- *Corresponding Author:
- M. S. R. Murty
Medicinal Chemistry and Pharmacology Division, Hyderabad-500 007, India
E-mail: msrmurty@ymail.com
| Date of Submission | 22 April 2017 |
| Date of Revision | 28 February 2018 |
| Date of Acceptance | 30 August 2018 |
| Indian J Pharm Sci 2018;80(5):930-939 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
A series of 1-substituted-1H-tetrazole-5-thiol building blocks were synthesized (6a–h) and coupled by S-alkylation with 2-bromo-1-(4-(substituted phenylsulfonyl)piperazin-1-yl)ethanone (4a–c) using triethylamine in ethanol under reflux conditions. The structures of newly synthesized compounds were characterised by nuclear magnetic resonance and mass spectral data. Further, the hybrid compounds (7a–x) were screened for in vitro inhibitory effect on human cervical carcinoma (SiHA), breast adenocarcinoma (MDA-MB-235) and human pancreatic carcinoma (PANC-1) cell lines using sulforhodamine B assay. Antiproliferative assay revealed that most of the target compounds exhibited significant growth inhibitory activity (GI50≤0.1 µM) against all tested cancer cell lines compared to the reference drug. The most promising active compounds in this series were 7e and 7n, which displayed antiproliferative activity with GI50≤0.2 µM against the SiHa and MIDA-MB-231 cancer cell lines, whereas compounds 7g, 7l, 7p, 7s and 7t exhibited antiproliferative activity with GI50≤0.1 µM against the PANC-1 cell line. Thus the tetrazole-piperazinesulfonamide hybrid compounds could be potential leads for the development of new antiproliferative agents.
Keywords
Tetrazoles, N-substituted sulfonyl piperazine, hybrid, antiproliferative activity
Cancer is a major cause of death around the world from decades. The WHO estimated 12 million deaths by cancer with current therapeutics by 2030. Among all the characterized cancers, only lung, stomach, liver, colon, and breast cancers are main cause of cancer mortality worldwide [1]. There probable causes for the difficulty in the control and treatment cancer could be the genetic instability and poor prognosis of cancer [2]. Among all types of cancer treatment, chemotherapy is an important option. Cancer cells become simultaneously resistant to different structural types of chemotherapeutic agents due to the multidrug resistance. Hence, the development of efficient, selective and less toxic anticancer agents are necessary to overcome the multidrug resistance of cancer cells [3].
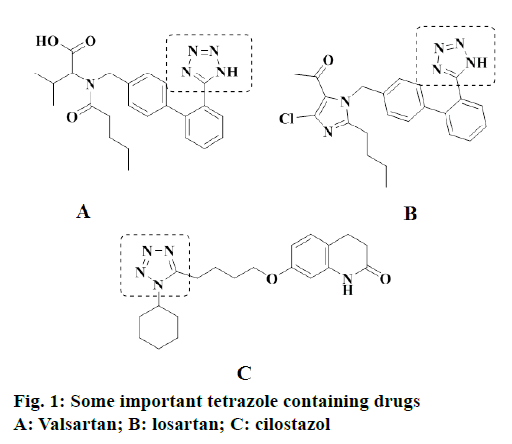
The tetrazoles are five-membered ring 6π-heterocycles, containing four contiguous nitrogen atoms and these are not found in nature, but there is scarce data available on their biological activity [4]. Further, 5-sulfenyl tetrazoles are congeners of tetrazoles that can be found as a distinguished scaffold in active pharmaceutical ingredients, such as cefamandol, latamoxef and cilostazol [5] (Figure 1). In addition, tetrazole derivatives displayed interesting pharmacological and biological properties such as antibacterial [6], antiinflammatory [6], anticonvulsant [7] and anticancer [8]. Moreover, tetrazoles commonly used in pharmaceuticals as lipophilic spacers and carboxylic acid surrogates [9].
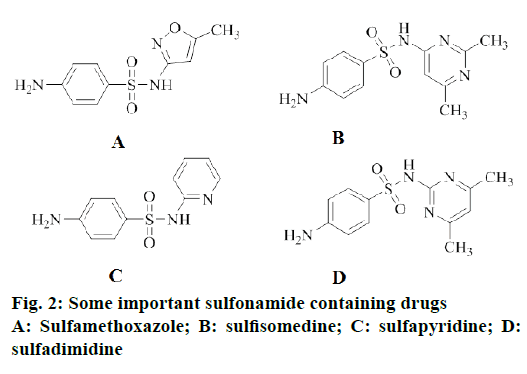
On the other hand, sulfonamides are a very important class of compounds in the pharmaceutical industry and a key pharmacophore in many marketed drugs (Figure 2) [10]. In addition, sulfonamide derivatives possess very interesting diversified pharmacological and biological properties, like antifungal [11], antiviral [12], antitumor [13], antiinflammatory [14] and as a carbonic anhydrase inhibitors [15]. Moreover, the sulfonamides combined with trimethoprim were established to significantly enhance their antibacterial efficacy. It was commonly well-known that sulfamethoxazole in combination with trimethoprim is a potent pharmaceutical compound and has been broadly used for the treatment of urinary tract and pneumonia bacterial infections, toxoplasmosis [16] and pneumocystis in HIV infected patients [17]. Numerous research reports indicated that the sulfanilamide moiety (-SO2NH-) retaining the structural feature, which was incorporated into nitrogen containing aromatic heterocyclic groups exhibited stronger anticancer or antitumor activity than the corresponding sulfanilamide precursor [11-14,18]. Thus, above results revealed that modification of sulfanilamide with nitrogen containing aromatic heterocyclic groups leads to the enhancement of antiproliferative activity and improve their potency.
