- *Corresponding Author:
- P. Ghosh
Natural Products and Polymer Chemistry Laboratory, Department of Chemistry, University of North Bengal, Raja Rammhunpur, Darjeeling-734 013
E-mail: pizy12@yahoo.com
| Date of Submission | 07 November 2013 |
| Date of Revision | 18 November 2014 |
| Date of Acceptance | 30 March 2015 |
| Indian J Pharm Sci 2015;77(2):202-207 |
Abstract
A simple synthesis and in vitro antileishmanial activity of 29,30-dibromo-28-oxoallobetulin against the parasitic protozoans, Leishmania donovani and Leishmania major is described. The structure of the compound is established on the basis of spectral data (IR, NMR, MS). Both the antiproliferative effect and the cell cycle progression were studied.
Keywords
Triterpenoid, halogen, leishmania, antiproliferative
Leishmaniasis constitutes a group of human diseases caused by obligate intracellular protozoan parasites of genus Leishmania. It is the second most parasitic disease in the modern world, behind malaria [1]. The parasites are transmitted between mammalian hosts by phlebotomus (Old World) and Lutzomyia (New World) sandflies. The disease Leishmaniasis affects the populations of 88 countries worldwide with symptoms ranging from disfiguring cutaneous and mucocutaneous lesions that can cause widespread destruction of mucous membranes to visceral disease affecting the haemopoetic organs. The disease leishmaniasis is present in four clinical forms: (i) visceral leishmaniasis (VL) or Kala Azar, (ii) cutaneous leishmaniasis (CL), (iii) mucocutaneous leishmaniasis (MCL) and (iv) diffuse cutaneous leishmaniasis (DCL). Leshmaniasis has a worldwide distribution with important foci of infection in Central and South America, Southern Europe, North and East Africa, the Middle East and the Indian subcontinent. In recent times the main foci of VL are in Sudan and India. [2]
Till today some antimony containing drugs are generally used to treat leishmaniasis. The toxicity of the available drugs and the emergence of strains that are not responsive to drug therapy make the discovery of novel therapeutic agents imperative [3,4]. In recent years, various new compounds with profound antileishmanial activity have been reported. Various compounds, such as atovaquone [5], WR6026 [6], licochalcone A [7], ilmfosine [8] and formycin B [9] have been reported to inhibit Leishmania donovani infection, the causative agent of visceral leishmaniasis. Unfortunately, all such compounds are effective only at higher doses and none of them have been recognized as oral drugs. Recently miltefosine (hexadecylphosphocholine or HPC), originally developed as anticancer agent, has been introduced as an effective antileishmanial drug. However, miltefosine-associated gastrointestinal toxicity and teratogenicity have already been identified during clinical trials in India. Long half-life of the drug might also encourage the emergence of resistance, as evidenced from selected miltefosine resistant line of L. donovani and L. tropica in vitro is also another concern [10]. Moreover, cases indicating the relapse of the disease, even after ten months of a full course of treatment with miltefosine [11] prompted the researchers in searching novel molecules active against the Leishmania infection. Therefore, development of new chemotherapeutics with lower toxicity and higher efficiency is the high demand in contemporary medicinal and pharmaceutical sciences.
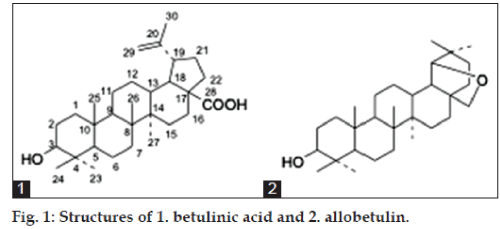
Triterpenoids are widely distributed throughout plant kingdom in different skeletal frame work. These are the plants secondary metabolites and have defensive physiological function.Due to their molecular complexity and diversity, secondary metabolites from natural sources, mainly plants, still inspire the design of drugs. In this context scientists throughout the world have reported potential activity of these compounds against different diseases [12,13] and leishmania parasite [14-16]. People have so far reported the antileshmonial activity of betulinic acid (1, fig. 1), a lupane derived triterpenoid. Because of its good activity it is now considered as a very promising new chemotherapeutic agent for the treatment of leishmniasis.
