- *Corresponding Author:
- S. K. Kashaw
Pharmaceutical Chemistry Division, Department of Pharmaceutical Sciences, Dr. H. S. Gour University, Sagar-470 003, India
E-mail: sushilkashaw@gmail.com
| Date of Submission | 05 May 2007 |
| Date of Revision | 02 February 2008 |
| Date of Acceptance | 21 August 2008 |
| Indian J Pharm Sci 2008, 70 (4): 535-538 |
Abstract
A series of 1,2,4-dithiazole were synthesized from 1,2,4-thiadiazoles in the presence of CS 2 and evaluated for their antimicrobial, anticonvulsant, analgesic and neurotoxicity potential. The compounds provided significant protection against maximal electroshock-induced seizures and seizures induced by 300 mg/kg of subcutaneous pentylenetetrazole administration. The designed compounds (3a-g) were screened in vitro for antibacterial activity against Staphylococcus aureus Escherichia coli , Bacillus subtilis and Pseudomonas aeruginosa and antifungal activity in fungal strains of Candida albicans and Aspergillus niger. Synthesized compounds exhibited moderate antibacterial and antifungal activity. N,N -Di-naphthalen-1-yl- N -(thioxo-5 H -[1,2,4]dithiazol-3-yl)-guanidine and N,N -Bis-(4-fluoro-phenyl)- N -(5-thioxo-5 H -[1,2,4]dithiazol-3-yl)-guanidine showed analgesic activity by tail flick method.
Keywords
1,2,4-Thiadiazoles, 1,2,4-dithiazole, antimicrobial, anticonvulsant, analgesic, neurotoxicity
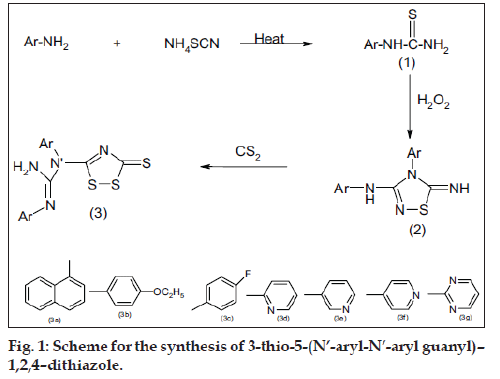
In recent years, several 1,2,4-dithiazole and their derivatives were found to have prominent pharmacological activities such as anticonvulsant, analgesic, antiinflammatory activity [1-3]. The present paper reports the synthesis and biological activity of some novel 1,2,4-Thiadiazoles and 1,2,4-dithiazoles. Aromatic/hetero aromatic amines were reacted with ammonium thiocyanate in the presence of concentrated HCl to give aryl thiourea (I). Comp I were oxidatively closed into 3-aryl amino-4-aryl-5-imino-D2-1,2,4- thiadiazoline (II). Compound (II) on reacting with carbon disulfide gave the corresponding 3-thio-5- (N’-aryl-N’-arylguanyl)-1,2,4-dithiazoles. All the compounds showed satisfactory elemental analysis. IR and NMR spectra were consistent with the assigned structure (fig. 1).
Melting points were determined by open capillary method by using Buchi 530 melting point apparatus and were uncorrected. The purity of the compounds was confirmed by thin layer chromatography using silica gel glass plates and a solvent system of benzene, ethanol (9:1). The spots were developed in iodine chamber and visualized under ultra violet lamp. All the compounds were subjected to elemental analysis (S and N) on Perkin Elemer-2400 instrument and the measured values agreed within ±0.4% with the calculated. 13C-NMR spectra were recorded on 13C Avance Bruker 300 MHz spectrophotometer. IR spectra in KBr disc were recorded on Testcan Shimadzu FTIR 8000. Mass spectra were obtained on Joel SX 102/M- 6000 mass spectrometer applying FAB method.
Aryl thiourea was synthesized using a procedure in which aryl amine (0.1 mol) was taken in a 250-ml beaker containing 100 ml distilled water. Concentrated HCl (10 ml) was added and contents were warmed to dissolve the amine. Ammonium thiocynate (7.6 g, 0.1 mol) was added to the amine solution and the mixture was heated. The mixture was poured on crushed ice, the precipitate thus obtained was filtered off by suction and recrystallised from ethanol. Spectral analysis, m.p. and elemental analysis confirm the formation of the corresponding thiourea
3-amino-4-aryl-5-imino--1,2,4–thiadiazoline (2a-2g) were synthesized by warming corresponding aryl thiourea (1a-1g, 0.5 mol) in a conical flask and dissolving in warm 10 ml of HCl. Hydrogen peroxide (60-70 ml, 20 vol.) was added drop wise from the separating funnel with continuous stirring, the mixture was kept aside for 2 h. The oxidized mixture was diluted with water and separated sulphur was then removed by filtration. The filtrate on neutralization with dilute ammonia, gave precipitate which was collected by filtration and recrystallised from ethanol.
