-1-yl)- 4-(1H-pyrrol-1-yl) benzamide (15e): Yield 45% mp 255-257°; IR (KBr cm−1): 3262 (NH), 1748 (C=O of β lactam), 1698 (amide C=O), 1577 (NO2); 1H NMR (300 MHz, DMSO-d6) δ ppm: 4.62 (d, 1H, CHCl), 5.92 (s, 1H, N-CH-Ar), 6.32 (s, 2H, pyrrole-C3 and C4-H), 7.43 (s, 2H, pyrrole-C2 and C5-H), 7.86- 8.68 (m, 8H, Ar-H), 9.40 (s, 1H, CONH, disappeared on D2O exchange); 13C NMR (500 MHz, DMSO-d6) δ ppm: 167.38 (CONH), 163.56 (azetidinone-C4), 149.52 (phenyl-C3 at C2 of azetidinone), 143.36 (bridging phenyl-C4), 142.88 (phenyl-C1 at C2 of azetidinone), 132.65 (phenyl-C6 at C2 of azetidinone), 130.56 (bridging phenyl-C1), 129.73 (phenyl-C5 at C2 of azetidinone), 127.99 (bridging phenyl-C2 and C6), 122.69 (phenyl-C2 at C2 of azetidinone), 121.56 (phenyl-C4 at C2 of azetidinone), 120.88 (bridging phenyl-C3 and C5), 119.44 (pyrrole-C2 and C5), 110.22 (pyrrole-C3 and C4), 64.16 (azetidinone-C3), 61.29 (azetidinone-C2); Mass (m/z): 410.81.
N-(3-Chloro-2-(2-nitrophenyl)-4-oxoazetidin-1-yl)- 4-(1H-pyrrol-1-yl) benzamide (15f): Yield 52% mp 248-250°; IR (KBr cm−1): 3250 (NH), 1752 (C=O of β lactam), 1692 (amide C=O), 1573 (NO2); 1H NMR (300 MHz, DMSO-d6) δ ppm: 4.66 (d, 1H, CHCl), 5.93 (s, 1H, N-CH-Ar), 6.34 (s, 2H, pyrrole-C3 and C4-H), 7.47 (s, 2H, pyrrole-C2 and C5-H), 7.82- 8.78 (m, 8H, Ar-H), 9.58 (s, 1H, CONH, disappeared on D2O exchange); 13C NMR (500 MHz, DMSO-d6) δ ppm: 166.29 (CONH), 163.22 (azetidinone-C4), 147.59 (phenyl-C2 at C2 of azetidinone), 143.80 (bridging phenyl-C4), 137.56 (phenyl-C1 at C2 of azetidinone), 134.44 (phenyl-C5 at C2 of azetidinone), 130.33 (bridging phenyl-C1), 128.05 (phenyl-C6 at C2 of azetidinone), 127.82 (bridging phenyl-C2 and C6), 127.23 (phenyl-C4 at C2 of azetidinone), 123.59 (phenyl-C3 at C2 of azetidinone), 120.36 (bridging phenyl-C3 and C5), 119.32 (pyrrole-C2 and C5), 110.18 (pyrrole-C3 and C4), 64.45 (azetidinone-C3), 50.26 (azetidinone-C2); Mass (m/z): 410.81.
N-(3-Chloro-2-(4-hydroxyphenyl)-4-oxoazetidin- 1-yl)-4-(1H-pyrrol-1-yl) benzamide (15g): Yield 60% mp 210-212°; IR (KBr cm−1): 3420 (OH), 3266 (NH), 1759 (C=O of β lactam), 1698 (amide C=O); 1H NMR (300 MHz, DMSO-d6) δ ppm: 4.60 (d, 1H, CH-Cl), 5.91 (s, 1H, N-CH-Ar), 6.39 (s, 2H, pyrrole-C3 and C4-H), 7.45 (s, 2H, pyrrole-C2 and C5-H), 7.80-8.74 (m, 8H, Ar-H), 9.69 (s, 1H, CONH, disappeared on D2O exchange), 11.10 (s, 1H, OH); 13C NMR (500 MHz, DMSO-d6) δ ppm: 164.22 (CONH), 163.45 (azetidinone-C4), 155.63 (phenyl-C4 at C2 of azetidinone), 143.86 (bridging phenyl-C4), 136.46 (phenyl-C1 at C2 of azetidinone), 129.36 (bridging phenyl-C1), 128.26 (phenyl-C2 and C6 at C2 of azetidinone), 128.12 (bridging phenyl-C2 and C6), 127.26 (bridging phenyl-C3 and C5), 120.15 (pyrrole-C2 and C5), 116.85 (phenyl-C3 and C5 at C2 of azetidinone), 110.87 (pyrrole-C3 and C4), 67.25 (azetidinone-C2), 64.28 (azetidinone-C3); Mass (m/z): 381.09.
N-(-3-Chloro-2-(4-(dimethylamino) phenyl-4- oxoazetidin-1-yl)-4-(1H-pyrrol-1-yl) benzamide (15h): Yield 45% mp 222-224°; IR (KBr cm−1): 3278 (NH), 1742 (C=O of β lactam), 1692 (amide C=O); 1H NMR (300 MHz, DMSO-d6) δ ppm: 2.90 (s, 6H, 2CH3), 4.54 (d, 1H, CH-Cl), 5.78 (s, 1H, N-CH-Ar), 6.31 (s, 2H, pyrrole-C3 and C4-H), 7.49 (s, 2H, pyrrole-C2 and C5-H), 7.88-8.88 (m, 8H, Ar-H), 9.40 (s, 1H, CONH, disappeared on D2O exchange); Mass (m/z): 408.88.
