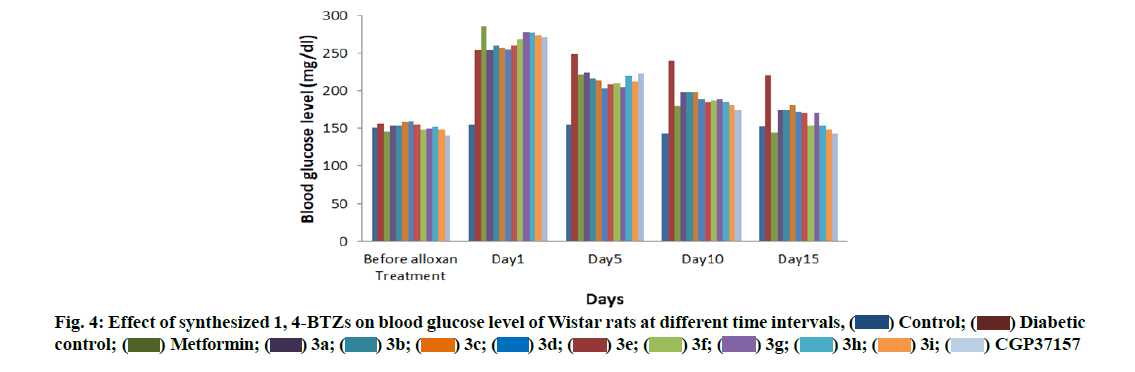- *Corresponding Author:
- Meena Tiwari
Department of Pharmacy,
Laboratory of Medicinal and Pharmaceutical Chemistry,
Shri Govindram Seksaria Institute of Technology and Science,
Indore,
Madhya Pradesh 452003,
India
E-mail:drmeenatiwari@gmail.com
| Date of Received | 30 March 2020 |
| Date of Revision | 18 July 2021 |
| Date of Acceptance | 17 October 2021 |
| Indian J Pharm Sci 2021;83(5):1033-1043 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study reported the synthesis and antihyperglycemic activity of 1,4-benzothiazepines (1,4-benzothiazepine-2-one derivatives) in alloxan-induced diabetic rats. 1,4-benzothiazepines (3a-i) were synthesized via Friedel-Crafts acylation, reduction and microwave assisted S-alkylation reactions and characterized by Fourier transform infrared spectroscopy, proton nuclear magnetic resonance, mass and elemental analysis. In silico absorption, distribution, metabolism, excretion and toxicity parameters of synthesized compounds 3a-i were found within their acceptable limits. In acute toxicity study, none of synthesized 1,4-benzothiazepines showed any toxicity risks such as tremor, convulsion, lacrimation, sedation, increased or decreased motor activity, etc., in Wistar rats. The antihyperglycemic activity showed that synthesized 1,4-benzothiazepines (3a-i) reduced the elevated blood glucose level significantly in Wistar rats. 3f, 3h and 3i with electronegative substitution at 7 position of benzothiazepine nucleus and at 2’ position of phenyl ring, showed better antihyperglycemic activity as compared to other 1,4-benzothiazepines having weak electronegative substituent at the same position. The antihyperglycemic activity of synthesized 1,4-benzothiazepines was comparable with antihyperglycemic activity of metformin and CGP37157. The present study may be helpful in development of novel antidiabetic agents.
Keywords
Diabetes, 1,4-benzothiazepine, Friedel-Craft acylation, S-alkylation, in silico absorption, distribution, metabolism, excretion and toxicity, antihyperglycemic activity
Diabetes mellitus is a clinical condition which accounts for about 90 % of diagnosed diabetic cases and is characterized by increased blood glucose level due to impaired insulin secretion, insulin action, insulin resistance and excessive hepatic gluconeogenesis [1,2]. Without enough insulin, body cells cannot take up sufficient glucose, which results in elevation of blood glucose level. Such conditions over long period of time can harm major organs such as liver, kidney, nerves, eyes, heart and blood vessels. Impairment in activity of some of these organs may lead to serious complications and even death [3].