3-thio-5-(N’-aryl-N’-aryl guanyl)–1,2,4–dithiazole (3a-3g) were synthesized by dissolving corresponding thiadiazolines (2a-2g) (0.01 mol) in ethanol (20 ml). Carbon disulphide (12 ml) was added to it and the content was reß uxed for 1h. The solution was cooled and thereafter yellow coloured crystals separate out. The products were recrystallised from ethanol. The physical properties of the title compounds are reported in Table 1.
| Code | % yield | mp° | Mol. formula | Elemental analysis (found %/ calculated %) | IR (KBr) cm-1/ Molecular ion peak | 13CNMR (δ ppm) |
|---|---|---|---|---|---|---|
| 3a | 70 | 130 | C23H16N4S3 | N(12.95/12.71) | 3446 (NH), 1605 (C=N), | 220 (C3), 163 (C5, C*), |
| S(21.63/21.64) | 1263 (C-N), 509 (S-S), | 147.8 (C1’), 141.2 (C1’’) | ||||
| 1240 (C=S), 658 (C-S)/444 | ||||||
| 3b | 55 | 181 | C19H20N4S3O2 | N(12.94/12.94) | 3334 (NH), 1635 (C=N), | 220 (C3), 163 (C5, C*), |
| S(22.20/22.19) | 1305 (C-N), 512 (S-S), | 140.6 (C1’), 132.9 (C1’’) | ||||
| 1246 (C=S), 672 (C-S)/432 | ||||||
| 3c | 66 | 172 | C15H10N4S3F2 | N(14.75/14.73) | 3410 (NH), 1583 (C=N), | 220 (C3), 163 (C5, C*), |
| S(25.25/25.23) | 1304 (C-N), 510 (S-S), | 144.6 (C1’), 136.9 (C1’’) | ||||
| 1216 (C=S), 707 (C-S)/380 | ||||||
| 3d | 60 | 115 | C13H10N6S3 | N(24.24/24.24) | 3428 (NH), 1624 (C=N), | 220 (C3), 163 (C5, C*), |
| S(27.60/27.70) | 1325 (C-N), 512 (S-S), | 156.5 (C2’), 147.7 (C2’’) | ||||
| 1240 (C=S), 723 (C-S)/346 | ||||||
| 3e | 64 | 118 | C13H10N6S3 | N(24.38/24.24) | 3410 (NH), 1634 (C=N), | 220 (C3), 163 (C5, C*), |
| S(27.39/27.70) | 1326 (C-N), 523 (S-S), | 139(C3’), 145.1 (C3’’) | ||||
| 1279 (C=S), 676 (C-S)/346 | ||||||
| 3f | 71 | 126 | C13H10N6S3 | N(24.34/24.24) | 3405 (NH), 1618 (C=N), | 220 (C3), 163 (C5, C*), |
| S(27.55/27.70) | 1282 (C-N), 512 (S-S), | 149 (C4’), 155.3 (C4’’) | ||||
| 1242 (C=S), 682 (C-S)/346 | ||||||
| 3g | 58 | 136 | C11H8N8S3 | N(32.23/32.16) | 3404 (NH), 1647 (C=N), | 220 (C3), 163 (C5, C*), |
| S(27.33/27.60) | 1293 (C-N), 522 (S-S), | 163.2 (C2’), 169.3 (C2’’) |
Table 1: Physical And Spectral Data Of The Compounds (3a-G)
Serial dilution method was used for determining minimum inhibitory concentration (MIC) [4,5] of the synthesized compounds. Bacterial strain of Staphylococcus aureus (MTCC no. 96), Pseudomonas aeruginosa (MTCC no. 424), Bacillus subtilis (MTCC no. 619) and Escherichia coli (MTCC no. 40) and fungal strain of Candida albicans (MTCC no. 227) and Aspergillus niger (MTCC no.1344) were procured from Institute of Microbial Technology, Chandigarh and used in the present study [6,7]. Nutrient broth (beef extract 1 g, yeast extract 2 g, peptone 5 g, sodium chloride 5 g, distilled water q.s. 1000 ml) was used as growth medium for bacteria and Sabouraund’s medium (dextrose 40 g, peptone 10 g, distilled water q.s. 1000 ml) was used for growth of fungus [8,9]. Cook’s procedure of serial dilution was used to determine the MIC. Dimethylformamide (DMF) was used as solvent. A blank test was conducted to check the antimicrobial activity of DMF. The results are in Table 2. The study was simultaneously performed for norfloxacin and clotrimazole as standard drug for antibacterial and antifungal activity respectively.