In 1995, it was established that allobetulin (2, fig 1), a pentacyclic triterpenoid, showed modest inhibitory activity against theinfluenza B virus. It was claimed in the patent literature that different derivatives of allobetulin, including 2 and its 3-O-acylated and phosphorylated derivatives exhibited significant antiviral activity and could be used to treat herpes virus (HSV-herpes simplex virus) infection [17]. Also in 2002, 28-oxoallobetulin was shown in cell culture to inhibit influenza A growth [18] and several allobetulin derivatives showed good cytotoxicity [19]. But there is no report relating to the antileishmanial activity of allobetulin or its derivatives.
Over the years insertion of halogen atoms has been used in several cases of hit-lead or lead to drug conversion. From the medicinal chemistry perspective, insertion of bulky groups into BioNCEs like halogens can induce antagonistic or agonist effects in comparison with the original BioNCEs [20-22]. Therefore, incorporation of bromine atom instead of chlorine may lead to cause more volumetric and conformational changes in the target molecules. Previously Moreau et al. had reported [21] the encouraging antitopoisomerase I and cell apoptosis effects of the dibrominated imide derivative of natural metabolite rebeccamycin. Cytotoxicity of several bromo derivatives have also been reported [22]. The synthesis of brominated ecdysteroids suggested that this kind of brominated derivatives could be used as genetic modulator for controlling pests.
In view of this and in continuation of our research on transformation of naturally occurring triterpenoids to potential bioactive molecules [12], here we are reporting the antileishmanial activity of a prepared bromo derivative of allobetulin against different strains of Leishmania; Leishmania donovani AG83 (MHOM/IN/83/AG83), causative agent of visceral leishmaniasis; a field isolate Leishmania donovani MS10, causative agent of visceral leishmaniasis; Leishmania major LV39 (MRHO/Sv/59/P strain), causative agent of cutaneous leishmaniasis.
Materials and Methods
Betulinic acid was isolated from the bark of Biscofia javanica through Soxhlet apparatus in toluene. All the chemicals, solvents used were reagent grade and purified, where applicable, prior to their use. Melting point was detected in open capillary method and was uncorrected. IR spectra were taken in Shimadzu 8300 FT-IR spectrophotometer in KBr disc. The NMR chemical shift was reported in ppm relative to 7.20 and 77.0 ppm of CDCl3 solvent as the standards. 1H spectra were recorded in 300 MHz frequencies and 13C NMR spectra were recorded in 75.4 MHz frequencies. Coupling constant J was calculated in Hz.
29,30-dibromo-28-oxoallobetulin, 3
N-bromosuccinimide (NBS) in CCl4-CHCl3 (1:1 mixture) was treated with the methyl ester of betulinic acid at room temperature for 48 h. The reaction mixture was then filtered and solvent was recovered in reduced pressure. It was then poured into ice cold water, a white precipitate appeared that on purification over a column of silica gel gave 29,30-dibromo-28-oxoallobetulin (3), in 22 % yield. The synthesis was presented in Scheme 1.
IR at 3452 (-OH), 2942, 2858, 1764 (lactone carbonyl), 1724, 1453, 1380 (gem dimethyl), 1024, 668 cm-1, molecular ion peak at m/z 614 corresponding to the molecular formula of C30H46Br2O3, 1H NMR δH 0.71 (24-Me), 0.79 (25-Me),0.84 (23-Me), 0.86 (27-Me) and 0.92 (26-Me), δH 3.15 (m, 1H,) and δH 3.48 (m, 2H) and 3.65 (m, 2H).