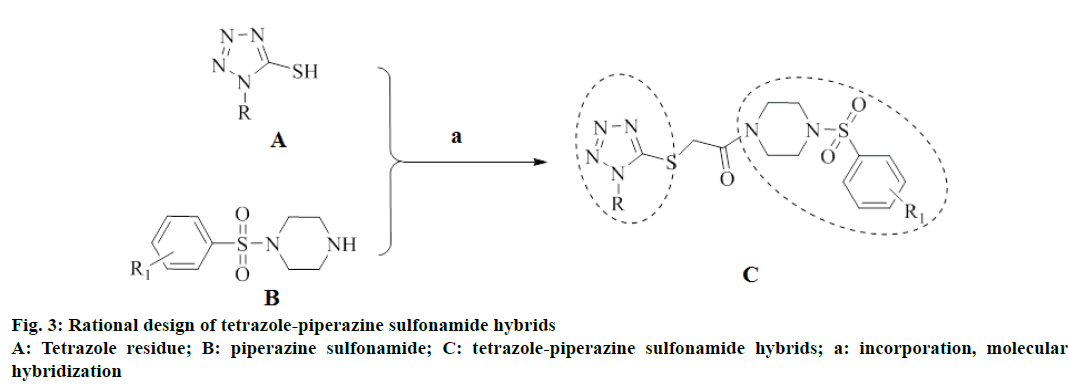
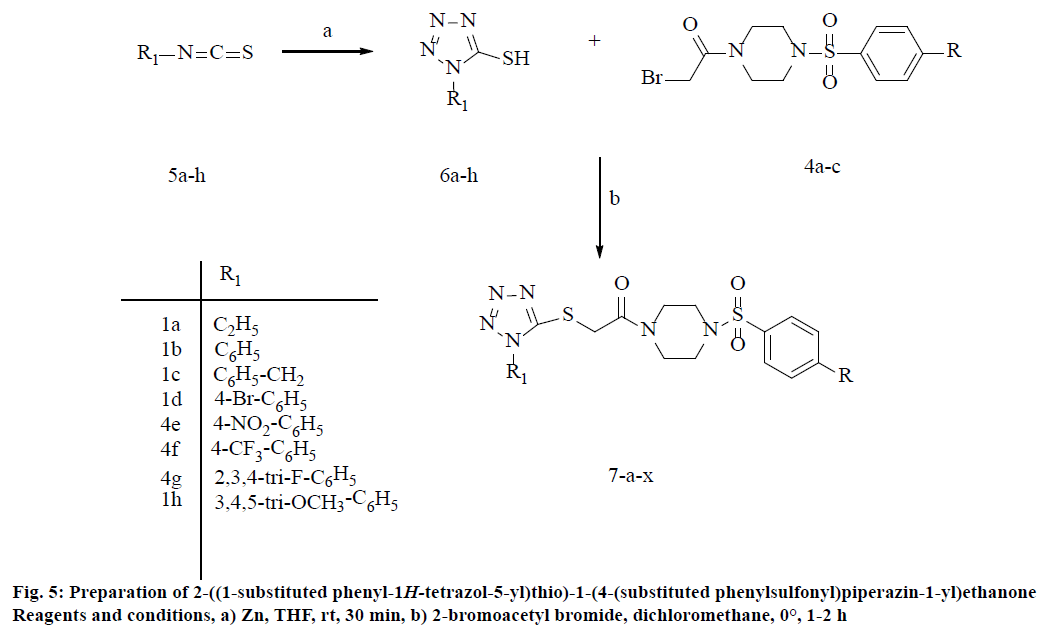
Prompted by the aforementioned important medicinal properties of tetrazole and piperazine sulfonamides, an attempt has been made to incorporate the structural features of piperazine sulfonamides and tetrazole by aiming at the discovery of new drug candidates with potent antiproliferative activity. The synthesis of aforesaid conjugates was done by a pharmacophore hybrid approach adopted in which piperazine sulfonamide incorporated with tetrazole were hybridized in one structure (Figure 3) often lead to synergistic effects [19-21]. Herein, a new series of 2-((1-substituted phenyl-1H-tetrazol-5-yl)thio)-1- (4-(substituted phenylsulfonyl)piperazin-1-yl) ethanone (7a-x) were synthesized by combining the two vital pharmacophores; sulfonamides and tetrazole with a view to create promising antiproliferative agent.
Materials and Methods
All the reagents and starting materials were obtained from commercial sources (Aldrich Chemicals, Spectrochem and Alfa-aesar) and were used without further purification. The progress of reactions was determined by analytical thin-layer chromatography (TLC) using silica gel 60 F254 pre-coated plates, and a UV lamp and I2 stain for visualization of the TLC plates. Column chromatography was done using Merck 60-120 sized mesh silica gel using chloroform and methanol as eluents. 1H nuclear magnetic resonance (NMR) spectra (300 and 500 MHz) and 13C NMR spectra (75, 101 and 126 MHz) were recorded on a Bruker Avance spectrometer using CDCl3 or DMSO-D6 as solvents and tetramethylsilane (TMS) as internal standard. Chemical shifts were reported in parts per million (ppm, δ) downfield from TMS. The following abbreviations are used for NMR signals: s=singlet, d=doublet, t=triplet, q=quartet, m=multiplet, dd=doublet of doublets. ESI-high resolution mass spectrometry (HRMS) spectra were recorded on “high-resolution QSTAR XL hybrid MS/MS system using methanol as a solvent. Melting points (MPs) were recorded on a Buchi R-535 apparatus and are uncorrected.
General procedure for the synthesis of 1-((4-substitutedphenyl)sulfonyl)piperazine (3a–c)
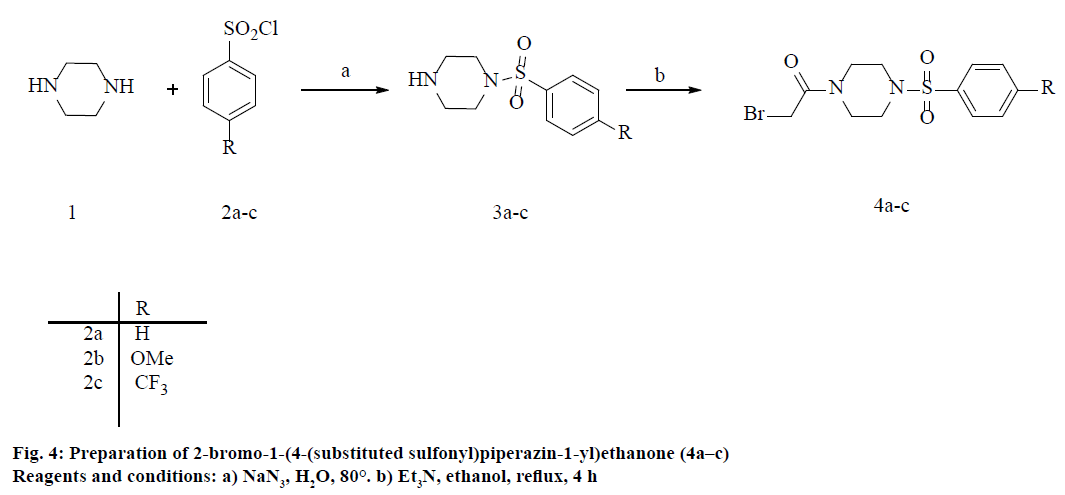
4-substituted aryl sulfonyl chloride (2a–c; 1.4 mmol) was added drop-wise over 4 min to a solution of piperazine (1.4 mmol) in tetrahydrofuran (THF, 3 ml) at 10-15°. The reaction mixture was stirred for 10 min and then zinc dust (2.8 mmol) was added to the reaction mixture and stirred at room temperature for 20 min. After the completion of reaction (confirmed by TLC), the reaction mixture was filtered and the filtrate was concentrated to give the crude product. The product was washed with ether and purified by crystallization using methanol to yield pure 1-((4-substitutedphenyl) sulfonyl)piperazine (3a–c).