Several classes of drugs are available to treat type-2 diabetes such as insulin secretagogues (sulfonylureas and meglitinides), insulin sensitizers (thiazolidinediones and biguanides), glucose uptake blockers (acarbose and pramlintide), insulin and modified insulin’s etc. Efficacy of these drugs is limited due to mechanism related side effects (i.e. hypoglycemia, weight gain, gastrointestinal distress etc.), inadequacy for use as monotherapy and development of tolerance [4-6]. Sulfonylureas lose efficacy as their prolonged use causes β-cell fatigue [4]. Thiazolidinediones banned in some countries due to serious cardiac ailments and bladder cancer [7,8]. Insulin secretagogues, when used as monotherapy stimulate insulin secretion under fasting condition and increase the risk of hypoglycemia [9]. Dipeptidyl peptidase-4 inhibitors or gliptins are also associated with pancreatitis as a risk [10-12]. Unfortunately, none of the oral hypoglycemic agent has been completely successful in controlling hyperglycemia and longterm complications. This provides an opportunity to medicinal chemists to explore other class of chemical compounds as novel antihyperglycemic agents which can treat type 2 diabetes effectively and safely.
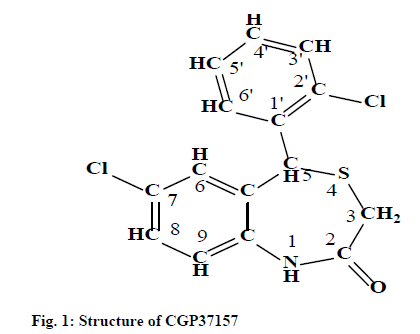
Compounds from 1,4-benzothiazepine-2-one and 1, 5-benzothiazepine-2-one chemical classes have been reported to inhibit mitochondrial sodium calcium exchange (mNCE) activity [13] (fig. 1). The mNCE inhibitors augment mitochondrial oxidative metabolism and stimulate glucose, stimulated insulin secretion in a glucose-dependent manner in rat islets and in a rat Insulinoma Cell Line-1 (INS-1) cell culture model [13-18], thus, can be explored as antihyperglycemic agent.
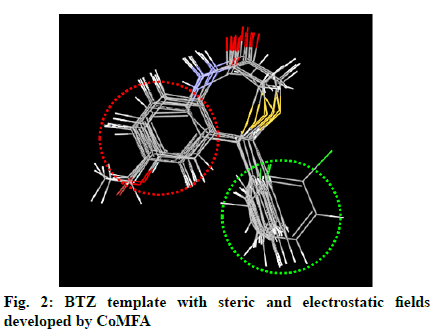
Previously, a Comparative Molecular Field Analysis (CoMFA) of Benzothiazepines (BTZs) as antidiabetic agents has been reported by our group [19]. As an ongoing effort, the present study reported the synthesis of some 1,4-BTZs and their antihyperglycemic activity was evaluated in Wistar rats. CGP37157, a potent mNCE inhibitor of 1,4-BTZ class was also synthesized and evaluated for its antihyperglycemic activity. Metformin was taken and reference antidiabetic drug and the antihyperglycemic activity of synthesized 1,4-BTZs was compared against it.
In the drug development process, most of the developed drug molecules failed in the clinical phases due to their poor pharmacokinetics and toxicity [20]. Thus, it is always beneficial to point out such failures at early stage of drug development. In this study, a preliminary drug-likeness, in silico Absorption, Distribution, Metabolism, Excretion and Toxicity (ADME/T) profiling and toxicity risk assessments (i.e. Mutagenicity (MUT), Tumorigenicity (TUM), Irritant (IRR) and Reproductive Effects (RPRD)) studies were performed to determine the pharmacokinetic and toxicity profile of synthesized 1,4-BTZs.