| Code. | S. aureus | B. subtilis | P. aeruginosa | E. coli | C. albicans | A. niger |
|---|---|---|---|---|---|---|
| 3a | 36** | 68 | 34** | > 100 | 100 | 52* |
| 3b | > 100 | 66 | 100 | > 100 | 18*** | >100 |
| 3c | 100 | 16*** | > 100 | > 100 | >100 | 96 |
| 3d | > 100 | 76 | 38** | > 100 | 52* | 14*** |
| 3e | > 100 | 24** | 26** | > 100 | 44** | 56* |
| 3f | 100 | 68 | > 100 | 66 | 36** | 12*** |
| 3g | 100 | 78 | > 100 | 12*** | 54* | 36** |
| Norfloxacin | 4 | 16 | 10 | 8 | - | - |
| Clotrimazole | - | - | - | - | 6 | 12 |
Table 2: Antibacterial and antifungal screening of 3-thio-5-(n’-aryl-n’-arylguanyl)-1,2,4- Dithiazoles
Initial anticonvulsant evaluation of the compounds was undertaken by the anticonvulsant drug development (ADD) program protocol at National Institute of Health, USA [10,11]. Male albino rats (90-110 g) of either sex maintained in standard conditions for temperature, relative humidity, light/day cycles and fed with normal diet and water ad libitum, were used. Maximal seizures were induced by application of an electrical current across the brain via corneal electrodes. The stimulus parameters were 50 mA, AC in a pulse of 60 Hz for 200 ms (0.2 s). The test compounds were suspended in 0.5% methyl cellulose/water. All the compounds were administered i.p. in doses of 30, 100 and 300 mg/kg to one to four animals. After 30 min and 4 h of drug administration electroshock was applied using corneal electrodes. Disappearance of the hind leg extensor component of convulsion was used as positive criteria.
The test compounds were administered i.p. to all animals in a group in doses of 30, 100 and 300 mg/kg. Thirty min after i.p. injection 60 mg/kg pentylenetetrazole was injected subcutaneously. Observations were taken for absence or presence of clonic convulsive seizures after 30 min and 4h of administration. The results are presented in Table 3
| Code | MES | ScPTZ | Neurotoxicity | Latent period of tail flick response (Sec.) (Mean ± SEM)a |
||||
|---|---|---|---|---|---|---|---|---|
| 0.5h | 4h | 0.5h | 4h | 0.5h | 4h | Control | Treated (after 30 min) |
|
| 3a | 300 | 300 | -- | -- | 100 | -- | 7.60 ± 0.25 | 10.52 ± 0.85* |
| 3b | 100 | 300 | -- | -- | 300 | -- | 4.82 ± 0.38 | 6.9 ± 1.38* |
| 3c | 300 | -- | 300 | -- | 100 | -- | 5.50 ± 0.21 | 9.69 ± 0.25* |
| 3d | 300 | -- | -- | -- | 100 | -- | 6.86 ± 0.92 | 7.6 ± 0.23 |
| 3e | 300 | -- | -- | -- | 100 | -- | 6.35 ± 0.23 | 7.80 ± 0.61 |
| 3f | 300 | -- | -- | -- | 100 | -- | 6.66 ± 0.41 | 8.39 ± 0.38* |
| 3g | -- | -- | -- | -- | 100 | -- | 4.86 ± 0.45 | 5.96 ± 1.3 |
| Phenytoin | 30 | 100 | -- | -- | 100 | 100 | ||
| Carbamazepine | 30 | -- | 100 | -- | 100 | 300 | ||
| Acetyl salicylic acid | ||||||||
| (10 mg/Kg) | 6.03 ± 0.55 | 10.22 ± 0.83* | ||||||
Table 3: Anticonvulsant, neurotoxicity and analgesic activity of 3-thio-5-(n’-aryl-n’-aryl Guanyl)-1,2,4-dithiazoles
Neurotoxicity studies were performed using the rotorod test [12,13]. Mice were grouped into groups of four animals each and trained to stay stable on an accelerating rotorod of diameter 3.2 cm revolving at the rate of 6 revolutions per min. Animals were administered 30, 100 and 300 mg/kg i.p. of the compounds. Thirty minutes after the injection the mice was placed individually on the rotating rod. Neurotoxicity was indicated by the inability of the animal to maintain equilibrium on the rod for at least one min in each of three trials (Table 3).
Analgesic activity was determined using hot wire ‘Techno’ anlgesiometer provided with an arrangement for circulation of cold water to avoid heating of the area surrounding the wire. Animals of either sex were grouped into groups of six animals each. The rats were then put into a rat holder individually with the tail protruding out of the hole. The tail was then kept on the hot wire of the instrument in such a way that it did not touch the wire. It is presumed that on feeling pain, the rat would withdraw its tail. After 30 minutes of the administration of synthesised compounds (10mg/kg.) the reaction time was determined. Acetyl salicylic acid (10 mg/ kg i.p.) was taken as reference drug. The results are reported Table 3.