General procedure for the synthesis of 2-bromo- 1-(4-(substituted phenylsulfonyl) piperazin-1-yl) ethanone (4a–c)
A mixture of substituted 1-((4-substitutedphenyl) sulfonyl)piperazine (3a–c; 10 mmol) and bromoacetylchloride (11.5 mmol) in dichloromethane were stirred at 0° for 30-45 min. Completion of reaction indicated by TLC, the compound was extracted with CH2Cl2. The organic layers were collected, washed with saturated brine solution, dried over anhydrous MgSO4 and concentrated in vacuo. The resultant crude was purified by column chromatograph (EtOAc and n-hexane, 3:6) to get the corresponding 2-bromo-1- (4-(substituted phenylsulfonyl)piperazin-1-yl) ethanone (4a–c).
General procedure for the synthesis of 2-((1-substituted phenyl-1H-tetrazol-5-yl)thio)- 1-(4-(substituted phenylsulfonyl)piperazin-1-yl) ethanone (7a–x)
To a solution of 1- substituted phenyl-1Htetrazole-5-thiol (6a–h; 0.9819 mmol) in ethanol (4 ml) and triethylamine (0.27 ml, 1.9638 mmol; 0.0981 mmol) were added at room temperature under N2 atmosphere. To the resultant mixture, 2-bromo- 1-(4-(substituedsulfonyl)piperazin-1-yl)ethanone (3a–c; 0.9819 mmol) was added and heated at 80°. Completion of reaction indicated by TLC, ethanol was evaporated in vacuo. The compound was extracted with CH2Cl2. The organic layers were collected, washed with saturated brine solution, dried over anhydrous MgSO4 and concentrated in vacuo. The resultant crude was purified by column chromatograph (EtOAc and n-hexane, 3:7) to get the title compound.
2-((1-ethyl-1H-tetrazol-5-yl)thio)-1-(4-(phenyl sulfonyl)piperazin-1-yl)ethanone (7a)
White solid; MP: 121-123°. 1H NMR (500 MHz, CDCl3) δ 7.80-7.69 (m, 2H), 7.68-7.51 (m, 5H), 4.34 (s, 3H), 4.28 (q, J=7.3 Hz, 3H), 3.79-3.63 (m, 4H), 3.17-2.98 (m, 7H), 1.51 (t, J=7.3 Hz, 4H). 13C NMR (75 MHz, CDCl3) δ 164.8, 152.5, 135.2, 133.3, 129.3, 127.6, 46.0, 45.4, 42.7, 41.7, 37.2, 13.9; ESI-HRMS (m/z): [M+H]+. Calcd. for C15H21O3N6S2: 397.11124, found: 397.11065.
2-((1-ethyl-1H-tetrazol-5-yl)thio)-1-(4-((4-methoxy phenyl)sulfonyl)piperazin-1-yl)ethanone (7b)
Yellow solid; MP: 138-140°. 1H NMR (500 MHz, CDCl3) δ 7.72-7.63 (m, 2H), 7.04-6.97 (m, 2H) 4.34 (s, 2H), 4.27 (q, J=7.2 Hz, 2H), 3.88 (s, 3H) 3.77-3.64 (m, 4H), 3.10-2.95 (m, 4H), 1.50 (t, J=7.3 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 164.7, 163.4, 152.6, 129.8, 126.5, 114.5, 55.7, 46.0, 45.6, 42.8, 41.7, 37.3, 13.9; ESI-HRMS (m/z): [M+H]+. Calcd. for C16H23O4N6S2: 427.12205, found: 427.12178.
2-((1-ethyl-1H-tetrazol-5-yl)thio)-1-(4-((4-(trifluoro methyl)phenyl)sulfonyl)piperazin-1-yl)ethanone (7c)
White solid; MP: 160-162°. 1H NMR (500 MHz, CDCl3) δ 7.81-7.69 (m, 2H), 7.68-7.51 (m, 2H), 4.34 (s, 2H), 4.27 (q, J=7.3 Hz, 2H), 3.78-3.66 (m, 4H), 3.08 (m, 4H), 1.51 (t, J=7.3 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 164.9, 152.5, 138.8, 128.2, 126.5, 45.4, 42.8, 41.6, 37.2, 14.1; ESI-HRMS (m/z): [M+H]+. Calcd. for C16H20O3N6F3S2: 465.09864, found: 465.09828.
2-((1-phenyl-1H-tetrazol-5-yl)thio)-1-(4-(phenyl sulfonyl)piperazin-1-yl)ethanone (7d)
White solid; MP: 157-159°. 1H NMR (500 MHz, CDCl3) δ 7.78-7.74 (m, 3H), 7.67-7.61 (m, 2H), 7.60-7.51 (m, 5H), 4.38 (s, 2H), 3.79-3.57 (m, 4H), 3.19- 3.07 (m, 2H), 3.01-2.95 (m, 2H); 13C NMR (126 MHz, CDCl3) δ 164.8, 153.5, 135.2, 133.3, 130.3, 129.9, 129.3, 127.7, 123.6, 46.0, 45.6, 41.7, 37.4; ESI-HRMS (m/z): [M+H]+. Calcd. for C19H21O3N6S2: 445.11134, found: 445.11105.
1-(4-((4-methoxyphenyl)sulfonyl)piperazin-1-yl)-2- ((1-phenyl-1H-tetrazol-5-yl)thio)ethanone (7e)
White solid; MP: 131-134°. 1H NMR (300 MHz, CDCl3) δ 7.68 (dd, J=6.7, 4.9 Hz, 2H), 7.61-7.51 (m, 5H), 7.02 (d, J=8.9 Hz, 2H), 4.39 (s, 2H), 3.88 (s, 3H), 3.80-3.75 (m, 4H), 3.14-2.96 (m, 4H); 13C NMR (101 MHz, CDCl3) δ 164.7, 163.4, 153.5, 133.4, 130.3, 129.9, 126.5, 123.6, 114.5, 55.7, 46.0, 45.5, 41.7, 37.5; ESI-HRMS (m/z): [M+H]+. Calcd. for C20H23O4N6S2: 475.12215, found: 475.12190.
2-((1-phenyl-1H-tetrazol-5-yl)thio)-1-(4-((4-(tri fluoromethyl)phenyl)sulfonyl)piperazin-1-yl) ethanone (7f)
White solid; MP: 165-167°. 1H NMR (500 MHz, CDCl3) δ 7.87-7.72 (m, 4H), 7.66-7.42 (m, 5H), 4.37 (s, 2H), 3.92-3.62 (m, 4H), 3.30-2.89 (m, 4H); 13C NMR (126 MHz, CDCl3) δ 162.3, 150.9, 136.4, 130.8, 127.8, 127.4, 125.6, 124.0, 121.1, 43.4, 43.0, 39.1, 34.7; ESIHRMS (m/z): [M+H]+. Calcd. for C20H20O3N6F3S2: 513.09889, found: 513.09854.