Antiproliferative effect on parasitic protozoa Leishmania donovani and Leishmania major
The cytotoxicity assay was performed on three different strain of Leishmania sp. in three independent experiments as per the guidelines of biosafety committee of West Bengal State University. Leishmania donovani AG83 (MHOM/ IN/83/AG83) was originally obtained from Indian Institute of Chemical Biology, Kolkata, India [23]. Leishmania donovani MS10, a field isolate, was obtained from IPGMER, Kolkata, India as a gift. L. donovani MS10 strain was an isolate recently transformed from L. donovani-infected patients from the geographical location of West Bengal, India. Leishmania major LV39 (MRHO/Sv/59/P strain) was obtained University of Lausanne, Switzerland as a gift [24]. The parasites were maintained in the animal facility as per the guidelines of institutional animal ethics committee of West Bengal State University. Promastigote morphs of Leishmania sp. were transformed from intracellular amastigotes, acquired from splenic aspirates of infected BALB/c mice in complete M 199 medium (Invitrogen) supplemented with 1% penicillin-streptomycin (Invitrogen) and 10% FCS (GIBCO) at requisite temperature, 22° for L. donovani (AG83 and MS10) and at 26° for L. major LV39. To estimate the percentage of inhibition the derivative, the 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide (MTT) micro method was used, as described previously [25]. Briefly, late-log-phase promastigotes were seeded in a 96-well flat-bottom plate (200 μl per well; BD Falcon) in complete M 199 medium in presence or absence of the derivative. To examine the effect of compound 3, the cultures were additionally supplemented without (DMSO control) or with increasing concentrations of the derivative (5 to 25 μg/ml) soluble in DMSO (0.2% v/v). Equal volume of DMSO only was added in control experiments. After 72 h of incubation in requisite temperature, MTT (10 mg/ml, 10 μl per well) was added to each well and the plates were incubated for an additional 4 h at 37°.The enzyme reaction was then stopped by addition of acidic isopropanol (0.4 ml 10 N HCl in 100 ml isopropanol, 100 μl per well), and the absorbance was measured at 570 nm. Degree of cytotoxicity of conventional antileishmanial drug, sodium antimony gluconate (SAG), was also measured on Leishmania donovani AG83 [25] at the increasing concentration of 5 μg/ml to 25 μg/ml. Percent inhibition was measured in respect to the proliferation of Leishmania promastigotes of DMSO control group [25]. Statistical analyses for all experimentswere performed by Student’s test with the program Sigma Plotusing alpha adjustment.
Analysis of cell cycle progression
The nuclear DNA content of a cell can be quantitatively measured at high speed by flow cytometry. Initially, a fluorescent dye that binds stoichiometrically to the DNA is added to a suspension of permeabilized single cells or nuclei. The principle is that the stained material has incorporated an amount of dye proportional to the amount of DNA. The stained material is then measured in the flow cytometer and the emitted fluorescent signal yields an electronic pulse with a height (amplitude) proportional to the total fluorescence emission from the cell. Thereafter, such fluorescence data are considered as measurement of the cellular DNA content. For flow cytometry analysis of DNA content, exponentially grown L. donovani promastigote cells (2×106) were incubated without or with 10 μg/ml and 15 μg/ml of test compound in complete M199 media for 48 h. Cells were then harvested and washed three times with 1X PBS, fixed in 45% ethanol (diluted in 1X PBS), treated with 500 μg/ml RNAse A and then suspended in 0.5 M sodium citrate containing 69 μM PI. These samples were analyzed through flow cytometry (Becton and Dickinson, USA) after keeping them in the dark for 45 min [26].
Results and Discussion
The modifications in the present work focused on the introduction of a halogen atom namely bromine into the pentacyclic triterpene skeleton. The compound 29,30-dibromo-28-oxoallobetulin was previously synthesized from methylbetulinate in a reaction that took at least ten days without any report of its biological activity. The present synthetic method involves the treatment of N-bromo succinimide (NBS) in CCl4-CHCl3 (1:1 mixture) on the methyl ester of betulinic acid efficiently in 22 % yield. Betulinic acid was isolated from the bark of Biscofia javanica by Soxhlet extractor.
Methyl ester of betulinic acid was treated with recrystallized NBS in a 1:1 mixture of CCl4-CHCl3 for 48 h. Purification of the reaction mixture over a column of silica gel (60-120 mesh) gave a white powdered compound of melting point 285o. In the IR spectrum it gave peaks at 3452 (-OH), 2942, 2858, 1764 (lactone carbonyl), 1724, 1453, 1380 (gem dimethyl), 1024, 668 cm-1. In the mass spectrum it showed a molecular ion peak at m/z 614. In the mass spectrum three distinct peaks atm/z 612, 614 and 616 appeared in the ratio of 1:2:1, thus signifying the presence of two bromine atoms in the synthesized molecule. From the elemental analysis and mass spectral data the molecular formula was determined to be C30H46Br2O3.
In the 1H NMR spectrum it showed the presence of five methyl groups at δH 0.71 (24-Me), 0.79 (25-Me), 0.84 (23-Me), 0.86 (27-Me) and 0.92 (26-Me). The absence of any methyl peak beyond δH 1.00 (C-30 methyl group of the isopropelene side chain of lupane skeleton) signified that during the reaction rearrangement of the isopropelene side chain could have been take place at the E ring of the pentacyclic triterpenoid. The aH at C-3 appeared as a multiplet centered at δH 3.15 (m, 1H). Upto this it was clear that the two newly incorporated bromine atoms might be at C-29 and C-30. This assumption was supported by the presence of two multiplets each with an integration of two protons centered at δH 3.48 (m, 2H) and 3.65 (m, 2H). The presence of bromine atoms might be the chief cause for the observed deshielding of the two methylene groups. Another sharp singlet at δH 4.29 (s, 1H) with an integration of one proton was due to the lactonic proton at C-19.