Synthesis of N-(4-oxo-2-substitutedthiazolidin-3-yl)- 4-(1H-pyrrol-1-yl) benzamides (16a-h)
A cool mixture of compound 14 (0.01 moles) and anhydrous zinc chloride (one pinch) in dry benzene (50 ml), thioglycolic acid (0.02 moles) was added drop wise with stirring at ambient temperature and the reaction mixture was kept for 3 days at room temperature and then refluxed for 14 h. The reaction mixture was filtered. The filtrate was concentrated and poured on crushed ice. The resultant solid was recrystallized from ethanol to give the products.
N-(4-Oxo-2-phenylthiazolidin-3-yl)-4-(1H-pyrrol- 1-yl) benzamide (16a): Yield 72% mp 278-280°; IR (KBr cm−1): 3250 (NH), 1730 (C=O of β thialactam), 1690 (C=O); 1H NMR (500 MHz, DMSO-d6) δ ppm: 2.86 (s, 2H, CH2-S), 5.92 (s, 1H, N-CHAr), 6.43 (dd, 2H, pyrrole-C3 and C4-H), 7.52 (dd, 2H, pyrrole-C2 and C5-H), 7.81-8.46 (m, 9H, 4 proton bridging phenyl-C2, C3, C5, and C6-H, 5 proton Ar-H), 9.94 (s, 1H, CONH, disappeared on D2O exchange); 13C NMR (500 MHz, DMSO-d6) δ ppm: 168.26 (thiazolidine-C4), 166.56 (CONH),143.46 (bridging phenyl-C4), 139.29 (phenyl-C1 at C2 of thiazolidine), 130.22 (bridging phenyl-C1), 128.92 (phenyl-C2 and C6 at C2 of thiazolidine), 128.22 (phenyl-C3 and C5 at C2 of thiazolidine), 128.03 (bridging phenyl-C2 and C6), 126.58 (phenyl-C4 at C2 of thiazolidine), 121.25 (bridging phenyl-C3 and C5), 119.26 (pyrrole-C2 and C5), 110.29 (pyrrole-C3 and C4), 57.56 (thiazolidine-C2), 39.29 (thiazolidine-C5); Mass (m/z): 363.43.
N-(2-(2-Chlorophenyl)-4-oxothiazolidin-3-yl)-4- (1H-pyrrol-1-yl) benzamide (16b): Yield 55% mp 286-288°; IR (KBr cm−1): 3242 (NH), 1725 (C=O of β thialactam), 1680 (C=O); 1H NMR (500 MHz, DMSO-d6) δ ppm: 2.84 (s, 2H, CH2-S), 5.90 (s, 1H, N-CHAr), 6.46 (dd, 2H, pyrrole-C3 and C4-H), 7.48 (dd, 2H, pyrrole-C2 and C5-H), 7.70-8.39 (m, 8H, 4 proton bridging phenyl-C2, C3, C5, and C6-H, 4 proton Ar-H), 9.90 (s, 1H, CONH, disappeared on D2O exchange); 13C NMR (500 MHz, DMSO-d6) δ ppm: 168.99 (thiazolidine-C4), 167.76 (CONH), 143.49 (bridging phenyl-C4), 138.56 (phenyl-C1 at C2 of thiazolidine), 134.23 (phenyl-C2 at C2 of thiazolidine), 130.56 (bridging phenyl-C1), 130.21 (phenyl-C6 at C2 of thiazolidine), 128.36 (phenyl-C3 at C2 of thiazolidine), 128.12 (phenyl-C4 at C2 of thiazolidine), 127.88 (bridging phenyl-C2 and C6), 126.15 (phenyl-C5 at C2 of thiazolidine), 121.69 (bridging phenyl-C3 and C5), 119.55 (pyrrole-C2 and C5), 110.36 (pyrrole-C3 and C4), 49.59 (thiazolidine-C2), 38.69 (thiazolidine-C5); Mass (m/z): 397.88.
N-(2-(4-Bromophenyl)-4-oxothiazolidin-3-yl)-4- (1H-pyrrol-1-yl) benzamide (16c): Yield 62% mp 286-288°; IR (KBr cm−1): 3248 (NH), 1728 (C=O of β thialactam), 1690 (C=O); 1H NMR (500 MHz, DMSO-d6) δ ppm: 2.82 (s, 2H, CH2-S), 5.96 (s, 1H, N-CHAr), 6.40 (dd, 2H, pyrrole-C3 and C4-H), 7.52 (dd, 2H, pyrrole-C2 and C5-H), 7.76-8.49 (m, 8H, 4 proton bridging phenyl-C2, C3, C5, and C6-H, 4 proton Ar-H), 9.98 (s, 1H, CONH, disappeared on D2O exchange); 13C NMR (500 MHz, DMSO-d6) δ ppm: 168.63 (thiazolidine-C4), 167.33 (CONH), 143.87 (bridging phenyl-C4), 137.29 (phenyl-C1 at C2 of thiazolidine), 131.56 (phenyl-C2 and C6 at C2 of thiazolidine), 131.20 (phenyl-C3 and C5 at C2 of thiazolidine), 130.86 (bridging phenyl-C1), 127.98 (bridging phenyl-C2 and C6), 121.26 (bridging phenyl-C3 and C5), 121.23 (phenyl-C4 at C2 of thiazolidine), 119.09 (pyrrole-C2 and C5),110.19 (pyrrole-C3 and C4), 57.59 (thiazolidine-C2), 38.40 (thiazolidine-C5); Mass (m/z): 442.33.