2-((1-benzyl-1H-tetrazol-5-yl)thio)-1-(4-(phenyl sulfonyl)piperazin-1-yl)ethanone (7g)
Yellow solid; MP: 133-135°. 1H NMR (300 MHz, CDCl3) δ 7.81-7.52 (m, 5H), 7.43-7.17 (m, 5H), 5.55 (s, 2H), 4.42 (s, 2H), 3.58-3.49 (m, 4H) 3.06-2.83 (m, 4H); 13C NMR (101 MHz, CDCl3) δ 164.9, 153.3, 135.2, 133.3, 132.6, 129.3, 129.1, 128.2, 127.6, 51.2, 45.9, 45.6, 41.7, 37.5; ESI-HRMS (m/z): [M+H]+. Calcd. for C20H23O3N6S2: 459.12703, found: 459.12659.
2-((1-benzyl-1H-tetrazol-5-yl)thio)-1-(4-((4- methoxyphenyl)sulfonyl)piperazin-1-yl)ethanone (7h)
White solid; MP: 123-125°. 1H NMR (500 MHz, CDCl3) δ 7.71-7.63 (m, 2H), 7.37-7.31 (m, 4H), 7.28-7.10 (m, 2H), 7.06-6.96 (m, 2H), 5.42 (s, 2H), 4.29 (s, 2H), 3.88 (s, 3H), 3.74-3.60 (m, 4H), 3.10-2.93 (m, 4H); 13C NMR (101 MHz, CDCl3) δ 164.8, 153.1, 139.0, 134.8, 132.5, 129.1, 128.2, 126.5, 124.4, 121.7, 51.2, 45.9, 45.6, 41.7, 37.2; ESI-HRMS (m/z): [M+H]+. Calcd. for C21H25O4N6S2: 489.13756, found: 489.13703.
2-((1-benzyl-1H-tetrazol-5-yl)thio)-1-(4-((4-(tri fluoromethyl)phenyl)sulfonyl)piperazin-1-yl) ethanone (7i)
White solid; MP: 135-137°. 1H NMR (500 MHz, CDCl3) δ 8.00-7.76 (m, 4H), 7.39-7.15 (m, 5H), 5.41 (s, 2H), 4.27 (s, 2H), 3.84-3.56 (m, 4H), 3.19-2.93 (m, 4H); 13C NMR (126 MHz, CDCl3) δ 164.8, 153.3, 139.1, 134.8, 132.5, 129.1, 128.2, 126.5, 124.0, 122.1, 51.2, 45.6, 41.7, 37.3; ESI-HRMS (m/z): [M+H]+. Calcd. for C21H22O3N6F3S2: 527.11456, found: 527.11402.
2-((1-(4-bromophenyl)-1H-tetrazol-5-yl)thio)-1-(4- (phenylsulfonyl)piperazin-1-yl)ethanone (7j)
White solid; MP: 154-156°. 1H NMR (400 MHz, CDCl3) δ 7.82-7.39 (m, 9H), 4.38 (s, 2H), 3.84-3.60 (m, 4H), 3.22-2.96 (m, 4H); 13C NMR (101 MHz, CDCl3) δ 164.5, 153.5, 135.1, 133.2, 132.3, 129.3, 127.7, 125.2, 124.4, 46.0, 45.6, 41.7, 37.6; ESI-HRMS (m/z): [M+H]+. Calcd. for C19H20O3N6BrS2: 523.02189, found: 523.02104.
2-((1-(4-bromophenyl)-1H-tetrazol-5-yl)thio)-1-(4- ((4-methoxyphenyl)sulfonyl)piperazin-1-yl) ethanone (7k)
White solid; MP: 155-157°. 1H NMR (500 MHz, CDCl3) δ 7.74-7.65 (m, 4H), 7.52-7.46 (m, 2H), 7.08- 6.97 (m, 2H), 4.39 (s, 2H), 3.88 (s, 3H), 3.78-3.67 (m, 4H), 3.14-3.06 (m, 2H), 3.02-2.92 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 164.6, 163.4, 153.5, 133.2, 132.3, 129.8, 126.5, 125.1, 124.4, 114.5, 55.7, 46.0, 45.5, 41.7, 37.7; ESI-HRMS (m/z): [M+H]+. Calcd. for C20H22O4N6BrS2: 553.03228, found: 553.03173.
2-((1-(4-bromophenyl)-1H-tetrazol-5-yl)thio)-1-(4- ((4-(trifluoromethyl)phenyl)sulfonyl)piperazin-1- yl)ethanone (7l)
White solid; MP: 140-142°. 1H NMR (500 MHz, CDCl3) δ 7.84 (d, J=8.5 Hz, 2H), 7.73-7.66 (m, 3H), 7.50-7.43 (m, 3H), 4.37 (s, 2H), 3.82-3.70 (m, 4H), 3.22-3.14 (m, 2H), 3.12-3.01 (m, 2H); 13C NMR (126 MHz, CDCl3) δ 164.7, 153.4, 139.0, 133.2, 132.3, 128.2, 126.6, 125.0, 124.4, 45.9, 45.6, 41.8, 37.3; ESIHRMS (m/z): [M+H]+. Calcd. for C20H19O3N6BrF3S2: 591.00905, found: 591.00838.
2-((1-(4-nitrophenyl)-1H-tetrazol-5-yl)thio)-1-(4- (phenylsulfonyl)piperazin-1-yl)ethanone (7m)
Yellow solid; MP: 145-147°. 1H NMR (500 MHz, CDCl3) δ 8.45 (t, J=10.8 Hz, 2H), 7.91 (t, J=10.8 Hz, 2H), 7.76-7.72 (m, 2H), 7.69-7.53 (m, 3H), 4.45 (s, 2H), 3.80-3.68 (m, 4H), 3.19-3.00 (m, 4H); 13C NMR (75 MHz, CDCl3+DMSO) δ 169.3, 158.9, 152.7, 142.9, 139.9, 138.1, 134.1, 132.3, 130.2, 129.0, 50.7, 50.1, 46.4, 42.8; ESI-HRMS (m/z): [M+H]+. Calcd. for C19H20O5N7S2: 490.09662, found: 490.09611.
1-(4-((4-methoxyphenyl)sulfonyl)piperazin-1-yl)-2- ((1-(4-nitrophenyl)-1H-tetrazol-5-yl)thio)ethanone (7n)
Yellow solid; MP: 161-163°. 1H NMR (500 MHz, CDCl3) δ 8.48-8.40 (m, 2H), 7.92-7.87 (m, 2H), 7.72- 7.62 (m, 2H), 7.06-6.96 (m, 2H), 4.45 (s, 2H), 3.89 (s, 3H), 3.79-3.67 (m, 4H), 3.16-2.98 (m, 4H); 13C NMR (126 MHz, DMSO-D6) δ 159.5, 158.5, 149.1, 143.2, 133.3, 125.0, 121.6, 120.6, 119.3, 109.7, 50.9, 41.1, 40.8, 36.8, 33.2; ESI-HRMS (m/z): [M+H]+. Calcd. for C15H21O3N6S2: 520.10713, found: 520.10640.