Materials and Methods
All reactions were carried out under anhydrous condition and in solvents dried over molecular sieves type 4 Å. The melting point (mp) of all synthesized 1, 4-BTZs was determined by open capillary method and is uncorrected. Reactions were monitored by Thin Layer Chromatography (TLC) using hexane:acetone (8:2) as mobile phase. Spots on TLC plate were detected in Ultraviolet (UV) cabinet. Compounds were purified by column chromatography. The wavelength of maximum absorption (λmax) of synthesized 1,4-BTZs was determined on Shimadzu 160A UV/Visible double beam spectrophotometer. Infrared (IR) spectra were recorded on Shimadzu Fourier Transform Infrared (FTIR)-8400S spectrophotometer. Proton Nuclear Magnetic Resonance (1H-NMR) spectra were recorded on Bruker II Avance NMR spectrometer in deuterated Chloroform (CDCl3). Chemical shift values (δ) were reported in Parts Per Million (ppm), where s, d, dd and m designate singlet, doublet, doublet of doublet and multiplet, respectively. Mass spectra were recorded on Bruker APEX III mass spectrometer and elemental analysis was done using Vario EL III CHNS elemental analyzer.
Synthesis of substituted benzophenones (1a-i):
Para substituted aniline (0.05 mol) was added with stirring to previously heated substituted-benzoyl chloride (0.11 mol) [21]. After 5 min, zinc chloride (0.064 mol) was added with continuous heating at 200°, until evolution of Hydrochloric Acid (HCl) gas ceased. After cooling the mixture, 50 ml of 3 N HCl was added cautiously with stirring and heated to reflux for 1 h. The hot acid layer was decanted to remove benzoic acid. The crude condensation product was dissolved in 30 ml of 75 % (v/v) sulfuric acid and refluxed again to complete the hydrolysis. The product formed was extracted with Methylene Chloride (CH2Cl2) and washed with HCl and sodium hydroxide solution to remove parasubstituted anilines and benzoic acid, respectively. The CH2Cl2 extract was filtered and dried to give substituted aminobenzophenones (1a-i), which was recrystallized with methanol.
Synthesis of substituted benzhydrols (2a-i):
Substituted aminobenzophenones (2.75 mmol) were dissolved in 10 ml of methanol. The solution was kept on an ice bath and Sodium Borohydride (NaBH4) 0.125 g was added with stirring. After 10-15 min, 15 ml of cold HCl (10 % v/v) was slowly added and stirred for 30 min. Crystals of substituted benzhydrols (2a-i) were collected by filtration and rinsed with small amount of cold water.
Synthesis of 1,4-BTZs (3a-i):
Basic alumina (7.0 g) was added to a mixture of substituted benzhydrols (3.75 mmol), methylthioglycolate (15.0 mmol) and trifluoroacetic acid (52.0 mmol) in a 50 ml flat bottom flask and subjected to microwave irradiation for 7 min at full power (800 W). The mixture was extracted with chloroform. The extract was concentrated under vacuum, redissolved in CH2Cl2, washed with saturated aqueous sodium carbonate and brine and passed through anhydrous sodium sulphate. Finally, the extract was concentrated under vacuum to obtain the final product, which was further purified by column chromatography. The physicochemical properties and spectral data of synthesized 1,4-BTZs are as follows:
7-methoxy-5-phenylbenzo [1, 4] thiazepin-2 (1H, 3H, 5H)-one (3a): A light brown solid product was formed by microwave assisted S-alkylation of 2-amino-5- methoxy benzhydrol; yield (%)=65.40; mp: 164-169°; Retention Factor (Rf) [hexane/acetone (8:2)]=0.67; λmax (nm): 336.6, 273.4; logP: 3.05; IR (Potassium Bromide (KBr), cm-1): 3286 (N-H), 3040 (C-H, argon (Ar)), 1681 (C=O), 1533 (C=C, Ar), 1183 (C-O-C); 1H-NMR (400 MHz, CDCl3, ppm): δ 7.33-7.09 (m, 6H, CH), 6.83 (d, J=1.3 Hz, 1H, CH), 6.78 (dd, J=7.4, 1.4 Hz, 1H, CH), 5.32 (s, 1H, NH), 5.08 (s, 1H, CH), 3.73 (s, 3H, Methyl Group (CH3)), 3.42 (d, J=12.4 Hz, 1H, CH), 3.23 (d, J=12.5 Hz, 1H, CH); calculated molecular weight: 285.36; found (Electron Ionization Mass Spectrum (EIMS)) mass to charge ratio (m/z): 285.08 (M+ peak), 286.09 (M+1 peak); molecular formula: C16H15NO2S, elemental analysis (%), calculated: C, 67.34; H, 5.30; N, 4.91; S, 11.24; found: C, 67.18; H, 5.23; N, 4.98; S, 11.02.