The 13C NMR spectrum indicates the presence of 30 carbon atoms. The lactone carbonyl appeared at dC 178.6 and the two bromine bearing carbon atoms appeared at dC 78.9 and 65.5. C-3 appeared at dC 80.9. All other peaks are in agreement to that of the allobetulin skeleton. All these data can only be explained by considering structure 3 to the synthesized compound.
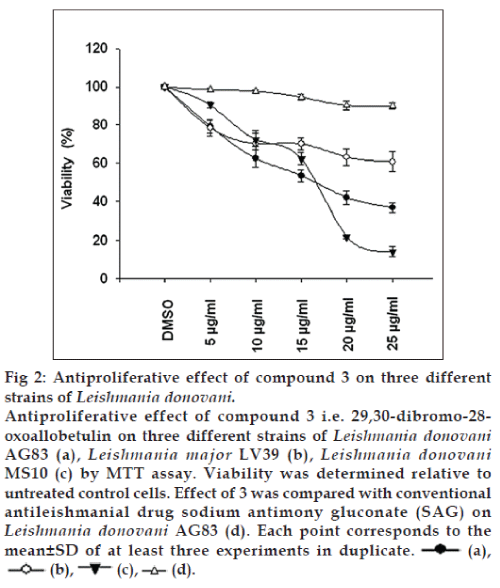
Antiproliferative effect of 3 was evaluated on Leishmania donovani, causative agent of visceral leishmaniasis and Leishmania major , causative agent of cutaneous leishmaniasis (fig. 2). It has been estimated that 25 μg/ml derivative inhibit the L. donovani AG83 promastigote proliferation by 63.27 % (P<0.001) in respect to DMSO control (Table 1). At the same dose the conventional drug SAG inhibited only by 10.02% in respect to DMSO control. Significantly, it has been found that 3 could inhibit the L. donovani MS10 strain, a field isolate obtained from an active visceral leishmaniasis patient very recently, by 85.92% (P<0.001) in respect to DMSO control. However, the effect of 3 was found costly against L. major LV39, causing 39.22% (P<0.001) inhibition in respect to DMSO control. Our data clearly indicated that 3 have a noteworthy antiproliferative effect on L. donovani AG83 and MS10 strain. We assumed that the finding is significant as 3 is highly effective against the recently isolated Leishmania donovani from active visceral leishmaniasis patient and should be further investigated for its immunomodulatory consequences.
| Leishmaniasp. (µg/ml) | % of inhibition vs. DMSO control | P value |
|---|---|---|
| LeishmaniadonovaniAG83 | ||
| (MHOM/IN/83/AG83) | ||
| SAG 5 | 1.375 | NS |
| SAG 10 | 2.275 | NS |
| SAG 15 | 5.45 | NS |
| SAG 20 | 9.8275 | NS |
| SAG 25 | 10.0275 | NS |
| LeishmaniadonovaniAG83 | ||
| (MHOM/IN/83/AG83) | ||
| B6 5 | 20.6675 | <0.012 |
| B6 10 | 37.56 | <0.004 |
| B6 15 | 46.5325 | <0.001 |
| B6 20 | 58.1075 | <0.001 |
| B6 25 | 63.2775 | <0.001 |
| Leishmania major LV39 | ||
| (MRHO/Sv/59/P strain) | ||
| B6 5 | 21.4925 | <0.014 |
| B6 10 | 29.775 | <0.023 |
| B6 15 | 29.92 | <0.003 |
| B6 20 | 36.8975 | <0.004 |
| B6 25 | 39.22 | <0.001 |
| LeishmaniadonovaniMS10 strain | ||
| B6 5 | 9.555 | <0.011 |
| B6 10 | 27.7925 | <0.004 |
| B6 15 | 37.5775 | <0.001 |
| B6 20 | 78.1645 | <0.001 |
| B6 25 | 85.925 | <0.001 |
DMSO: Dimethyl sulphoxide, NS: nonsignificant, SAG: sodium antimony gluconate
Table 1: Percentage of proliferation inhibition of leishmania promastigotes in respect to dmso control.