N-(2-(2,6-Dichlorophenyl)-4-oxothiazolidin-3-yl)-4- (1H-pyrrol-1-yl) benzamide (16d): Yield 48% mp 259-261°C; IR (KBr cm−1): 3234 (NH), 1722 (C=O of β thialactam), 1684 (C=O); 1H NMR (500 MHz, DMSO-d6) δ ppm: 2.89 (s, 2H, CH2-S), 5.92 (s, 1H, N-CHAr), 6.48 (dd, 2H, pyrrole-C3 and C4-H), 7.60 (dd, 2H, pyrrole-C2 and C5-H), 7.80-8.56 (m, 7H, 4 proton bridging phenyl- C2, C3, C5, and C6-H, 3 proton Ar-H), 9.88 (s, 1H, CONH, disappeared on D2O exchange); 13C NMR (500 MHz, DMSO-d6) δ ppm: 168.88 (thiazolidine-C4), 165.56 (CONH), 143.81 (bridging phenyl-C4), 138.56 (phenyl-C1 at C2 of thiazolidine), 135.44 (phenyl-C2 and C6 at C2 of thiazolidine), 129.56 (bridging phenyl-C3 and C5), 129.49 (bridging phenyl-C1), 128.18 (bridging phenyl-C2 and C6), 127.18 (phenyl-C3 and C5 at C2 of thiazolidine), 127.03 (phenyl-C4 at C2 of thiazolidine), 120.19 (pyrrole-C2 and C5), 110.89 (pyrrole-C3 and C4), 55.29 (thiazolidine-C2), 35.46 (thiazolidine-C5); Mass (m/z): 432.32.
N-(2-(3-Nitrophenyl)-4-oxothiazolidin-3-yl)-4-(1Hpyrrol- 1-yl) benzamide (16e): Yield 52% mp 252- 254°; IR (KBr cm−1): 3239 (NH), 1729 (C=O of β thialactam), 1680 (C=O), 1570 (NO2); 1H NMR (500 MHz, DMSO-d6) δ ppm: 2.84 (s, 2H, CH2-S), 5.86 (s, 1H, N-CHAr), 6.42 (dd, 2H, pyrrole-C3 and C4-H), 7.68 (dd, 2H, pyrrole-C2 and C5-H), 7.86-8.42 (m, 8H, 4 proton bridging phenyl- C2, C3, C5 and C6-H, 4 proton Ar-H), 9.80 (s, 1H, CONH, disappeared on D2O exchange); 13C NMR (500 MHz, DMSO-d6) δ ppm: 168.78 (thiazolidine-C4), 163.12 (CONH), 145.86 (phenyl-C3 at C2 of thiazolidine), 143.84 (bridging phenyl-C4), 140.45 (phenyl-C1 at C2 of thiazolidine), 133.48 (phenyl-C6 at C2 of thiazolidine), 129.85 (bridging phenyl-C3 and C5), 129.68 (phenyl-C5 at C2 of thiazolidine), 129.40 (bridging phenyl-C1), 128.36 (bridging phenyl-C2 and C6), 126.78 (phenyl-C2 at C2 of thiazolidine), 123.45 (phenyl-C4 at C2 of thiazolidine), 120.59 (pyrrole-C2 and C5), 110.80 (pyrrole-C3 and C4), 63.54 (thiazolidine-C2), 35.66 (thiazolidine-C5); Mass (m/z): 408.43.
N-(2-(2-Nitrophenyl)-4-oxothiazolidin-3-yl)-4- (1H-pyrrol-1-yl) benzamide (16f): Yield 56% mp 254-256°; IR (KBr cm−1): 3245 (NH), 1733 (C=O of β thialactam), 1688 (C=O), 1576 (NO2); 1H NMR (500 MHz, DMSO-d6) δ ppm: 2.82 (s, 2H, CH2-S), 5.96 (s, 1H, N-CHAr), 6.44 (dd, 2H, pyrrole-C3 and C4-H), 7.72 (dd, 2H, pyrrole-C2 and C5-H), 7.81-8.36 (m, 8H, 4 proton bridging phenyl-C2, C3, C5 and C6-H, 4 proton Ar-H), 9.78 (s, 1H, CONH, disappeared on D2O exchange); Mass (m/z): 408.43.
N-(2-(4-Hydroxyphenyl)-4-oxothiazolidin-3-yl)-4- (1H-pyrrol-1-yl) benzamide (16 g): Yield 52% mp 265-267°; IR (KBr cm−1): 3452 (OH), 3220 (NH), 1738 (C=O of β thialactam), 1690 (C=O); 1H NMR (500 MHz, DMSO-d6) δ ppm: 2.88 (s, 2H, CH2-S), 5.92 (s, 1H, N-CHAr), 6.48 (dd, 2H, pyrrole-C3 and C4-H), 7.78 (dd, 2H, pyrrole-C2 and C5-H), 7.88-8.38 (m, 8H, 4 proton bridging phenyl-C2, C3, C5 and C6-H, 4 proton Ar-H), 9.92 (s, 1H, CONH, disappeared on D2O exchange), 11.65 (s, 1H, OH); Mass (m/z): 379.43.
