- *Corresponding Author:
- S. S. Panda
Department of Pharmaceutical Chemistry, Manipal College of Pharmaceutical Sciences, MAHE Manipal-576 104, India
E-mail: sivashankarpanda@yahoo.com
| Date of Submission | 24 February 2009 |
| Date of Revision | 28 August 2009 |
| Date of Acceptance | 29 November 2009 |
| Indian J Pharm Sci, 2009, 71 (6): 684-687 |
Abstract
Chalcones were synthesized by reacting indole-3-aldehyde, prepared by Vilsemeir Haack reaction with 4-substituted acetophenone in ethanolic KOH solution. These chalcones were immediately reacted with hydroxylamine hydrochloride in presence of glacial acetic acid as reagent to obtain the corresponding isoxazole derivatives. The synthesized heterocycles were characterized on the basis of physical, chemical tests and spectroscopic data. These compounds were tested for the acute antiinflammatory activity and antibacterial activity using carrageenan-induced rat paw edema method and cup-plate method, respectively.
Keywords
Antibacterial activity, antiinflammatory activity, indolyl-isoxazoles
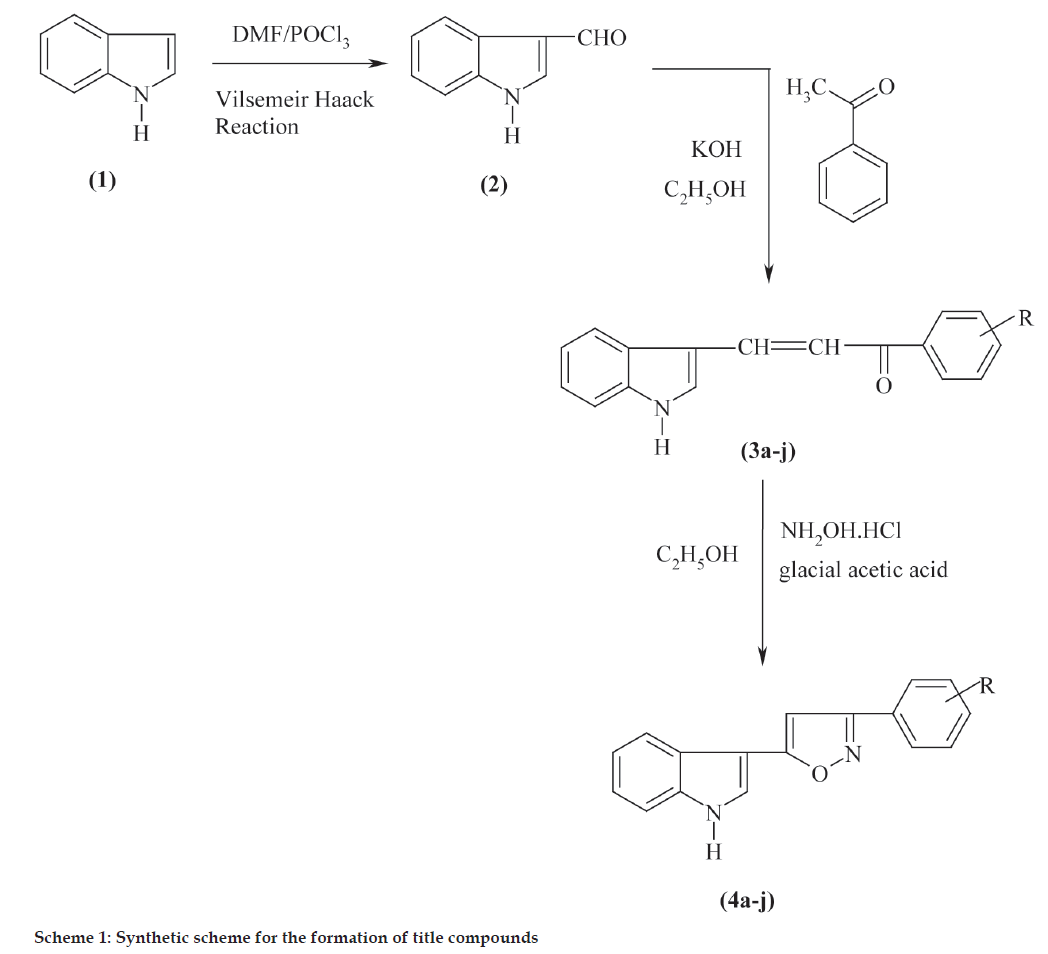
Isoxazoles were reported for their various biological activities [1-3]. The reactive intermediate chalcones involved in their synthesis also exhibit wide range of biological activities [4-6]. The ability of indole to exhibit antiinflammatory, antimicrobial, antifungal activities [7-9] prompted the selection of indole as starting compound. In the light of these interesting biological activities, it appeared of interest to synthesize some new indolyl-isoxazole derivatives and to evaluate their antibacterial and antiinflammatory activities. Indole-3-aldehyde (2) prepared using Vilsemeir Haack reaction by reacting indole (1) with substituted