2-((1-(4-nitrophenyl)-1H-tetrazol-5-yl)thio)-1-(4- ((4-(trifluoromethyl)phenyl)sulfonyl)piperazin-1- yl)ethanone (7o)
Yellow solid; MP: 154-156°. 1H NMR (300 MHz, CDCl3) δ 8.64-8.22 (m, 4H), 8.16-7.54 (m, 6H), 4.47 (s, 2H), 3.85-3.48 (m, 4H), 3.34-2.94 (m, 4H); 13C NMR (101 MHz, CDCl3) δ 164.3, 153.7, 148.1, 138.1, 136.7, 130.8, 130.2, 128.2, 125.5, 124.6, 124.0, 121.7, 45.9, 45.6, 41.8, 37.7, 29.7; ESI-HRMS (m/z): [M+H]+. Calcd. for C20H19O5N7F3S2: 558.08395, found: 558.08339.
1-(4-(phenylsulfonyl)piperazin-1-yl)-2-((1-(4- (trifluoromethyl)phenyl)-1H-tetrazol-5-yl)thio) ethanone (7p)
White solid; MP: 175-177°. 1H NMR (500 MHz, CDCl3) δ 7.93-7.68 (m, 6H), 7.64-7.57 (m, 3H), 4.43 (s, 2H), 3.85-3.58 (m, 4H), 3.24-2.92 (m, 4H); 13C NMR (126 MHz, CDCl3) δ 164.5, 153.7, 136.2, 135.2, 133.3, 129.3, 127.6, 127.24, 123.8, 46.0, 45.5, 41.7, 37.9; ESI-HRMS (m/z): [M+H]+. Calcd. for C20H20O3N6F3S2: 513.09878, found: 513.09834.
1-(4-((4-methoxyphenyl)sulfonyl)piperazin-1-yl)- 2-((1-(4-(trifluoromethyl)phenyl)-1H-tetrazol-5-yl) thio)ethanone (7q)
White solid; MP: 175-177°. 1H NMR (500 MHz, CDCl3) δ 7.85 (d, J=8.6 Hz, 2H), 7.78 (d, J=8.5 Hz, 2H), 7.73-7.66 (m, 2H), 7.07-6.98 (m, 2H), 4.42 (s, 2H), 3.88 (s, 3H), 3.79-3.65 (m, 4H), 3.14-3.07 (m, 2H), 3.05-2.98 (m, 2H); 13C NMR (126 MHz, CDCl3) δ 164.5, 163.4, 153.7, 136.2, 129.8, 127.2, 126.5, 123.8, 114.5, 55.7, 46.0, 45.5, 41.7, 37.9; ESI HRMS (m/z): [M+H]+. Calcd. for C21H22O4N6F3S2: 543.10946, found: 543.10901.
2-((1-(4-(trifluoromethyl)phenyl)-1H-tetrazol-5- yl)thio)-1-(4-((4-(trifluoromethyl)phenyl)sulfonyl) piperazin-1-yl)ethanone (7r)
White solid; MP: 175-177°. 1H NMR (500 MHz, CDCl3) δ 7.94-7.88 (m, 4H), 7.85 (d, J=8.9 Hz, 2H), 7.78 (d, J=8.4 Hz, 2H), 4.40 (s, 2H), 3.76-3.69 (m, 4H), 3.22-3.14 (m, 2H), 3.12-3.04 (m, 2H); 13C NMR (126 MHz, CDCl3) δ 164.6, 153.5, 139.0, 136.2, 128.2, 127.2, 126.5, 123.7, 45.9, 45.6, 41.8, 37.4; ESIHRMS (m/z): [M+H]+. Calcd. for C21H19O3N6F6S2: 581.08618, found: 581.08575.
1-(4-(phenylsulfonyl)piperazin-1-yl)-2-((1-(2,3,4- trifluorophenyl)-1H-tetrazol-5-yl)thio)ethanone (7s)
White solid; MP: 144-146°. 1H NMR (400 MHz, CDCl3) δ 7.89-7.47 (m, 6H), 7.41-7.09 (m, 2H), 4.39 (s, 2H), 3.80-3.60 (m, 4H), 3.12-2.98 (m, 4H); 13C NMR (126 MHz, CDCl3) δ 164.4, 155.7, 135.1, 133.4, 129.3, 127.6, 122.1, 113.0, 45.9, 45.6, 41.7, 37.9; ESI-HRMS (m/z): [M+H]+. Calcd. for C19H18O3N6F3S2: 499.08303, found: 499.08259.
1-(4-((4-methoxyphenyl)sulfonyl)piperazin-1-yl)- 2-((1-(2,3,4-trifluorophenyl)-1H-tetrazol-5-yl)thio) ethanone (7t)
White solid; MP: 175-177°. 1H NMR (500 MHz, CDCl3) δ 7.71-7.66 (m, 4H), 7.05-6.97 (m, 4H), 4.39 (s, 2H), 3.88 (s, 3H), 3.76-3.64 (m, 4H), 3.14-3.06 (m, 2H), 3.03-2.97 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 164.4, 163.4, 155.7, 129.8, 126.4, 122.3, 118.1, 114.5, 113.2, 113.0, 55.7, 46.0, 45.6, 41.6, 37.9; ESI-HRMS (m/z): [M+H]+. Calcd. for C20H20O4N6F3S2: 529.09372, found: 529.09318.
1-(4-((4-(trifluoromethyl)phenyl)sulfonyl)piperazin -1-yl)-2-((1-(2,3,4-trifluorophenyl)-1H-tetrazol-5- yl)thio)ethanone (7u)
White solid; MP: 143-145°. 1H NMR (500 MHz, CDCl3) δ 8.01-7.80 (m, 4H), 7.31-7.12 (m, 2H), 4.38 (s, 2H), 3.82-3.65 (m, 4H), 3.25-2.97 (m, 4H); 13C NMR (126 MHz, CDCl3) δ 164.6, 153.6, 139.1, 136.2, 128.2, 127.2, 126.6, 123.7, 45.9, 45.6, 41.8, 37.5; ESIHRMS (m/z): [M+H]+. Calcd. for C20H17O3N6F6S2: 567.07058, found: 567.07005.
1-(4-(phenylsulfonyl)piperazin-1-yl)-2-((1-(3,4,5- trimethoxyphenyl)-1H-tetrazol-5-yl)thio)ethanone (7v)
White solid; MP: 145-148°. 1H NMR (500 MHz, CDCl3) δ 7.81-7.71 (m, 2H), 7.67-7.52 (m, 3H), 6.76 (s, 2H), 4.37 (s, 2H), 3.90 (s, 9H), 3.77-3.68 (m, 4H), 3.18-3.14 (m, 2H), 3.07-3.01 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 164.6, 153.9, 138.9, 130.8, 130.3, 128.2, 127.2, 126.5, 123.8, 101.4, 61.0, 56.4, 45.9, 45.6, 41.7, 37.6; ESI-HRMS (m/z): [M+H]+. Calcd. for C22H27O6N6S2: 535.14306, found: 535.14268.
