- *Corresponding Author:
- Suvarna kini
Dept. of Pharmaceutical Chemistry, Manipal College of Pharmaceutical Sciences, MAHE, Manipal-576 104, India.
E-mail: suvarna.gk@manipal.edu
| Date of Submission | 2 September 2005 |
| Date of Revision | 19 July 2006 |
| Date of Acceptance | 21 January 2007 |
| Indian J Pharm Sci,2007, 69 (1) : 46-50 |
Abstract
The 2-arylsubstituted benzothiazole derivatives were synthesized by refluxing o-aminothiophenol with substituted benzoic acids in the presence of polyphosphoric acid at 220°. 2-Mercaptothiazole was used along with thionyl chloride to get the carbothioates. The physical and spectral data such as mp, Rf, IR, NMR was obtained for the synthesized compounds and the structures were confirmed. The screening for antitumour activity was done as per the National Cancer Institute drug screening strategy 3 compounds were found to be significantly cytotoxic as compared to [2-(3-bromo-4-aminophenyl) benzothiazole] against the human cervical cancer cell lines.
Introduction
The substituted benzothiazole derivatives have antitumor [1],vasodilator [2], antitubercular [3], antifungal [4], CNS [5] activities. The 2-(4-aminophenyl) benzothiazoles are a novel class of potent and selective antitumor agents and display a characteristic profile of cytotoxicity response across the cell lines; sensitive cell lines show GI50 values <10-8 M and insensitive cell lines >10-4 M [6]. Since they display potent and selective antitumor activity against interalia breast, ovarian, colon and renal cell lines, we thought it worthwhile to check for their antitumor activity against human cervical cancer cell lines. A compound NCB [2-(3-bromo-4-aminophenyl) benzothiazole showed substantial in-vitro activity against breast cancer cell lines MCF-7 (ER+) [6] NCB, 2-arylsubstituted benzothiazoles and their carbothioates with various substituents on the phenyl ring were therefore synthesized. Comparison of antitumor activities of the synthesized