N-(2-(4-(Dimethylamino) phenyl)-4-oxothiazolidi n-3-yl)-4-(1H-pyrrol-1-yl) benzamide (16h): Yield 58% mp 252-254°; IR (KBr cm−1): 3232 (NH), 1730 (C=O of β thialactam), 1684 (C=O); 1H NMR (500 MHz, DMSO-d6) δ ppm: 2.78 (s, 2H, CH2-S), 2.98 (s, 6H, 2CH3), 5.88 (s, 1H, N-CHAr), 6.44 (dd, 2H, pyrrole-C3 and C4-H), 7.76 (dd, 2H, pyrrole-C2 and C5-H), 7.84-8.45 (m, 8H, 4 proton bridging phenyl-C2, C3, C5 and C6-H, 4 proton Ar-H), 9.90 (s, 1H, CONH, disappeared on D2O exchange); Mass (m/z): 406.50.
in vitro evaluation of antimicrobial activity
The MIC determination of the tested compounds (4a-h, 5, 6, 7, 8, 9, 10, 11, 15a-h, 16a-h) was carried out simultaneously in comparison with ciprofloxacin, norfloxacin against Gram-positive (Staphylococcus aureus, Streptococcus faecalis, Bacillus subtilis) and Gram-negative bacteria (Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa) by broth microdilution method [22]. The antifungal activity was assayed against yeasts (Candida tropicalis, Saccharomyces cerevisiae) and moulds (Aspergillus niger). Serial dilutions of the test compounds and reference drugs were prepared in Mueller-Hinton broth for bacteria and Sabouraud Liquid Medium for fungi. Drugs (10 mg) were dissolved in dimethylsulfoxide (DMSO, 1 ml). Further progressive dilutions were done to obtain final concentrations of 1, 2, 4, 8, 16, 31.25, 62.5, 125, 250, and 500 μg/ml. The tubes were inoculated with 105 cfu ml−1 (colony forming unit/ml) and incubated at 37° for 18 h. The MIC was the lowest concentration of the tested compound that yields no visible growth on the plate. To ensure that the solvent had no effect on the bacterial growth, a control was performed with the test medium supplemented with DMSO at the same dilutions as used in the experiments and DMSO had no effect on the microorganisms in the concentrations studied.
in vitro antiMycobacterium tuberculosis assay
The preliminary antitubercular screening for test compounds (4a-h, 5, 6, 7, 8, 9, 10, 11, 15a-h, 16a-h) was carried for Mycobacterium tuberculosis H37Rv, the MIC of each drug was determined by broth dilution assay method [23,24] and is defined as the lowest concentration of drug, which inhibits ≥99% of bacterial population present at the beginning of the assay. A frozen culture in Middlebrook 7H9 broth supplemented with 10% albumin-dextrose-catalase and 0.2% glycerol was thawed and diluted in broth to 105 cfu/ml for M. tuberculosis and used as the inoculum. In the assay U-tubes were used to accommodate compounds in 1-500 μg/ml dilutions. Each test compound was dissolved in DMSO and then diluted in broth at twice the desired concentration. The final concentration of DMSO in the assay medium was 1.3%. Each U-tube was then inoculated with 0.05 ml of standardized culture and then incubated at 37° for 21 days. The growth in the U-tubes was compared with visibility against positive control (without drug), negative control (without drug and inoculum) and with a standard drug isoniazid. The tests were carried out in triplicate.
Cytotoxic activity
The cellular conversion of MTT [3-(4,5-dimethylthiazo- 2-yl)-2,5-diphenyl-tetrazolium bromide] into a formazan product [25] was used to evaluate cytotoxic activity (IC50) of some synthesized compounds (4b-d, 5, 6, 7, 10, 15b-d and 16b-d) against mammalian Vero cell lines and A549 (lung adenocarcinoma) cell lines up to concentrations of 62.5 µg/ml using the Promega Cell Titer 96 non-radioactive cell proliferation assay [26]. The IC50 values are means±SEM of three independent experiments.
Results and Discussion
In the IR spectrum of 3, broad stretching bands around 3285 cm−1 and 3195 cm−1 were due to amine/amide NH while strong stretching band at 1655 cm−1 was assigned to amide carbonyl. The 1H NMR spectrum of 3 showed a singlet at δ 1.95 which was accounted for two methyl groups on pyrrole ring. A singlet at δ 4.51 was assigned to NH2 group which disappeared on D2O exchange. The C3 and C4 protons of pyrrole ring appeared as singlet at δ 5.80. Four protons of phenyl moiety resonated as two doublets at δ 7.94 and δ 7.33. A broad singlet appeared at δ 10.0 was accounted for NH which vanished on D2O exchange. The mass spectrum of 3 displayed a molecular ion peak at m/z 229 which confirmed its molecular weight.
Disappearance of NH2 stretching band in the IR spectrum of 4a confirmed the formation of triazole. A stretching band at 3245 cm−1 was due to triazole NH. The 1H NMR spectrum of 4a showed a singlet at δ 1.96 which was accounted for two methyl groups. The C3 and C4 protons of pyrrole ring appeared as singlet at δ 5.79. The nine aromatic protons resonated as multiplets between δ 7.55 and δ 7.95. A singlet appeared at δ 11.01 was accounted for NH which vanished on D2O exchange. The 13C NMR data of 4a also supported the structure which displayed a peak at δ 10.24 due to two methyl carbons of pyrrole. Peaks at δ 162.40 and δ 148.20 were due to C5 and C3 of carbons of triazole. The FAB mass spectrum of 4a showed a molecular ion peak at m/z 314.38 which confirmed its molecular weight.