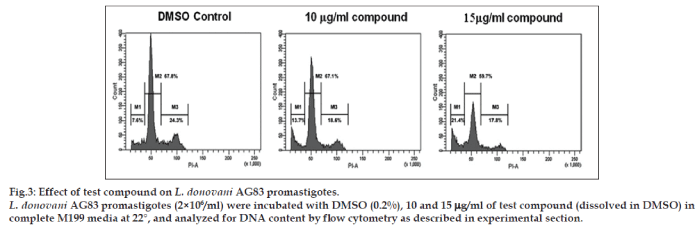
Cell-cycle analysis complemented the previous data obtained by MTT assay. It demonstrated that at 48 h of culture, both 10 and 15 μg/ml of test compound caused L. donovani promastigotes to remain as resting G0/G1 (M2) cells and inhibited their entry into the S phase (M3). The percent dead cells (M1) increased during this incubation period and growth arrest was also visible. Test compound at a concentration of 10 μg/ml started to block the entry of L. donovani promastigotes into S phase from G0/G1, however, at 15 μg/ml completely blocked the entry dose dependently (Table 2 and fig. 3). Both doses caused substantial increases in cell death, compared with DMSO treated cultures. Our results suggested that 29,30-dibromo-28-oxoallobetulin, 3 preferentially active against L. donovani promastigotes at inducing cell-cycle arrest followed by death in vitro.
| L. donovaniAG83 incubated with | M1 (sub G0/G1 or dead) (%) | M2 (G0/G1 or resting phase) (%) | M3 (S/G2/M or replicating phase) (%) |
|---|---|---|---|
| DMSO alone | 7.4 | 67.8 | 24.3 |
| 10 µg/ml compound | 13.7 | 67.1 | 18.6 |
| 15 µg/ml compound | 21.4 | 59.7 | 17.8 |
DMSO: Dimethyl sulphoxide
Table 2: The percentage of cells in different phases of life cycle after treatment with test compound.
We have developed a simple method for the synthesis of 29,30-dibromo-28-oxoallobetulin. The compound can inhibit the growth of L. donovani AG83 promastigote proliferation by 63.27% (P<0.001) in respect to DMSO control. At the same dose the conventional drug SAG inhibited only by 10.02% in respect to DMSO control. Our results suggested that compound 3 preferentially active against L. donovani promastigotes at inducing cell-cycle arrest followed by death in vitro . We assumed that the finding is significant as 3 is highly effective against the recently isolated Leishmania donovani from active visceral leishmaniasis patient and should be further investigated for its immunomodulatory consequences.
Acknowledgements
The authors thank Dr. Mitali Chatterjee, IPGMER, Kolkata, India for providing Leishmania donovani MS10 strain and Prof. Fabienne Tacchini-Cottier, Director, WHO-IRTC, University of Lausanne, Switzerland for a gift sample of Leishmania major LV39, MRHO/Sv/59/P strain.
References
- Desjeux P. Leishmaniasis: Current situation and new perspectives.Comp ImmunMicrobiolInfec Dis 2004;27:305-18.
- Report of the working group for communicable diseases in the 12th plan (WG-3.1 Communicable Diseases Report). Available from: http:// www.leishrisk.net. [Last accessed on 2013 Nov 07].
- Walton BC, Peters W, Killick-Kendrick R. The Leishmaniasis in Biology and Medicine. London: Academic Press; 1987. p. 636-44.
- Iwu MM, Jackson JE, Schuster BG. Medicinal plants in the fight against leishmaniasis. Parasitol Today 1994;10:65-8.
- Murray HW, Hariprashad J. Effect of oral atovaquone alone and in combination with antimony in experimental visceral leishmaniasis. Antimicrob Agents Chemother 1996;40:586-7.
- Dietze R, Carvalho SF, Valli LC, Berman J, Brewer T,Milhous W, et al. Phase 2 trial of WR6026, an orally administered 8-aminoquinoline, in the treatment of visceral leishmaniasis caused by Leishmaniachagasi. Am J Trop Med Hyg 2001;65:685-9.
- Chen M, Christensen SB, Theander TG, Kharazmi A. Antileishmanialactivity of licochalcone A in mice infected with Leishmania majorandin hamsters infected with Leishmaniadonovani. Antimicrob Agents Chemother 1994;38:1339-44.