5-(2-chlorophenyl)-7-methoxybenzo [1,4]thiazepin- 2(1H, 3H, 5H)-one (3b): A white solid product was formed by microwave assisted S-alkylation of 2-amino- 5-methoxy-2’-chlorobenzhydrol; yield (%)=75.92, mp: 179-185°, Rf [hexane/acetone (8:2)]=0.73; λmax (nm): 339.2, 285.2; logP: 3.61; IR (KBr, cm-1): 3288 (N-H), 3025 (C-H, Ar), 1657 (C=O), 1498 (C=C, Ar), 1164 (C-O-C), 1085 (C-Cl); 1H-NMR (400 MHz, CDCl3, ppm): δ 7.78–7.02 (m, 7H, CH and NH), 6.19 (dd, J=7.5, 1.4 Hz, 1H, CH), 5.11 (s, 1H, CH), 3.78 (s, 3H, CH3), 3.31 (d, J=12.5 Hz, 1H, CH), 3.25 (d, J=12.5 Hz, 1H, CH); calculated molecular weight: 319.81; found (EIMS) m/z: 319.04 (M+ peak), 321.04 (M+2 peak); molecular formula: C16H14ClNO2S, elemental analysis (%), calculated: C, 60.09; H, 4.41; N, 4.38; S, 10.03; found: C, 59.98; H, 4.30; N, 4.32; S, 9.95.
7-methyl-5-phenylbenzo [1,4] thiazepin-2 (1H, 3H, 5H)-one (3c): A brown solid product was formed by microwave assisted S-alkylation of 2-amino-5- methyl-benzhydrol; yield (%)=68.91; mp: 170-175°; Rf [hexane/acetone (8:2)]=0.76; λmax (nm): 235.4, 312.4; logP: 3.67; IR (KBr, cm-1): 3292 (N-H), 3032 (C-H, Ar), 1676 (C=O), 1493 (C=C, Ar); 1H-NMR (400 MHz, CDCl3, ppm): δ 7.32–7.23 (m, 1H, CH), 7.21– 7.11 (m, 7H, CH), 6.94 (s, 1H, NH), 5.12 (s, 1H, CH), 3.31 (d, J=12.3 Hz, 1H, CH), 3.29 (d, J=12.5 Hz, 1H, CH), 2.43 (s, 3H, CH3); calculated molecular weight: 269.36; found (EIMS) m/z: 269.09 (M+ peak), 270.09 (M+1 peak); molecular formula: C16H15NOS, elemental analysis (%), calculated: C, 71.34; H, 5.61; N, 5.20; S, 11.90; found: C, 71.08; H, 5.69; N, 4.98; S, 11.69.
5-(2-chlorophenyl)-7-methylbenzo [1,4] thiazepin- 2(1H, 3H, 5H)-one (3d): A yellowish white solid product was formed by microwave assisted S-alkylation of 2-amino-5-methyl-2’-chlorobenzhydrol; yield (%)=78.39; mp: 184-192°; Rf [hexane/acetone (8:2)]=0.69, λmax (nm): 234.3, 301.2; logP: 4.22; IR (KBr, cm-1): 3288 (N-H), 3038 (C-H, Ar), 1651 (C=O), 1503 (C=C, Ar), 1078 (C-Cl); 1H-NMR (400 MHz, CDCl3, ppm): δ 7.89–7.13 (m, 7H, CH), 5.43 (s, 1H, NH), 5.13 (s, 1H, CH), 3.42 (d, J=12.5 Hz, 1H, CH), 3.29 (d, J=12.3 Hz, 1H, CH), 2.23 (s, 3H, CH3); calculated molecular weight: 303.81; found (EIMS) m/z: 303.05 (M+ peak), 305.05 (M+2 peak); molecular formula: C16H14ClNOS, elemental analysis (%), calculated: C, 63.25; H, 4.64; N, 4.61; S, 10.55; found: C, 63.12; H, 4.52; N, 4.39; S, 10.59.