acetophenones (a-j) in ethanolic KOH to obtain chalcones (3a-j), which were condensed with hydroxylamine hydrochloride in presence of sodium acetate and glacial acetic acid to obtain isoxazoles (4a-j). The synthetic sequence leading to the formation of targeted compounds is depicted in Scheme 1.
Melting points of the compounds were determined on a Toshniwal electric melting point apparatus and the values were uncorrected. IR spectra of the synthesized compounds were recorded on a Shimadzu-FTIR 8300 using KBr disc method. 1H NMR spectra were recorded on a Jeol-GSX 400, (IIT Chennai) using DMSO-d6 as solvent. Mass spectra were recorded on a Shimadzu-GCMS 50508. All the solvents used were of analytical grade.
Indole-3-aldehyde (2) and chalcones (3a-j) were prepared following the literature method [10]. The general procedure used for synthesis of 5-(indol-3-yl)- 3-(substituted phenyl) isoxazole (4a-j) is as follows. A mixture of chalcone (0.01 mol) and hydroxylamine hydrochloride (0.01 mol) along with sodium acetate and glacial acetic acid in 50 ml ethanol was stirred and refluxed for 8-10 h. The progress of the reaction was monitored by TLC. The reaction mixture was cooled and poured into ice cold water, filtered and dried to get final product, which recrystallized from aqueous ethanol. The solvent system used for TLC was a 8:2 mixture of chloroform:methanol. The yield and melting point are given in Table 1. 5-(Indol-3-yl)- 3-(4-methoxyphenyl) isoxazole (4h) IR (KBr) cm-1: 3225 (-NH-), 3069 (Ar C-H), 1569 (-C=N-), 1016, 754. 1HNMR (DMSO-d6): δ 3.82 (s, 3H, -OCH3), 6.00(s, 1H, isoxazole ring proton), 7.12 (s, 1H, indolyl proton), 7.41-7.68 (m, 8H, aromatic protons), 11.06 (s, 1H, N-H). Mass m/z: 290 (M+), 291 (M+ + 1).