1-(4-((4-methoxyphenyl)sulfonyl)piperazin-1-yl)-2- ((1-(3,4,5-trimethoxyphenyl)-1H-tetrazol-5-yl)thio) ethanone (7w)
White solid; MP: 159-161°. 1H NMR (500 MHz, CDCl3) δ 7.69 (d, J=8.7 Hz, 2H), 7.04 (d, J=8.6 Hz, 2H), 6.78 (s, 2H), 4.39 (s, 2H), 3.91 (s, 9H), 3.83-3.78 (m, 4), 3.24-2.87 (m, 4H); 13C NMR (126 MHz, CDCl3) δ 164.7, 163.4, 153.9, 153.6, 139.3, 129.8, 128.6, 126.5, 114.5, 101.5, 61.0, 56.5, 55.7, 46.0, 45.6, 41.7, 37.4; ESI-HRMS (m/z): [M+H]+. Calcd. for C23H29O7N6S2: 565.15371, found: 565.15323.
1-(4-((4-(trifluoromethyl)phenyl)sulfonyl)piperazin -1-yl)-2-((1-(3,4,5-trimethoxyphenyl)-1H-tetrazol- 5-yl)thio)ethanone (7x)
White solid; MP: 155-157°. 1H NMR (500 MHz, CDCl3) δ 8.05-7.84 (m, 3H), 7.74-7.70 (m, 1H), 6.76 (s, 2H), 4.36 (s, 2H), 3.92 (s, 9H), 3.83-3.69 (m, 4H), 3.25-3.19 (m, 2H), 3.14-3.01 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 164.9, 153.9, 153.6, 139.3, 136.7, 130.8, 130.2, 130.0, 128.6, 124.5, 101.4, 61.0, 56.5, 45.9, 45.6, 41.7, 37.1; ESI-HRMS (m/z): [M+H]+. Calcd. for C23H26O6N6F3S2: 603.13056, found: 603.13009.
In vitro antiproliferative activity
The cell lines, SiHa, MDA-MB-231 and PANC1, which were used in this study, were procured from American Type Culture Collection (ATCC), United States. The synthesized tetrazole-arylpiperazinesulfonamide hybrid compounds were evaluated for in vitro antiproliferative activity against these three human cancer cell lines. A protocol of 48-h continuous drug exposure was used, and the sulforhodamine B (SRB) cell proliferation assay was used to estimate cell viability or growth [21,22]. All cell lines were grown in Dulbecco’s modified Eagle’s medium containing 10 % fetal bovine serum in a humidified atmosphere of 5 % CO2 at 37°. Cells were trypsinized when subconfluent from T25 flasks/60-mm dishes and seeded in 96-well plates in 100 μl aliquots at plating densities depending on the doubling time of individual cell lines. The microtiter plates were incubated at 37°, 5 % CO2, 95 % air, and 100 % relative humidity for 24 h prior to addition of experimental drugs and were incubated for 48 h with different doses (0.01, 0.1, 1, 10, 100 μM) of the prepared derivatives. After 48-h incubation at 37°, cell monolayers were fixed by the addition of 10 % (wt/vol) cold trichloroacetic acid, incubated at 4° for 1 h, and then stained with 0.057 % SRB dissolved in 1 % acetic acid for 30 min at room temperature. Unbound SRB was washed with 1 % acetic acid. The proteinbound dye was dissolved in 10 mM Tris base solution for OD determination at 510 nm using a microplate reader (Enspire, Perkin Elmer, USA). Using the seven absorbance measurements, time zero, (Tz), control growth (C), and test growth in the presence of drug at the five concentration levels (Ti), the percent growth was calculated at each level of the drug concentration. Percent growth inhibition was calculated as; [(Ti-Tz)/ (C-Tz)]×100 for concentrations for which Ti>/=Tz; [(Ti-Tz)/Tz]×100 for concentrations for which Ti<Tz.
The dose-response parameters were calculated for each experimental agent. Growth inhibition of 50 % (GI50) was calculated from [(Ti−Tz)/(C−Tz)]×100 = 50, which is the drug concentration resulting in a 50 % reduction in the net protein increase (as measured by SRB staining) in control cells during incubation with the test or standard agents. The values were calculated for this parameter if the level of activity is reached; however, if the effect is not reached or is exceeded, the value for that parameter was expressed as greater or less than the maximum or minimum concentration tested.
Results and Discussion
The 1-substituted-1H-tetrazole-5-thiols (6a–h) coupled with substituted phenylsulfonyl piperazine acetamide derivatives (3a–c) were synthesized by a converging synthesis route that requires the preparation of the 4-substituted phenylsulfonylpiperazines and 1-substituted-1H-tetrazole-5-thiol precursors independently that can be subsequently coupled together. Initially, 4-substituted phenylsulfonylpiperazines (3a–c) were prepared by starting with the appropriate arylsulfonyl chloride (2) reacted with simple piperazine in THF solvent using zinc dust under mild and neutral conditions at room temperature [23]. The excess piperazine was removed by washing with saturated aq. NaHCO3 solution to afford the required product with good yields. Then, the substituted phenylsulfonyl piperazines (3a–c) were treated with bromoacetyl bromide in CH2Cl2 solvent at 0° to room temperature stirring for 90-120 min to afford the 2-bromo-1- (4-(substitutedsulfonyl)piperazin-1-yl)ethanone (4a–c) with excellent yield range from 85-90 %, the overall reaction sequence described in Figure 4.
On the other hand, 1-substituted 5-mercaptotetrazoles [24] (6a–h) derivatives were synthesized by the reaction of NaN3 with isocyanates (5a–g) in water at 80° for 3 h. To obtain final compounds (7a–x), the compounds 2-bromo-1-(4-(substituted sulfonyl)piperazin-1-yl) ethanone (3a–c) and 1-substituted 5-mercaptotetrazoles (6a–h) were refluxed with ET3N in ethanol to afford corresponding compounds yields ranging from 79 to 88 % (Table 1, Figure 5). The formation of S-alkylated products (7a–x) were confirmed by the presence of S–CH2 characteristic peak appeared at δ 41.2- 41.7 ppm in 13C NMR and appearance of signal as a singlet for the methylene group at δ 3.39-3.47 ppm in 1H NMR spectrum, which is typical for connectivity. The spectroscopic data of all the newly synthesized compounds are in full accordance with their depicted structures. The detailed general synthesis procedure of the compounds is mentioned in the experimental section.