- Croft SL, Neal RA, Thornton EA, Herrmann DB. Antileishmanial activity of the ether phospholipid ilmofosine.Trans R Soc Trop Med Hyg 1993;87:217-9.
- Berman JD, Keenan CM, Lamb SR, Hanson WL, Waits VB. Leishmaniadonovani: Oral efficacy and toxicity of formycin B in theinfected hamster. ExpParasitol 1983;56:215-21.
- Seifert K, Pérez-Victoria FJ, Stettler M, Sánchez-Cañete MP, Castanys S, Gamarro F, et al. Inactivation of the miltefosine transporter,LdMT, causes miltefosine resistance that is conferred to the amastigote stage of Leishmaniadonovani and persists in vivo. Int J Antimicrob Agents 2007;30:229-35.
- Pandey BD, Pandey K, Kaneko O, Yanagi T, Hirayama K. Relapse of visceral leishmaniasis after miltefosine treatment in a Nepalese patient. Am J Trop Med Hyg 2009;80:580-2.
- Mandal A, Ghosh S, Bothra AK, Nanda AK, Ghosh P. Synthesis of friedelantriterpenoid analogs with DNA topoisomerase IIα inhibitory activity and their molecular docking studies. Eur J Med Chem 2012;54:137-43.
- Ghosh P, Mandal A, Ghosh J, Pal C, Nanda AK. Synthesis of bioactive 28-hydroxy-3-oxolup-20(29)-en-30-al with antileukemic activity. J AsianNat Prod Res 2012;14:141-53.
- Chan-Bacab MJ, Peña-Rodríguez LM. Plant natural products with leishmanicidal activity. Nat Prod Rep 2001;18:674-88.
- Inocêncio da Luz RA, Vermeersch M, Deschacht M, Hendrickx S,Van Assche T, Cos P, et al. In vitro and in vivo prophylactic and curative activity of the triterpenesaponin PX-6518 against cutaneous Leishmania species. J AntimicrobChemother 2011;66:350-3.
- Lai TK, Biswas G, Chatterjee S, Dutta A, Pal C, Banerji J, et al.Leishmanicidal and anticandidal activity of constituents of Indian edible mushroom Astraeushygrometricus.ChemBiodivers 2 012;9:1517-24.
- Carlson RM. U.S. Patent US 6369101, 2002, (CA 2002, 136, 279583).
- Flekhter OB, Nigmatullina LR, Baltina LA, Karachurina LT, Galin FZ, Zarudii FS, et al. Synthesis of betulinic acid from betulin extract and study of the antiviral and antiulcer activity of some related terpenoids.Pharm Chem J 2002;36:484-7.
- Urban M, Sarek J, Kvasnica M, Tislerova I, Hajduch M. Triterpenoidpyrazines and benzopyrazines with cytotoxic activity. JNat Prod 2007;70:526-32.
- Zhan P, Liu X, Li Z, Fang Z, Li Z, Wang D, et al. Novel1,2,3-thiadiazole derivatives as HIV-1NNRTIs with improved potency: Synthesis and preliminary SAR studies. Bioorg Med Chem 2009;17:5920-7.
- Moreau P, Anizon F, Sanceleme M, Prodhomme M, Severe D, Riou JF, et al.Synthesis, mode of action, and biological activities ofrebeccamycinbromo derivatives. J Med Chem 1999;42:1816-22.
- Nofal ZM, El-Zahar MI, El-Karim SS. Derivatives with expected biological activity. Molecules 2000;5:99-113.
- Ghosh AK, Rakshit MM, Ghosh DK. Effect of berberine chloride onLeishmaniadonovani. Indian J Med Res 1983;78:407-16.
- Charmoy M, Megnekou R, Allenbach C, Zweifel C, Perez C, Monnat K, et al. Leishmania major induces distinct neutrophil phenotypes in mice that are resistant or susceptible to infection. J LeukocBiol 2007;82:288-99.
- Mallick S, Dutta A, Ghosh J, Maiti S, Mandal AK, Banerjee R, et al.Protective therapy with novel chromone derivative against Leishmaniadonovaniinfection induces Th1 response in vivo. Chemotherapy2011;57:388-93.
- Sahu NP, Pal C, Mandal NB, Banerjee S, Raha M, Kundu AP, et al. Synthesis of a novel quinoline derivative, 2-(2-methylquinolin-4-ylamino)-N-phenylacetamide a potential antileishmanial agent. Bioorg Med Chem 2002;17:1687-93.