| Compd. | R | Mol. formula | M.P (°C) | Rf * Value | Yield (%) |
|---|---|---|---|---|---|
| 4a | -H | C17H12N2O | 147 | 0.59 | 77 |
| 4b | -NH2 (p) | C17H13N3O | 151 | 0.53 | 65 |
| 4c | -Br (p) | C17H11N2OBr | 119 | 0.45 | 67 |
| 4d | -Cl (p) | C17H11N2OCl | 128 | 0.47 | 60 |
| 4e | -OH (o,p) | C17H12N2O3 | 175 | 0.38 | 75 |
| 4f | -F (p) | C17H11N2OF | 157 | 0.41 | 71 |
| 4g | -CH3 (p) | C18H14N2O | 138 | 0.45 | 58 |
| 4h | -OCH3 (p) | C18H14N2O2 | 183 | 0.35 | 78 |
| 4i | -OH (p) | C17H12N2O2 | 161 | 0.52 | 72 |
| 4j | -NO2 (p) | C17H11N3O3 | 167 | 0.57 | 91 |
All the synthesized compounds were recrystallized from ethanol and * solvent system in TLC was chloroform:methanol (8:2)
Table 1: Physical Data Of 5-(Indol-3-Yl)-3- (Substituted Phenyl) Isoxazole
Antiinflammatory activity was measured using the carrageenan-induced paw edema test in rats [11]. Animals were divided into different groups each consisting of six animals. Out of twelve synthesized compounds, five compounds (4a, 4c, 4f, 4g, 4j) were selected as test compounds and standard ibuprofen were administered orally at a dose of 200 mg/kg as an aqueous suspension in 1% CMC, while the control group was fed with the same volume of 1% CMC suspension. The paw volume were measured using a plethysmograph immediately before and 3 h after the carrageenan injection. The percent inhibition of paw volume was calculated by using the formula, percent inhibition= (1-Vt/Vc)×100, where Vt is the mean volume of the test drug, Vc is the mean volume of the control Table 2. The one-way ANOVA (Scheffe's method [12]) test was applied, and the test compounds were found to be significantly active compared to the control (P <0.05). The institutional animal ethics committee of Kasthurba Medical College has approved the experimental protocol (No. IACE/ KMC/030/2004-05).
| Group | Dose in µg | Mean edema ± SE | % Reduction in edema volume |
|---|---|---|---|
| Control | -- | 0.406 ± 0.047 | -- |
| Ibuprofen | 200 | 0.170 ± 0.019a | 68.08 |
| 4a | 200 | 0.153 ± 0.021ab | 69.5 |
| 4c | 200 | 0.268 ± 0.022a | 32.9 |
| 4f | 200 | 0.105 ± 0.019ab | 73.7 |
| 4g | 200 | 0.126 ± 0.014ab | 70.7 |
| 4j | 200 | 0.175 ± 0.026a | 36.6 |
5% allowance value is 0.28 (Scheffe’s method), *P<0.05 Vs control. Note: Any two means showing a difference of 0.28 are statistically significant.
Table 2: Antiinflammatory Activity Of Synthesized Compounds
All compounds synthesized (4a-4j) were screened for antibacterial activity using the cup-plate agar diffusion method [13] by measuring the zone of inhibition in mm. Ciprofloxacin (50 mg/ml and 100 mg/ml) was used as standard for antibacterial activity. The compounds were screened for antibacterial activity against S. aureus, B. subtilis, E. coli and P. aeruginosa in Muller-Hinton agar medium. This sterilized agar medium was poured into Petri dishes and allowed to solidify. On the surface of the media microbial suspensions were spread with the help of sterilized triangular loop. A stainless steel cylinder of 8 mm diameter (pre-sterilized) was used to bore the cavities. All the synthesized compounds (50 mg/ml and 100 mg/ml) were placed serially in the cavities with the help of micropipette and allowed to diffuse for 1 h. Dimethylsulfoxide (DMSO) was used as a solvent for all compounds and as control. The plates were incubated at 37° for 14 h. The zone of inhibition observed around the cups after incubation was measured. The results are presented in Table 3.
| Compd. | S. aureus | B. | subtilis | E. coli | P. aeruginisa | |||
|---|---|---|---|---|---|---|---|---|
| 50* | 100* | 50* | 100* | 50* | 100* | 50* | 100* | |
| 4a | 12 | 16 | - | - | 14 | 16 | 11 | 13 |
| 4b | 22 | 26 | - | 09 | 14 | 17 | 17 | 24 |
| 4c | 14 | 20 | - | - | 12 | 14 | 14 | 19 |
| 4d | 13 | 19 | - | - | - | 10 | 13 | 18 |
| 4e | - | 14 | - | - | - | - | 10 | 14 |
| 4f | 14 | 18 | - | - | 14 | 16 | 14 | 17 |
| 4g | - | 10 | - | - | 10 | 15 | 12 | 13 |
| 4h | 14 | 21 | - | - | 12 | 14 | 13 | 17 |
| 4i | 12 | 16 | - | 10 | - | - | 10 | 11 |
| 4j | 20 | 22 | 13 | 15 | 13 | 16 | 16 | 23 |
| Cipro | 28 | 34 | 14 | 20 | 15 | 22 | 18 | 25 |
| floxacin | ||||||||
*Indicates concentration of drug in μg/ml. Zone of inhibition in mm
Table 3: Antibacterial Activities Of Synthesized Compounds
All six compounds evaluated for antiinflammatory activity exhibited good activity ranging from 36.6 to 73.7% reduction in edema volume. The compounds 4a, 4f and 4g showed significant antiinflammatory activity comparable to ibuprofen and the other compounds 4c and 4j showed moderate activity. The test compounds were found to be significantly active when compared to control group.