compounds against human cervical cancer cell lines was performed by in vitro cytotoxicity study on Ehrlich Ascitic Carcinoma (Tryphan blue exclusion assay) and MTT cell proliferation assay according to the protocol of ATCC (American Type Cell Culture collection) using human cervical cancer cell lines (SiHa). In vivo cytotoxicity study on Ehrlich Ascitic Carcinoma induced Swiss albino mice was also performed.
Materials and Methods
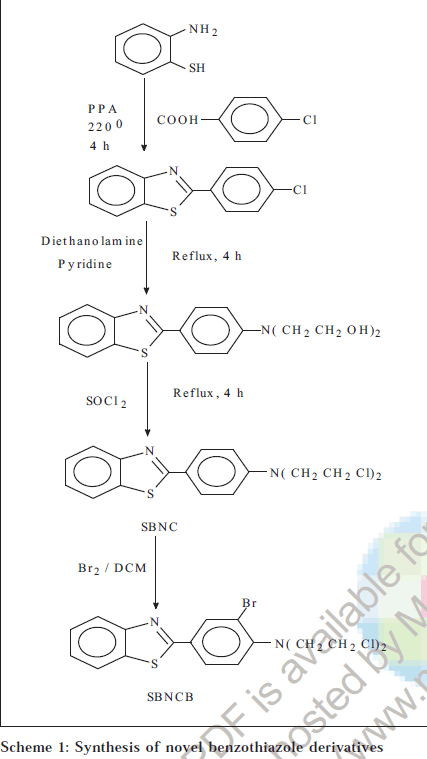
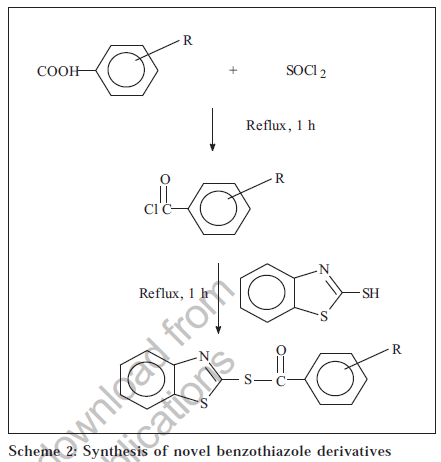
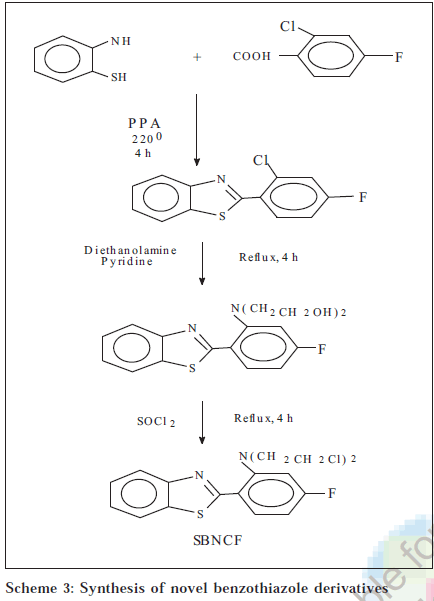
Melting points were determined on a Toshniwal Scientific melting point apparatus and are uncorrected; UV spectra were recorded on UV-1601 PC,UV/Vis spectrophotometer (Shimadzu). IR spectra were recorded in KBr disc on a FTIR 8300, KBr Press (Shimadzu) spectrometer at MCOPS, Manipal. 1H NMR spectra (DMSO-d6) were obtained from IISc Bangalore. The physical data of the derivatives are listed in Table 1. The scheme of synthesis is given in Schemes 1, 2 and 3.
| Compound name | Yield(%) | M.P.(°) | Rf | λmax | Molecular formula |
|---|---|---|---|---|---|
| SBCF | 74 | 105 | 0.9 | 295 | C13H7NSCl2F |
| SBNCF | 82 | 110 | 0.88 | 295 | C17H16N2SCl2F |
| SBNC | 63 | 111 | 0.91 | 341 | C17H16N2SCl2 |
| SBNCB | 72 | 114 | 0.89 | 304 | C17H16N2SCl2Br |
| SBSNO | 76 | 180 | 0.62 | 257 | C14H8N2S2O3 |
| SBSNH | 42 | 140 | 0.5 | 306 | C14H10N2S2O |
Table 1: Physical Data Of The Synthesized Compounds
2-(2-chloro-4-fluorophenyl)-benzothiazole (SBCF)
Equimolar quantities of o-aminothiophenol (0.04 mol) and 2-chloro-4-fluorobenzoic acid were added to 15 g of polyphosphoric acid and refluxed for 4 h at 220°. The reaction mixture was cooled and poured in ice cold 10% sodium carbonate solution. The precipitate was filtered and recrystallized from methanol. IR (KBr) cm-1: 3050.0 (ArH), 1593.1 (C=C), 1311.5 (C-N), 1691.5 (C=N), 1490.90 (C-C), 1047.3 (C-F), 719.4 (C-Cl), 1H NMR (DMSO-d6): δ ppm 7.18- 8.26 (m, 7H, ArH).