The structures of compounds 5-11 were ascertained by the IR, 1H NMR, 13C NMR, and mass spectral data. Electron impact mass spectra showed an accurate molecular ion peak at m/z 257.29, 239.27, 295.34, 270.35, 279.34, 287.40, and 293.36 for compounds 5-11, respectively. IR spectrum of 15a exhibited characteristic bands at 1745 cm−1 (C=O of β-lactam) and amide carbonyl stretching at 1680 cm−1. In the PMR spectra of compound 15a, the peak was observed at δ 4.60 ppm due to the CH-Cl in β-lactam ring; in 13C NMR spectra of compound 15a, peaks at δ 65.32 was observed due to CH-Cl, δ 163.29 (C=O of β-lactam) and δ 63.58 due to C4 carbon in β-lactam moiety. The mass spectra of compound 15a showed the molecular ion peak at 365.81.
The compound 16a showed characteristic absorptions for the cyclic carbonyl group at 1730 cm−1 and amide carbonyl group at 1690 cm−1 in the IR spectrum. The 1H NMR spectrum aroused our attention and clearly indicates the presence of the two active methylene protons in the thiazolidine ring at δ 2.86 ppm. The 13C NMR spectrum of compound 16a also supported the fact that cyclic carbonyl group was present and signal appeared at δ 168.26. All these facts were also supported by the two evidences that are (a) disappearance of N=CH proton and (b) appearance of N-CH proton at δ 5.92 ppm in the 1H NMR spectrum of compound 16a.
The results of antimicrobial activities of the synthesized compounds against selected three Gram-positive, three Gram-negative bacteria, yeasts, moulds, and M. tuberculosis H37Rv are illustrated in Tables 1 and 2, respectively. The antimicrobial and antifungal activity results are expressed in MIC values (minimum inhibitory concentration) along with the activity of ciprofloxacin, norfloxacin, and flucanazole for comparison.
| Compound | MIC values (µg/ml) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram‑positive | Gram‑negative | Fungic | |||||||||
| organismsa | organismsb | ||||||||||
| Sa | Sf | Bs | Kp | Ec | Pa | Sc | Ct | An | |||
| 4a | 16 | 62.5 | 31.25 | 500 | 500 | 500 | 32.5 | 32.5 | 32.5 | ||
| 4b | 2 | 16 | 4 | 125 | 250 | 250 | 8 | 8 | 8 | ||
| 4c | 1 | 8 | 4 | 125 | 125 | 125 | 4 | 4 | 4 | ||
| 4d | 1 | 8 | 2 | 125 | 62.5 | 62.5 | 2 | 2 | 2 | ||
| 4e | 8 | 31.25 | 16 | 125 | 250 | 250 | 31.25 | 31.25 | 31.25 | ||
| 4f | 8 | 31.25 | 16 | 250 | 250 | 250 | 31.25 | 31.25 | 31.25 | ||
| 4g | 8 | 31.25 | 16 | 250 | 250 | 250 | 31.25 | 31.25 | 31.25 | ||
| 4h | 8 | 31.25 | 16 | 250 | 250 | 250 | 62.5 | 62.5 | 62.5 | ||
| 5 | 16 | 31.25 | 31.25 | 31.25 | 31.25 | 125 | 250 | 250 | 250 | ||
| 6 | 2 | 2 | 2 | 4 | 4 | 4 | 125 | 125 | 125 | ||
| 7 | 125 | 125 | 125 | 250 | 250 | 250 | 250 | 250 | 250 | ||
| 8 | 4 | 4 | 4 | 8 | 8 | 8 | >500 | >500 | >500 | ||
| 9 | 4 | 4 | 4 | 2 | 4 | 8 | >500 | >500 | >500 | ||
| 10 | 1 | 1 | 1 | 2 | 2 | 2 | 62.5 | 62.5 | 125 | ||
| 11 | 125 | 125 | 125 | 250 | 250 | 250 | >500 | >500 | >500 | ||
| 15a | 8 | 8 | 16 | 16 | 8 | 8 | >500 | >500 | >500 | ||
| 15b | 1 | 1 | 1 | 1 | 1 | 1 | >500 | >500 | >500 | ||
| 15c | 1 | 1 | 1 | 1 | 1 | 1 | >500 | >500 | >500 | ||
| 15d | 1 | 1 | 1 | 1 | 1 | 1 | >500 | >500 | >500 | ||
| 15e | 4 | 4 | 4 | 4 | 4 | 4 | >500 | >500 | >500 | ||
| 15f | 4 | 8 | 4 | 4 | 4 | 4 | >500 | >500 | >500 | ||
| 15g | 4 | 4 | 4 | 4 | 4 | 4 | >500 | >500 | >500 | ||
| 15h | 2 | 2 | 2 | 2 | 2 | 2 | >500 | >500 | >500 | ||
| 16a | 8 | 8 | 16 | 16 | 16 | 8 | >500 | >500 | >500 | ||
| 16b | 2 | 2 | 2 | 2 | 2 | 2 | >500 | >500 | >500 | ||
| 16c | 2 | 2 | 2 | 2 | 2 | 2 | >500 | >500 | >500 | ||
| 16d | 2 | 2 | 2 | 2 | 2 | 2 | >500 | >500 | >500 | ||
| 16e | 4 | 4 | 4 | 4 | 4 | 4 | >500 | >500 | >500 | ||
| 16f | 4 | 4 | 4 | 4 | 4 | 4 | >500 | >500 | >500 | ||
| 16g | 4 | 4 | 4 | 4 | 4 | 4 | >500 | >500 | >500 | ||
| 16h | 2 | 4 | 2 | 2 | 2 | 2 | >500 | >500 | >500 | ||