The compound 4b and 4j showed good activity against S. aureus. Compounds 4b, 4c, 4d and 4j showed moderate activity against P. aeruginosa. Compounds 4b, 4c, 4d and 4j did not possess any encouraging activity against B. subtilis.
Acknowledgements
The authors are thankful to IIT-Chennai for carrying out PMR study. We are also grateful to the Principal, Manipal College of Pharmaceutical Sciences, Manipal, for providing all the facilities.
References
- Jung HK, Doddareddya MR, Cha JH, Rhim H, Cho YS, Koh HY, et al. Synthesis and biological evaluation of novel T-type Ca2+channelblockers. Bioorg Med Chem 2004;12:3965-70.
- Popat KH, Nirmavat KS, Kachhadia VV, Joshi HS. Synthesis and biological activity of 3-aryl-5-(3'-bromo/chlorophenyl) isoxazoles. J Indian Chem Soc 2003;80:707-8.
- Norman BH, Lander PA, Gruber JM, Kroin JS. Cyclohexyl-linked tricyclic isoxazoles are potent and selective modulators of the multidrug resistance protein (MRP1). Bioorg Med Chem Lett 2005;15:5526-30.
- Raman K, Pandey BR, Barthwal JP, Parmar SS. Antiinflammatory and antiproteolytic properties of 1,3-disubstituted-5-(2-arylindol-3-yl)-∆2-pyrazolines. Eur J Med Chem Chem Ther 1980;15:567-9.
- Kumar A, Sinha JN, Bhargava KP, Shanker K. Synthesis of 1-acetyl-5-aryl-3-[ortho-(3-chloro-2-indol-3-yl-4-oxo-1-azetidinyl)phenyl]2-pyrazolines. Indian J Chem 1984;23B:589-91.
- Gupta DP, Kumar P, Ahmad S, Shanker K. Synthesis, antiacetylcholinesterase, and anthelmintic activities of newer indolyl derivatives. Indian J Chem 1990;29B:194-6.
- Lather V, Chowdary PVR. Synthesis and antimicrobial activity of N1-(arylidine hydrazidomethyl)-indoles,2-(substitutedaryl)-3-(N1-indolylacetamidyl)-4-oxo-thiazolidines and 5-benzylidine derivatives of thiazolidinones. Indian J Pharm Sci 2003;65:576-9.
- Gadaginamath GS, Shyadligeri AS, Kavali RR. Chemoselectivity of indoledicarboxylates towards hydrazine hydrate: part III- synthesis and antimicrobial activity of novel 4-thiazolidinonylindoles. Indian J Chem 1999;38B:156-9.
- Renukadevi P, Biradar JS. Synthesis and antimicrobial activity of 3,5-disubstituted-2-[1'-phenyl-5'-thioalkyl-s-triazol-2'-yl]indoles and 3,5-disubstituted-2-[1'-substituted aminomethyl-4'-phenyl-5',4'-phenyl-5'(4'H)-thion-s-triazol-3-yl]indoles. Indian J Heterocycl Chem 1999;9:107-12.
- Panda SS, Chowdary PV. Synthesis of novel indolyl-pyrimidine antiinflammatory, antioxidant and antibacterial agents. Indian J Pharm Sci 2008;70:208-15.
- Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Expt Biol 1962;111:544-7.
- Clarke GM, Cooke D. A Basic Course in Statistics. 4th ed. London: Arnold; 1998. p. 520-46.
- Kavanagh F. Analytical Microbiology. New York: Academic Press; 1963. p. 313-46.