| S. No. | R | R1 | Yielda (%) |
|---|---|---|---|
| 7a | H | C2H5 | 84 |
| 7b | OCH3 | C2H5 | 85 |
| 7c | CF3 | C2H5 | 82 |
| 7d | H | Ph | 86 |
| 7e | OCH3 | Ph | 87 |
| 7f | CF3 | Ph | 83 |
| 7g | H | Ph-CH2 | 88 |
| 7h | OCH3 | Ph-CH2 | 86 |
| 7i | CF3 | Ph-CH2 | 83 |
| 7j | H | 4-Br-Ph | 86 |
| 7k | OCH3 | 4-Br-Ph | 87 |
| 7l | CF3 | 4-Br-Ph | 82 |
| 7m | H | 4-NO2-Ph | 83 |
| 7n | OCH3 | 4-NO2-Ph | 84 |
| 7o | CF3 | 4-NO2-Ph | 79 |
| 7p | H | 4-CF3-Ph | 82 |
| 7q | OCH3 | 4-CF3-Ph | 84 |
| 7r | CF3 | 4-CF3-Ph | 79 |
| 7s | H | 2,3,4-tri-F-Ph | 83 |
| 7t | OCH3 | 2,3,4-tri-F-Ph | 82 |
| 7u | CF3 | 2,3,4-tri-F-Ph | 80 |
| 7v | H | 3,4,5-tri-OMe-Ph | 88 |
| 7w | OCH3 | 3,4,5-tri-OMe-Ph | 90 |
| 7x | CF3 | 3,4,5-tri-OMe-Ph | 81 |
‘a’After purification by column chromatography
Table 1: Synthesis of 2-((1-substituted phenyl-1h-tetrazol-5-yl)thio)-1-(4-(substituted phenylsulfonyl)piperazin-1-yl)ethanone
The novel target compounds (7a–x) were tested in vitro for inhibitory effect on human cancer cell lines, cervix (SiHa), breast (MDA-MB-231) and pancreatic carcinoma (PANC-1) using the SRB assay method. The GI50 values are listed in Table 2. From the screening results in Table 2, it was observed that all the compounds showed antiproliferative activities against these cell lines in a concentration-dependent manner.
| Compound No | SiHA | MDA-MB -231 | PANC-1 |
|---|---|---|---|
| 7a | 0.685 ± 0.02 | 0.239 ± 0.01 | 0.491 ± 0.01 |
| 7b | 0.731 ± 0.04 | 0.858 ± 0.03 | 0.681 ± 0.01 |
| 7c | 3.365 ± 0.07 | 0.102 ± 0.05 | 0.228 ± 0.03 |
| 7d | 0.073 ± 0.002 | 1.877 ± 0.06 | 0.42 ± 0.02 |
| 7e | 0.098 ± 0.003 | 0.229 ± 0.03 | 0.827 ± 0.05 |
| 7f | 12.527 ± 0.06 | 11.47 ± 0.09 | 9.77 ± 0.06 |
| 7g | 3.675 ± 0.02 | 3.627 ± 0.05 | 0.483 ± 0.01 |
| 7h | 0.2 ± 0.01 | 7.783 ± 0.06 | 0.2 ± 0.03 |
| 7i | 2.234 ± 0.05 | 2.395 ± 0.02 | 0.375 ± 0.03 |
| 7j | 1.765 ± 0.06 | 1.632 ± 0.01 | 0.642 ± 0.02 |
| 7k | 0.948 ± 0.02 | 2.394 ± 0.03 | 0.334 ± 0.07 |
| 7l | 1.641 ± 0.02 | 2.021 ± 0.04 | 0.678 ± 0.04 |
| 7m | 1.883 ± 0.03 | 1.885 ± 0.06 | 0.154 ± 0.03 |
| 7n | 0.071 ± 0.002 | 0.193 ± 0.02 | 1.967 ± 0.06 |
| 7o | 8.182 ± 0.09 | 10 ± 0.05 | 0.388 ± 0.01 |
| 7p | 0.582 ± 0.02 | 1.24 ± 0.01 | 0.155 ± 0.03 |
| 7q | 0.817 ± 0.04 | 1.833 ± 0.03 | 0.884 ± 0.05 |
| 7r | 0.221 ± 0.01 | 0.496 ± 0.02 | 0.2 ± 0.01 |
| 7s | 0.639 ± 0.03 | 2.761 ± 0.01 | 0.072 ± 0.002 |
| 7t | 9.961 ± 0.08 | 2.845 ± 0.06 | 0.1 ± 0.01 |
| 7u | 0.991 ± 0.02 | 0.571 ± 0.03 | 0.261 ± 0.03 |
| 7v | 2.482 ± 0.06 | 2.363 ± 0.05 | 2.92 ± 0.04 |
| 7w | 5.982 ± 0.06 | 1.627 ± 0.02 | 1.1 ± 0.03 |
| 7x | 0.699 ± 0.03 | 0.223 ± 0.01 | 1.15 ± 0.01 |
| Tamoxifen | 0.12 ± 0.01 | 0.24 ± 0.01 | 0.15 ± 0.02 |
‘a’GI50 was defined as the concentration resulting in 50 % growth
inhibition. Data are means ± SD of three independent experiments.
‘b’All compounds were characterized by NMR and mass spectroscopy
Table 2: In Vitro Antiproliferative Activity (GI50 µM)a of the synthesized compoundsb
Among the tested cell lines, compounds such as 7d, 7e and 7n showed 1-2 fold greater activity than reference drug (GI50=0.12 μM), as evidenced by GI50 values of 0.071-0.098 μM against SiHa cancer cell line. On the other hand, compounds 7a, 7c, 7e and 7n showed comparable activity (GI50=0.193-0.239 μM) than reference drug (GI50=0.24 μM) against MIDAMB- 231 cancer cell line, while the compounds 7h, 7s and 7m showed equal activity (GI50=0.072-0.15 μM) to that of the reference drug (GI50=0.15 μM) against PANC-1 cancer cell line
Different substituents such as alkyl, phenyl, benzyl, electron-withdrawing Br, CF3, F, NO2 and electrondonating OCH3 on a phenyl ring were applied on tetrazole moiety as well as different substituents (CF3, OCH3) were employed on sulfonylpiperazines to investigate antiproliferative activity. However the interesting inhibitory behaviour of these compounds is relatively dependent on electronic nature of substituents on the tetrazole and sulfonylpeparazine hybrids (7a–x). The overall results of antiproliferative activity indicated that the compounds bearing methoxy group (OCH3) sulfonylpeparazine attached to the tetrazole which were having unsubstituted phenyl (7d and 7e) and substituted electron withdrawing (NO2, F) groups (7n, 7s and 7t) showed 50 % inhibition at a concentrations ranges from 0.071-0.22 μM against various tested cancer cell lines. From the obtained results it revealed that compounds bearing methoxy group (OCH3) sulfonylpeparazine attached to the tetrazole, which were having either unsubstituted phenyl or substituted electron withdrawing (NO2, F) groups contributed to the promising antiproliferative activity.