| Ciprofloxacin | <5 | <5 | ≤1 | ≤1 | ≤1 | >5 | ‑ | ‑ | ‑ | ||
| Norfloxacin | <5 | <5 | ≤1 | ≤1 | ≤1 | >5 | ‑ | ‑ | ‑ | ||
| Flucanazole | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ≤1 | ≤1 | ≤1 | ||
Minimal inhibition concentration is expressed in μg/ml; aGram-positive bacteria=Staphylococcus aureus ATCC 11632 (Sa), Streptococcus faecalis ATCC 14506 (Sf), Bacillus subtilis ATCC 60511 (Bs), bGram-negative bacteria=Klebsiella pneumoniae ATCC 10031 (Kp), Escherichia coli ATCC 10536 (Ec) Pseudomonas aeruginosa ATCC 10145 (Pa), cYeasts=Saccharomyces cerevisiae (ATCC 9763, Sc) and Candida tropicalis (ATCC 1369, Ct), mould=Aspergillus niger (ATCC 6275, An)
Table 1: In Vitro Antimicrobial Activity
| Compound | MIC values | IC50 (µM) | |
|---|---|---|---|
| M. tuberculosis H37Rv | |||
| MV cell linesa | A549b | ||
| 4a | 31.25 | NT | NT |
| 4b | 8 | 222.1+1.2 | 228.1+0.2 |
| 4c | 2 | 230.7+0.8 | 225.2+1.5 |
| 4d | 8 | 228.6+0.2 | 230.8+0.9 |
| 4e | 16 | NT | NT |
| 4f | 16 | NT | NT |
| 4g | 16 | NT | NT |
| 4h | 16 | NT | NT |
| 5 | 4 | 210.6+0.2 | 218.8+0.2 |
| 6 | 4 | 215.7+0.6 | 226.2+1.2 |
| 7 | 4 | 201.6+1.8 | 208+1.6 |
| 8 | 2 | NT | NT |
| 9 | 2 | NT | NT |
| 10 | 1 | 220.7+1.8 | 223+0.9 |
| 11 | 8 | NT | NT |
| 15a | 4 | NT | NT |
| 15b | 2 | 258.1+0.9 | 251.2+1.7 |
| 15c | 1 | 268.4+0.8 | 271.3+1.8 |
| 15d | 1 | 257.7+1.4 | 259.4+0.5 |
| 15e | 2 | NT | NT |
| 15f | 2 | NT | NT |
| 15g | 2 | NT | NT |
| 15h | 2 | NT | NT |
| 16a | 4 | NT | NT |
| 16b | 2 | 258.4+1.9 | 252+0.6 |
| 16c | 1 | 260.3+0.8 | 265+0.8 |
| 16d | 1 | 252.1+0.4 | 258+1.5 |
| 16e | 4 | NT | NT |
| 16f | 4 | NT | NT |
| 16g | 4 | NT | NT |
| 16h | 4 | NT | NT |
| Isoniazid | 0.25 | >450 | >450 |
Minimal inhibition concentration is expressed in μg/ml; Cytotoxicity is expressed as IC50, is the concentration of compound, which is reduced by 50% of the optical density of treated cells with respect to untreated cells using the MTT assay, aMammalian Vero cell lines, bA549 (lung adenocarcinoma) cell lines, NT=Not tested
Table 2: Cytotoxicity And Antimycobacterial Activity Of Pyrrole Derivatives
The investigation of antimicrobial screening data revealed that all the compounds showed moderate to good microbial inhibition. Compounds 4a-h and 5-11 (figs. 1 and 2) showed antimicrobial activity at MIC values of 1 to 500 µg/ml. Compounds showed enhanced activity against Gram positive bacteria than the Gram negative bacteria. Compounds 4c, 4d, and 10 were found to be more active than t
he others at an MIC value of 1 µg/ml against Staphylococcus aureus. The synthesized compounds showed antimicrobial activity with MIC values between 2 and 125 µg/ml against Bacillus subtilis. Compounds exhibited antimicrobial activity with MIC values between 1 and 125 µg/ml Streptococcus faecalis. All the compounds exhibited moderate activity against Klebsiella pneumoniae. Compounds showed lower activity against Escherichia coli and Pseudomonas aeruginosa. Compounds 15a-h which have azetidinone ring were more active than the thiazolidinone derivatives 16a-h against some of the test microorganisms.
Compounds 15a-h and 16a-h (fig. 3) showed antimicrobial activity at MIC values of 1 to 16 µg/ml. Compounds 15b, 15c, and 15d were found to be more active than the others at an MIC value of 1 µg/ml against all the tested bacteria. The synthesized compounds showed antimicrobial activity with MIC values between 1 and 8 µg/ml against Streptococcus faecalis. Compounds 15b-h and 16b-h which have electron withdrawing substituents on phenyl ring were found to be the most active compounds against the tested bacteria. The majority of compounds exhibited good inhibitory activity against both Gram-positive and Gram-negative bacteria. Compounds showed poor activity (MIC>500 µg/ml) against moulds and yeasts.