7-bromo-5-phenylbenzo [1,4] thiazepin-2 (1H, 3H, 5H)-one (3e): A white solid product was formed by microwave assisted S-alkylation of 2-amino-5- bromo-benzhydrol; yield (%)=69.09; mp: 176-183°; Rf [hexane/acetone (8:2)]=0.81; λmax (nm): 265.2, 295.3; logP: 3.57; IR (KBr, cm-1): 3289 (N-H), 3042 (C-H, Ar), 1673 (C=O), 1499 (C=C, Ar), 1045 (C-Br); 1H-NMR (400 MHz, CDCl3, ppm): δ 7.62–7.52 (m, 2H, CH and NH), 7.43 (dd, J=7.5, 1.4 Hz, 1H, CH), 7.29- 7.13 (m, 6H, CH), 5.12 (s, 1H, CH), 3.42 (d, J=12.5 Hz, 1H, CH), 3.33 (d, J=12.5 Hz, 1H, CH); calculated molecular weight: 334.23; found (EIMS) m/z: 334.98 (M+ peak), 332.98 (M-2 peak); molecular formula: C15H12BrNOS; elemental analysis (%), calculated: C,53.90; H, 3.62; N, 4.19; S, 9.59; found: C, 53.56; H, 3.79; N, 4.01; S, 9.42.
7-bromo-5-(2-chlorophenyl) benzo [1,4] thiazepin- 2-(1H, 3H, 5H)-one (3f): A brownish white solid product was formed by microwave assisted S-alkylation of 2-amino-5-bromo-2’-chlorobenzhydrol; yield (%)=72.79; mp: 188-195°; Rf [hexane/acetone (8:2)]=0.71; λmax (nm): 312.3, 287.1; logP: 4.01; IR (KBr, cm-1): 3291 (N-H), 3048 (C-H, Ar), 1681 (C=O), 1512 (C=C, Ar), 1095 (C-Cl), 1060 (C-Br); 1H-NMR (400 MHz, CDCl3, ppm): δ 8.22 (s, 1H, NH), 7.46 (d, J=1.6 Hz, 1H, CH), 7.33 (dd, J=7.5, 1.5 Hz, 1H, CH), 7.32–6.22 (m, 5H, CH), 5.13 (s, 1H, CH), 3.43 (d, J=12.3 Hz, 1H, CH), 3.33 (d, J=12.3 Hz, 1H, CH); calculated molecular weight: 368.68; found (EIMS) m/z: 368.94 (M+ peak), 366.94 (M-2 peak); molecular formula: C15H11BrClNOS; elemental analysis (%), calculated: C, 48.87; H, 3.01; N, 3.80; S, 8.70, found: C, 48.45; H, 2.93; N, 3.59; S, 8.55.
7-fluoro-5-phenylbenzo [1,4] thiazepin-2(1H, 3H, 5H)-one (3g): A white solid product was formed by microwave assisted S-alkylation of 2-amino-5- fluoro-benzhydrol; yield (%)=72.79; mp: 169-176°; Rf [hexane/acetone (8:2)]=0.59; λmax (nm): 323.0, 239.4; logP: 2.89; IR (KBr, cm-1): 3261 (N-H), 3043 (C-H, Ar), 1656 (C=O), 1534 (C=C, Ar), 1195 (C-F); 1H-NMR (400 MHz, CDCl3, ppm): δ 7.78 (s, 1H, NH), 7.23-7.13 (m, 7H, CH), 7.02 (d, J=7.8, 1.3 Hz, 1H, CH), 5.13 (s, 1H, CH), 3.44 (d, J=12.4 Hz, 1H, CH), 3.33 (dt, J=12.4 Hz, 1H); calculated molecular weight: 273.33; found (EIMS) m/z: 273.06 (M+ peak), 274.03 (M+1 peak); molecular formula: C15H12FNOS; elemental analysis (%), calculated: C, 65.91; H, 4.43; N, 5.12; S, 11.73; found: C, 65.82; H, 4.21; N, 4.99; S, 11.34.