2-[4-(N,N-dichloroethylamino) phenyl] benzothiazole (SBNC)
Equimolar quantities of o-aminothiophenol (0.04 mol) and 4-chlorobenzoic acid were added to 15 g of polyphosphoric acid and refluxed for 4 h at 220°. The reaction mixture was cooled and poured in ice cold 10% sodium carbonate solution. The precipitate was filtered and recrystallized from methanol to get 2-(4’chlorophenyl)-benzothiazole. 0.01 mol of 2-(4’chlorophenyl)- benzothiazole and 0.01 mol of diethanolamine were dissolved in 25 ml of pyridine and refluxed for 4 h, cooled and poured in cold water. The mixture was filtered after 1 h and the precipitate recrystallized from methanol to get N-[4-(1,3- benzothiazol-2-yl)phenyl]-N,N-bis-(2-hydroxyethyl)amine. 0.01 mol of N-[4-(1,3-benzothiazol-2-yl)phenyl]-N,N-bis- (2-hydroxyethyl)amine was refluxed with 0.03 mol of thionyl chloride for 4 h. The excess of thionyl chloride was removed by distilling with benzene. After distillation, the residue was collected, washed with cold water and recrystallized from ethanol. IR (KBr) cm-1: 3053.1 (ArH), 1544.9 (C=C), 1313.4 (C-N), 1649.0 (C=N), 1433.0 (C-C), 2854.5 (C-H), 2920.0 (C-Cl). 1H NMR (DMSO-d6): δ ppm 7.20- 8.23 (m,8H, ArH), 2.5-3.5 [m, 8H, 2 (CH2 CH2)]
2-[3-bromo-4-(N,N-dichloroethylamino) phenyl] benzothiazole (SBNCB)
Equimolar quantity of 2-[4-(N,N-dichloroethylamino) phenyl] benzothiazole (0.04 mol) was dissolved in 50 ml of dichloromethane and bromine in 10 ml of dichloromethane was added to it. The reaction mixture was vigorously stirred for 2 min at -5°. Then 400 ml of cold water was added to it and stirred for 1 h the organic layer was shaken with sodium thiosulfate solution, washed several times with water and evaporated to get the crude product which was recrystallized from ethanol. IR (KBr) cm-1: 3057.1 (ArH), 1560.3 (C=C), 1311.5 (C-N), 1652.9 (C=N), 1425.3 (C-C), 2856.4 (C-H), 719.4 (C-Cl), 530.4 (C-Br). 1H NMR (DMSO-d6): δ ppm 7.15-8.27 (m,7H, ArH), 2.5-3.8 [m, 8H, 2 (CH2 CH2)].
2-[2-(N,N-dichloroethylamino)-4-fluorophenyl)-benzothiazole (SBNCF)
Equimolar quantities of o-aminothiophenol (0.04 mol) and 2-amino-4-fluorobenzoic acid were added to 15 g of polyphosphoric acid and refluxed for 4 h at 220°. The reaction mixture was cooled and poured in ice cold 10% sodium carbonate solution. The precipitate was filtered and recrystallized from methanol to get 2-(2’-amino-4’-fluorophenyl)-benzothiazole. 2-(2’-Amino-4’-fluorophenyl)-benzothiazole (0.01 mol) and 0.01 mol of diethanolamine were dissolved in 25 ml of pyridine and refluxed for 4 h, cooled and poured in cold water. The mixture was filtered after 1 h and the precipitate recrystallized from methanol to get 2-[2-(N,N-dihydroxyethylamino)-4-fluorophenyl)benzothiazole. 2-[2-(N,N-Dihydroxyethylamino)-4fluorophenyl)-benzothiazole (0.01 mol) was refluxed with 0.03 mol of thionyl chloride for 4 h. The excess of thionyl chloride was removed by distilling with benzene. After distillation, the residue was collected, washed with cold water and recrystallized from ethanol. IR (KBr) cm-1: 3058.9 (ArH), 1595.0 (C=C), 1311.5 (C-N), 1695.3 (C=N), 1425.3 (CC), 2922.0, 2822.5 (C-H), 1047.3 cm-1 (C-F). 1H NMR (DMSO-d6): δ ppm 7.13- 8.22 (m,7H, ArH), 2.5-3.9 [m, 8H, 2 (CH2 CH2)].
1,3-benzothiazol-2-yl-4-nitrobenzene carbothiaote (SBSNO)
A quantity equivalent to 0.01 mol of 4-nitrobenzoic acid and 0.04 mol of thionyl chloride were magnetically stirred and refluxed at 700 for 1 h. The excess of thionyl chloride was removed from the reaction mixture by distilling with benzene to get the acid chloride. The acid chloride (0.01 mol) and 0.01 mol of 2-mercaptobenzothiazole were dissolved in 25 ml pyridine and heated on water-bath for 15 min. The reaction mixture was cooled and poured in ice-cold water to get the precipitate that was later recrystallized from methanol.IR (KBr) cm-1: 3050.0 (ArH), 1602.7 (C=C), 1348.1 (C-N), 1425.3 (C-C), 1693.4 (C=O), 1320.0, 1531.4 (N-O). 1H NMR (DMSO-d6): δ ppm 7.13-8.28 (m, 8H, ArH).