The tested compounds (figs. 1, 2, and 3) showed activities against mycobacteria with MIC values ranging from 1 to 31.25 µg/ml (Table 2). Preliminary examination of the antimycobacterial activity revealed that compounds 15a-h and 16a-h (fig. 3) containing the azetidinone and thiazolidionone rings showed better activity against M. tuberculosis H37Rv and compounds 15c, 15d, 16c, and 16d exhibited highest activity (MIC 1 µg/ml). The compounds 4a-h and 5-11 (figs. 1 and 2) showed activities against mycobacteria with MIC values ranging from 1 to 31.25 µg/ml (Table 2). Compounds 4c, 7, and 8 showed anti-tubercular activity at MIC value of 2 μg/ml. Compound 10 showed the highest activity at MIC value of 1 μg/ml when compared with first line drug isoniazid (MIC=0.25 µg/ml).
Some compounds 4b-d, 5-7, 10, 15b-d, and 16b-d were further examined for toxicity (IC50) in mammalian Vero cell lines and A549 (lung adenocarcinoma) cell lines up to 62.5 µg/ml concentrations. After 72 h of exposure, viability was assessed on the basis of cellular conversion of MTT into a formazan product using the Promega Cell Titer 96 non-radioactive cell proliferation assay and results are summarized in Table 2. Among the 13 derivatives tested, showed IC50 values ranging from 201.6 to 271.3 µM against mammalian Vero cell lines. All compounds did not show significant activity against mammalian Vero cell line at concentrations <200 µM. Among the test compounds, azetidinone and thiazolidinone derivatives 15b-d and 16b-d showed inferior toxicity with IC50 values of >250 µM against both mammalian Vero cell lines and A549 (lung adenocarcinoma) cell lines. A comparison of the substitution pattern on pyrrole nucleus demonstrated that 2,5-dimethyl substituted analogs were more cytotoxic than the 2,5-unsubstituted pyrrole analogs. These results are important as these compounds with their increased cytoliability are much striking in the development of new chemical entities for the treatment of TB. This is primarily due to the fact that the disease is chronic by its nature; the therapy needs to be continued for at least about 1-2 years in most of the cases and the need for an agent with a high margin of safety becomes a major concern. The IC50 for the compound 15c was found to be 271.3 µM against the A549 (lung adenocarcinoma) cell lines tested.
In conclusion, this work demonstrates the synthesis of novel pyrrole derivatives and in vitro evaluation of antimicrobial (antibacterial and antifungal) and antitubercular activity against Mycobacterium tuberculosis H37Rv strain by broth dilution assay method. Further, some title compounds were also assessed for their cytotoxic activity (IC50) against mammalian Vero cell lines and A549 (lung adenocarcinoma) cell lines using MTT assay method. The results indicated that these compounds exhibit antitubercular activity at non-cytotoxic concentrations. Due to the better activity against tested microorganisms and mycobacteria, compounds 4c, 4d, 10, 15c, 15d, 16c and 16d have been selected for further development and studies to acquire more information about structure-activity relationships are in progress in our laboratories. The promising in vitro antimicrobial activity and low-toxicity profile of clubbed pyrrole class of compounds make them certain promising molecules for further lead optimization in the development of novel antitubercular agents.
Acknowledgements
The authors thank Shri. H. V. Dambal, President, S. E. T’s College of Pharmacy, Dharwad, India, for providing the facilities. We also thank Dr. T. M. Aminabhavi, Director, Research and Development, S. E. T’s College of Pharmacy, Dharwad, India, for his valuable suggestions. We gratefully acknowledge the support for this research from Indian Council of Medical Research, New Delhi (File No. 64/4/2011-BMS, IRIS Cell No. 2010-08710). We thank Dr. K. G. Bhat, Maratha Mandal’s Dental College, Hospital and Research Centre, Belgaum, Karnataka, India for providing the facilities for antibacterial and antitubercular activity. We also thank the Director, SAIF, Indian Institute of Technology, Chennai, India and the Director, USIC, Karnataka University Dharwad, India for providing NMR and Mass spectral data. We also thank Mr. S. A. Tiwari for his technical assistance.
References
- Padmavathi V, PremaKumari C, Venkatesh BC, Padmaja A. Synthesis and antimicrobial activity of amido linked pyrrolyl and pyrazolyl-oxazoles, thiazoles and imidazoles. Eur J Med Chem 2011;46:5317-26.
- Hilmy KM, Khalifa MM, Hawata MA, Keshk RM, el-Torgman AA. Synthesis of new pyrrolo [2,3-d] pyrimidine derivatives as antibacterial and antifungal agents. Eur J Med Chem 2010;45:5243-50.
- Biava M, Porretta GC, Poce G, De Logu A, Meleddu R, De Rossi E, et al. 1,5-Diaryl-2-ethyl pyrrole derivatives as antimycobacterial agents:Design, synthesis, and microbiological evaluation. Eur J Med Chem 2009;44:4734-8.
- Kamal A, Prabhakar S, JanakiRamaiah M, Venkat Reddy P, Ratna Reddy Ch, Mallareddy A, et al. Synthesis and anticancer activity of chalcone-pyrrolobenzodiazepine conjugates linked via 1,2,3-triazole ring side-armed with alkane spacers. Eur J Med Chem 2011;46:3820-31.