In summary, a straightforward synthetic strategy was developed for the preparation of novel 2-((1-substitued phenyl-1H-tetrazol-5-yl)thio)-1-(4-(substituted phenyl sulfonyl)piperazin-1-yl)ethanone derivatives (7a–x) with good yields and evaluated for their in vitro antiproliferative studies. The preliminary studies revealed that a few of the synthesized hybrids were active on all the tested cancer cell lines with GI50 values less than 1.5 μM. The growth inhibition of cancer cell lines profile revealed that compounds 7e and 7n displayed broader spectrum of antiproliferative activity with GI50≤0.2 μM against the SiHa and MIDA-MB-231 cell lines compared to reference drug whereas compounds 7g, 7l, 7p, 7s and 7t exhibited antiproliferative activity with GI50≤0.1 μM against the PANC-1 cell lines. Overall, these findings proposed that tetrazole-containing substituted phenylsulfonylpiperazine hybrids have the potential to be developed as lead molecule and further structural modification might create promising new antiproliferative agents.
Acknowledgements
The authors thank the Director, Indian Institute of Chemical Technology, Hyderabad for constant encouragement, KD is grateful to CSIR, New Delhi, India for the award of research fellowship. Authors also thank CSIR for financial support under the 12th Five Year plan projects ‘‘Affordable Cancer Therapeutics (ACT)’’ (CSC 0301) and ‘‘Small Molecules in Lead Exploration (SMiLE)’’ (CSC0111).
Conflict of interest
There is no conflict of interest among authors.
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29.
- Despaigne AA, Parrilha GL, Izidoro JB, da Costa PR, dos Santos RG, Piro OE, et al. 2-Acetylpyridine- and 2-benzoylpyridine-derived hydrazones and their gallium(III) complexes are highly cytotoxic to glioma cells. Eur J Med Chem 2012;50:163-72.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90.
- Butler RN, Katritzky AR, Rees CW, Scriven EFV. Comprehensive heterocyclic chemistry II. Oxford, United Kingdom: Oxford University Press; 1996. p. 621.
- da Silva FM, dos Santos JC, Campos JL, Mafud AC, Polikarpov I, Figueira AC, et al. Structure-based identification of novel PPAR gamma ligands. Bioorg Med Chem Lett 2013;23:5795-802.
- Bekhit AA, El-Sayed OA, Aboulmagd E, Park JY. Tetrazolo[1,5-a]quinoline as a potential promising new scaffold for the synthesis of novel anti-inflammatory and antibacterial agents. Eur J Med Chem 2004;39:249-55.
- Wagle S, Adhikari AV, Kumari SK. Synthesis of some new 4-styryltetrazolo [1,5-a] quinoxaline derivatives as potent anticonvulsants. Eur J Med Chem 2009;44:1135-43.
- Kumar CNSSP, Parida DK, Santhoshi A, Kota AK, Sridhar B, Rao VJ. Synthesis and biological evaluation of tetrazole containing compounds as possible anticancer agents. Med Chem Commun 2011;2:486-92.
- Herr RJ. 5-Substituted-1H-tetrazoles as carboxylic acid isosteres: medicinal chemistry and synthetic methods. Bioorg Med Chem 2002;10:3379-93.
- Feng M, Tang B, Liang SH, Jiang X. Sulfur Containing Scaffolds in Drugs: Synthesis and Application in Medicinal Chemistry. Curr Top Med Chem 2016;16:1200-16.
- Zoumpoulakis P, Camoutsis Ch, Pairas G, Sokovic M, Glamoclija J, Potamitis C, et al. Synthesis of novel sulfonamide-1,2,4-triazoles, 1,3,4-thiadiazoles and 1,3,4-oxadiazoles, as potential antibacterial and antifungal agents. Biological evaluation and conformational analysis studies. Bioorg Med Chem 2012;20:1569-83.
- Al-Ansary GH, Ismail MA, Abou El Ella DA, Eid S, Abouzid KA. Molecular design and synthesis of HCV inhibitors based on thiazolone scaffold. Eur J Med Chem 2013;68:19-32.
- Al-Dosari MS, Ghorab MM, Alsaid MS, Nissan YM, Ahmed AB. Synthesis and anticancer activity of some novel trifluoromethylquinolines carrying a biologically active benzenesulfonamide moiety. Eur J Med Chem 2013;69:373-83.
- Weber A, Casini A, Heine A, Kuhn D, Supuran CT, Scozzafava A, et al. Unexpected nanomolar inhibition of carbonic anhydrase by COX-2-selective celecoxib: new pharmacological opportunities due to related binding site recognition. J Med Chem 2004;47:550-7.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168-81.
- Brun-Pascaud M, Chau F, Garry L, Farinotti R, Derovin F, Girard PM. Altered trimethoprim-sulfamethoxazole ratios for prophylaxis and treatment of Toxoplasma gondii and Pneumocystis carinii dual infections in rat model. J Acquir Immune Defic Syndr Hum Retrovirol 1996;13:201-7.
- Wininger DA, Fass R. Impact of trimethoprim-sulfamethoxazole prophylaxis on etiology and susceptibilities of pathogens causing human immunodeficiency virus-associated bacteremia. Antimicrob Agents Chemother 2002;46:594-7.
- Casini A, Scozzafava A, Mastrolorenzo A, Supuran LT. Sulfonamides and sulfonylated derivatives as anticancer agents. Curr Cancer Drug Targets 2002;2:55-75.
- Dileep K, Katiki MR, Rao BR, Vardhan VPSV, Sistla R, Nanubolu JB, et al. Regioselective synthesis and preliminary cytotoxic activity properties of tetrazole appendage N-substituted piperazine derivatives. Org Commun 2017;10(3):178-89.
- Murty MS, Katiki MR, Nanubolu JB, Garimella S, Polepalli S, Jain N, et al. Synthesis and biological evaluation of novel tamoxifen-1,2,4-triazole conjugates. Mol Divers 2016;20:687-703.
- Dileep K, Polepalli S, Jain N, Buddana SK, Prakasham RS, Murty MSR. Synthesis of novel tetrazole containing hybrid ciprofloxacin and pipemidic acid analogues and preliminary biological evaluation of their antibacterial and antiproliferative activity. Mol Divers 2017;1-11:83-93.
- Reddy MA, Jain N, Yada D, Kishore C, Vangala JR, Surendra PR, et al. Design and synthesis of resveratrol-based nitrovinylstilbenes as antimitotic agents. J Med Chem 2011;54:6751-60.
- Murty MSR, Rami Reddy N, Yadav JS. Zinc-mediated novel and efficient method for Nsulfonylation of amines in the absence of base. J Sulfur Chem 2006;27:589-93.
- Altland HW. The Smiles rearrangement of 2-tetrazolylthio-3-aminopyridines. J Org Chem 1976;41:3395-98.