1,3-benzothiazol-2-yl-4-aminobenzene carbothiaote (SBSNH)
A quantity equivalent to 0.01 mol of 4-aminobenzoic acid and 0.04 mol of thionyl chloride were magnetically stirred and refluxed at 70° for 1 h. The excess of thionyl chloride was removed from the reaction mixture by distilling with benzene to get the acid chloride. 0.01 mol of the acid chloride and 0.01 mol of 2- mercaptobenzothiazole were dissolved in 25 ml pyridine and heated on water-bath for 15 min. The reaction mixture was cooled and poured in ice-cold water to get the precipitate that was later recrystallized from methanol. IR (KBr) cm-1: 3024.2 (ArH), 1596.9 (C=C), 1336.6 (C-N), 1444.6 (C-C), 1703.0 (C=O), 3159.2, 3234.4 cm-1 (N-H). 1H NMR (DMSO-d6): δ ppm 7.14-8.22 (m, 8H, ArH).
In vitro cytotoxicity of compounds on EAC (Ehrlich Ascitic Carcinoma)
All the synthesized compounds were screened for antitumor activity [7] by tryphan blue exclusion method. The compounds were dissolved in DMSO to obtain the concentration of 1000 μg/ml. An aliquot (500 μl) of the EAC cell suspension in phosphate buffer saline (1×10-6cells per ml) was taken and 50 μl of the solution of the compounds was added to it. It was incubated at 37° for 4 h in 5% CO2 atmosphere. Then 25 μl of tryphan blue solution was added to it. The dead (blue coloured) and live (no colour) cells were counted in haemocytometer. The results are expressed as % cell death (Table 2).
| Compound | % Cell death |
|---|---|
| SBCF | 88 |
| SBNCF | 81 |
| SBNC | 91 |
| SBNCB | 92 |
| SBSNO | 44 |
| SBSNH | 90 |
Table 2: In Vitro Cytotoxicity Studies On Eac
In vitro cytotoxicity studies on human cervical cell lines
In vitro cytotoxicity studies were carried out on human cervical cell lines (SiHa). Cell suspension (100 μl×10-6 cells per ml) was transferred to each well of a 96 well flat bottom micro plate. It was incubated at 37° for 24 h in 5% CO2. Then 10 μl of concentration of drug at different concentrations were added and incubated for 44 h. MTT solution (20 μl) was added to each well and incubated for 4 h. Purple color was developed after incubation. Isopropyl alcohol: HCl (4 N) (1:100) mixture (100 μl) was added to each well. The absorbances were noted at 570 nm and at 630 nm and compared with control. The results are expressed as % cell death at different conc. viz. 1000, 500, 250, 100 μg/ml (Table 3)
| Compound | % Cell death at different concentration | |||
|---|---|---|---|---|
| 1000µg/ml | 500µg/ml | 250µg/ml | 100µg/ml | |
| NCB | 10 | 0 | 0 | 0 |
| SBNCB | 38 | 18 | 0 | 0 |
| SBCF | 18 | 2 | 0 | 0 |
| SBSNH | 25 | 5 | 0 | 0 |
| SBNCF | 11 | 0 | 0 | 0 |
| Control | 0 | 0 | 0 | 0 |
% cervical cell death produced by the benzothiazole derivatives at different concentrations. Zero indicates that no cells have died at that concentration
Table 3: In Vitro Cytotoxicity Studies On Human Cervical Cancer Cell Lines
Acute toxicity studies
The compounds were weighed and formulated into a suspension using 2% acacia. The solvent used was double distilled water. The required dose of the drugs was given daily for 9 d intraperitonially. The LD50 for all the compounds was found out.