- Joshi SD, Vagdevi HM, Vaidya VP, Gadaginamath GS. Synthesis of new 4-pyrrol-1-yl benzoic acid hydrazide analogs and some derived oxadiazole, triazole and pyrrole ring systems, a novel class of potential antibacterial and antitubercular agents. Eur J Med Chem 2008;43:1989-96.
- Joshi SD, More Y, Vagdevi HM, Vaidya VP, Gadaginamath GS, Kulkarni VH. Synthesis of new 4-(2,5-dimethylpyrrol-1-yl)/4-pyrrol-1-yl benzoic acid hydrazide analogs and some derived oxadiazole, triazole and pyrrole ring systems a novel class of potential antibacterial, antifungal and antitubercular agents. Med Chem Res 2013;22:1073-89.
- Abdulla RF, Fuhr KH. Monocyclic antibiotic beta-lactams. J Med Chem 1975;18:625-7.
- Halve AK, BhadauriaD,Dubey R. N/C-4 substituted azetidin-2-ones: Synthesis and preliminary evaluation as new class of antimicrobial agents. Bioorg Med ChemLett 2007;17:341-5.
- Veinberg S, Vorona K. Synthesis of antitumor 6-alkylidenepenicillanate sulfones and related 3-alkylidene-2-azetidinones. Bioorg Med Chem 2004;12:147-50.
- Singh GS, Mmolotsi BJ. Synthesis of 2-azetidinones from 2-diazo-1, 2-diarylethanones and N-(2-thienylidene) imines as possible antimicrobial agents. Farmaco 2005;60:727-30.
- Dubey A, Srivastava SK, Srivastava SD. Conventional and microwave assisted synthesis of 2-oxo-4-substituted aryl-azetidine derivatives of benzotriazole: A new class of biological compounds. Bioorg Med ChemLett 2001;21:569-73.
- Havrylyuk D, Mosula L, Zimenkovsky B, Vasylenko O, Gzella A, Lesyk R. Synthesis and anticancer activity evaluation of 4-thiazolidinones containing benzothiazole moiety. Eur J Med Chem 2010;45:5012-21.
- Pathak RB, Chovatia PT, Parekh HH. Synthesis, antitubercular and antimicrobial evaluation of 3-(4-chlorophenyl)-4-substituted pyrazole derivatives. Bioorg Med ChemLett 2012;22:5129-33.
- Mohamed MS, Kamel MM, Kassem EM, Abotaleb N, Abd El-Moez SI, Ahmed MF. Novel 6,8-dibromo-4 (3H) quinazolinone derivatives of antibacterial and antifungal activities. Eur J Med Chem 2010;45:3311-9.
- Bonde CG, Gaikwad NJ. Synthesis and preliminary evaluation of some pyrazine containing thiazolines and thiazolidinones as antimicrobial agents. Bioorg Med Chem 2004;12:2151-61.
- Omar K, Geronikaki A, Zoumpoulakis P, Camoutsis C, Sokovic M, Ciric A, et al. Novel 4-thiazolidinone derivatives as potential antifungal and antibacterial drugs. Bioorg Med Chem 2010;8:426-32.
- Wang XL, Wan K, Zhou CH. Synthesis of novel sulfanilamide-derived 1,2,3-triazoles and their evaluation for antibacterial and antifungal activities. Eur J Med Chem 2010;45:4631-9.
- Joshi SD, Vagdevi HM, Vaidya VP, Gadaginamath GS, Purohit SS. Synthesis antibacterial and antitubercular activities of some new 3-substituted phenyl-5 (4-pyrrol-1-ylphenyl)-4H-1,2,4-triazoles. Indian J HeterocyclChem 2008;17:367-8.
- Tripathi RP, Yadav AK, Ajay A, Bisht SS, Chaturvedi V, Sinha SK. Application of Huisgen (3+2) cycloaddition reaction: Synthesis of 1-(2,3-dihydrobenzofuran-2-yl-methyl [1,2,3]-triazoles and their antitubercular evaluations. Eur J Med Chem 2010;45:142-8.
- Duan YC, Ma YC, Zhang E, Shi XJ, Wang MM, Ye XW, et al. Design and synthesis of novel 1,2,3-triazole-dithiocarbamate hybrids as potential anticancer agents. Eur J Med Chem 2013;62:11-9.
- Piotrowska DG, Balzarini J, Głowacka IE. Design, synthesis, antiviral and cytostatic evaluation of novel isoxazolidine nucleotide analogues with a 1,2,3-triazole linker. Eur J Med Chem 2012;47:501-9.
- Goto S, Jo K, Kawakita T, Mitsuhashi S, Nishino T, Ohsawa N, et al. Determination method of minimum inhibitory concentrations.Chemotherapy 1981;29:76-9.
- Suling WJ, Seitz LE, Pathak V, Westbrook L, Barrow EW, Ginkel SZ, et al. Antimycobacterial Activities of 2,4-diamino-5-deazapteridinederivatives and effects on mycobacterial dihydrofolatereductase. Antimicrob Agents Chemother 2000;44:2784-93.
- Yajko DM, Madej JJ, Lacaster MV, Sanders CA, Cawthon VL, Gee B, et al. Colorimetric method for determining MICs of antimicrobial agents for Mycobacterium tuberculosis. J ClinMicrobiol 1995;33:2324-7.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55-63.
- Gundersen LL, Nissen-Meyer J, Spilsberg B. Synthesis and antimycobacterial activity of 6-arylpurines: the requirements for the N-9 substituent in active antimycobacterial purines. J Med Chem 2002;45:1383-6.