The anticonvulsant activities of the synthesised compounds were established after i.p. administration in two seizure models in mice viz., maximal electroshock-induced seizures (MES) and seizures induced by subcutaneous pentylenetetrazole administration (ScPTZ). The animals were dosed with 30, 100 and 300 mg/kg of the test compounds and examined at 0.5 and 4 h after the injections for anticonvulsant activity. The minimum dose whereby bioactivity was demonstrated in half or more of the mice is presented in Table 3 along with the activity data of phenytoin and carbamazepine. In this series 90% of the compounds tested were found to be active in MES screen, while only one compound 3c showed activity in ScPTZ screen. Hence the compound exhibits MES selectivity. All the compounds showed activity after 0.5 h hence onset of the action is rapid. Compound 3b was found to be moderately active showing activity at 100 mg/kg. Only one compound 3c showed broad spectrum of activity whereas compound 3a and 3b showed the prolong duration of action, showing activity at 300 mg/kg after 4 h. It was observed that compounds having napthyl group as substituents increases the potency of compounds. This may be attributed to bulkiness of the naphthyl ring which increases the hydrophobicity of the molecules thereby increasing the penetration power of the compounds across the blood brain barrier. All compounds showed neurotoxicity at some dose level. The compound 3b, showed neurotoxicity at higher dose level at 300 mg/kg. All remaining compounds were found to be neurotoxic at 100 mg/kg. None of the compound showed neurotoxicity at the end of 4 h. The compounds 3c and 3a showed significant analgesic activity (P<0.01) in tail ß ick response test similar to the acetyl salicylic acid. Compound 3a and 3f of this series were also good analgesic effect with significant value P<0.05 as compared to the standard drug acetyl salicylic acid. Other compounds of this series showed insignificant analgesic activity. Analgesic study was undertaken with a view to find whether the compounds showing good potency so far as anticonvulsant activity is concerned. It was observed that generally a good anticonvulsant had analgesic property too. Compound 3a was found to be moderately active against S. aureus and P. aeruginosa. Compound 3e was found to be moderately active against B. subtilis and E. coli. Compound 3d also showed moderate activity against P. aeruginosa. Compound 3b was found to be active against C. albicans whereas 3d, 3f were found to be active against A. niger. 3e and 3f were also found to be moderately active against C. albicans and compounds 3d and 3g were found to be also mild active against C. albicans and moderately active against A. niger.
Acknowledgements
The authors are thankful to RSIC, CDRI Lucknow and SAIF, Punjab for spectral analysis. Thanks are due to Dr J. P. Stables and other members of the anticonvulsant drug development program, NIH, Bethesda, USA, for extraordinary help in anticonvulsant evaluation.
References
- Bohme H, Ahrens KH. 5-Aaryl-3H-1,2,4-dithiazoles: A new class ofhighly potent fungicides. Arch Pharm 1974;307:828-36.
- Pandeya SN. Kumar A, Singh BN, Mishra DN. Synthesis and biological activity of isodithiobiurets, dithiobiurets, and dithiazoles. Pharm Res1978;4:321-6.
- MacDonald JW, McKinnon DM. 1,2,4-Dithiazole-3-thiones and Derivatives. Can J Chem 1967;45:1225-9.
- Cook AM, Brown MR. The relation between heat activation andcolony formation for the spores of bacillus stearothermophilus. J Pharm Pharmacol 1954;6:725-32.
- Davis BD, Dubelcco R, Eisen HN, Gimsberg HS. Microbiology. 3rded. Philadelphia: Harper and Row Publications; 1980.
- Catalogue of MTCC Institute of Microbial Technology (IMTECH), Chandigarh, 1992.
- Pelczar MJ, Chan EC, Kreig NR.Microbiology. 5th ed. New York:McGraw Hill Publications Co.; 1993.
- Collins CH Lyne PM. Microbiological Methods. 4th ed. London: Butterworth and Co. Publications Ltd.; 1976.
- Aneja KR. Experiments in Microbiology, Plant Pathology, TissueCulture and Mushroom Cultivation. 2nd ed., New Delhi: Wishwa Prakashan; 1996.
- Krall PL, Penry JK, White BG, Kupferburg HJ, Swinyard EA. Antiepileptic drug development: II, anticonvulsant drug screening. Epilepsia 1978;19:409-17.
- Conley JP, Kohn H. Functionalized DL-Amino acid derivatives: Potentnew agents for the treatment of epilepsy. J Med Chem 1987;30:567-74.
- Kinnard WJ, Carr CJ. A preliminary procedure for the evaluationof central nervous system depressants. J Pharmacol ExpTher 1957;116:354-61.
- Dunham MS, Miya TA. A note on a simple apparatus for detecting neurological deÞcit in rats and mice. J Am Pharmac Assoc Sci Edit 1957;46:208-9.