In vivo studies on EAC induced Swiss Albino mice
The ascitic fluid from ascitic tumor bearing mice (donor) was injected intraperitonially to obtain ascitic tumor in the Swiss albino mice. The drug administration was started 24 h of the tumor inoculation. The drug was administered daily for 9 d and the mice were weighed on every day. The tumor response was assessed on the basis of mean survival time (MST) and % increase in life span (%ILS). %ILS={MST(treated)–MST(control)}×100/MST(control). As per the literature survey if the %ILS is more than 125% then the drug is considered to be an effective antitumor agent. For statistical analysis one way ANOVA with post hoc Scheffe’s test was applied to all the parameters. (Table 4)
| Compound | MST (Days) Mean± S.E.M | % ILS |
|---|---|---|
| Control | 18.83 ± 0.30 | 100 |
| Cisplatin | 37.33± 0.33a | 198.24 |
| SBCF | 25± 0.36a | 132.76 |
| SBNCB | 32.16± 0.30a | 170.79 |
| SBSNH | 29.33± 0.49a | 155.76 |
One way ANOVA by using SPSS 9.0 computer package. a -values in mean±SEM, p< 0.05
Table 4: Response Of Ehrlich Ascitic Carcinoma In Mice To Benzothiazole Derivatives
Results and Discussion
Screening for antitumor activity included measurement of % cell death of EAC by tryphan blue exclusion assay.The compounds SBCF, SBNCB, SBSNH were found to be significantly cytotoxic compared to the other derivatives. So, these were selected for in vivo screening of antitumor activity using Swiss mice. Cisplatin was the standard drug for comparison and it was found that SBNCB, SBCF, SBSNH had significantly increased the %ILS i.e., ILS>125%. The difference in the mean between cisplatin, SBCF, SBNCB, SBSNH groups and control groups was found to be significant (p> 0.05). At 1000 μg/ml, only SBNCB showed good activity on cell growth inhibition of cervical cancer cell lines. SBSNH and SBCF showed moderate cytotoxicity whereas others showed poor activity. At 500 μg/ml, the activity of all the compounds was reduced and at 250 μg/ml, no drug showed cytotoxicity.
The cytotoxicities of the compounds were compared with [2-(3-bromo-4-aminophenyl) benzothiazole] (NCB) due to similarity in structure and which is a potent antitumor drug against mammary cancer cell lines according to literature survey [1] . SBNCB, SBSNH, SBCF showed higher cytotoxicity than NCB.
The cytotoxicity of the compound containing N(CH2CH2Cl)2 at para position is more than the compounds having -NH2 at para position on the phenyl ring attached to the benzothiazole. Replacing chlorine in SBCF by -N(CH2CH2Cl)2 reduced the activity in in vitro assay on EAC cells to one half the original activity. Compounds with -NH2 at meta position on the phenyl ring decrease the cytotoxicity. Compounds with more than one halogen exhibited better cytotoxicity. Meta substituted derivatives were less cytotoxic. Nitro-substituted derivatives were the least cytotoxic of the compounds synthesized.
Acknowledgements
We acknowledge the Animal Ethical Committee for giving permission to carry out the antitumor activity on animals. We are grateful to Dr. Satyamurthy, Head of Biotechnology Dept., Science Centre, Manipal for helping us with the anticancer activity against the Human cervical cancer cell lines. We also acknowledge IISc, Bangalore for providing spectral details in time.
References
- Dong- Fang S., Bradshaw, T.D., Wrigley, S., McCall, C.J., Lelieveld, P., Fitchner, I. and Stevens, M.F.G., J. Med. Chem. , 1996, 39, 3375.
- Kochichiro, Y., Katsumi, G., Kazuya, Y., Tominori, M. and Goro, T., J. Med. Chem. , 1990, 33, 2192.
- Bhusari, K.P., Khadekar, P.B., Umathe, S.N., Bahekar, R.H. and Rao, A.R., Indian. J. Heterocycl. Chem. , 2000, 9, 213.
- Klein, L.L., Yeuns, C.M., Weissing, D.E., Lartey, P.A., Tonaka, S.K., Plattner, J.J. and Mulford, D.J., J. Med. Chem., 1994, 37, 572.
- Verma, R.S. and Singh, A.P., Indian. J. Chem. , 1998, 27B, 438.
- Mei-Sze C., Dong- Fang S., Wrigley, S., Bradshaw, T.D., and Hutchinson, I., Shaw, P.N., Barrett, D.A., Stanley, L.A. and Stevens, M.F.G., J. Med. Chem., 1999, 42, 381.
- Goldin, A., Schepartz, S.A., Venditti, J.M. and Devita, V.T., In; Methods in Cancer Research, Vol.16, Academic Press, 1979, 165.