5-(2-chlorophenyl)-7-fluorobenzo [1,4] thiazepin- 2(1H, 3H, 5H)-one (3h): A yellow solid product was formed by microwave assisted S-alkylation of 2-amino- 5-fluoro-2’-chlorobenzhydrol; yield (%)=83.34; mp: 182-190°; Rf [hexane/acetone (8:2)]=0.68; λmax (nm): 287.3, 253.4; logP: 3.45; IR (KBr, cm-1): 3235 (N-H), 3053 (C-H, Ar), 1645 (C=O), 1543 (C=C, Ar), 1089 (C-Cl), 1184 (C-F); 1H-NMR (400 MHz, CDCl3, ppm): δ 7.92 (s, 1H, NH), 7.21 (dtdd, J=16.4, 14.5, 7.4, 1.6 Hz, 5H, CH), 7.09–6.92 (m, 2H, CH), 5.11 (s, 1H, CH), 3.34 (d, J=12.4 Hz, 1H, SH); calculated molecular weight: 307.77; found (EIMS) m/z: 307.2 (M+ peak), 309.02 (M+2 peak); molecular formula: C15H11ClFNOS; elemental analysis (%): C, 58.54; H, 3.60; N, 4.55; S, 10.42; found: C, 58.44; H, 3.34; N, 4.34; S, 10.32.
7-fluoro-5-(2-fluorophenyl)benzo [1,4] thiazepin- 2(1H, 3H, 5H)-one (3i): A white solid product was formed by microwave assisted S-alkylation of 2-amino- 5-fluoro-2’-fluorobenzhydrol; yield (%)=82.43; mp: 193-200°; Rf [hexane/acetone (8:2)]=0.65; λmax (nm): 245.3, 310.4; logP: 3.05; IR (KBr, cm-1): 3298 (N-H), 3037 (C-H, Ar), 1679 (C=O), 1530 (C=C, Ar), 1089 (C-Cl), 1232(C-F); 1H-NMR (400 MHz, CDCl3, ppm): δ 7.58 (s, 1H, NH), 7.19 (dd, J=9.9, 7.5, 6.2, 3.1 Hz, 4H, C-H, Ar), 7.11–6.97 (m, 3H, C-H, Ar), 5.11 (s, 1H, CH), 3.43 (d, J=12.4 Hz, 1H, CH), 3.32 (d, J=12.4 Hz, 1H, CH); calculated molecular weight: 291.32; found (EIMS) m/z: 291.05 (M+ peak), 292.06 (M+1 peak); molecular formula: C15H11F2NOS; elemental analysis (%), calculated: C, 61.84; H, 3.81; N, 4.81; S, 11.01; found: C, 61.34; H, 3.43; N, 4.45; S, 10.94.
7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1- benzothiazepin-2(3H)-one (CGP37157): A yellowish white solid product was formed by microwave assisted S-alkylation of 2-amino-5, 2’dichloro benzhydrol; yield (%)=88.34; mp: 125-130°; Rf [hexane/acetone (8:2)]=0.80, λmax (nm): 235.5, 287.3; logP: 3.85; IR (KBr, cm-1): 3298 (N-H), 3037 (C-H, Ar), 1679 (C=O), 1530 (C=C, Ar), 1089 (C-Cl); 1H-NMR (400 MHz, CDCl3, ppm): δ 7.90 (s, 1H, NH), 7.43 (d, J=1.4 Hz, 1H, CH), 7.33–6.89 (m, 6H, CH), 5.12 (s, 1H, CH), 3.42 (d, J=12.3 Hz, 1H, CH), 3.32 (d, J=12.5 Hz, 1H, CH); calculated molecular weight: 324.22; found (EIMS) m/z: 324.22(M+ peak), 322.99 (M-2 peak); molecular formula: C15H11Cl2NOS; elemental analysis (%), calculated: C, 55.57; H, 3.42; N, 4.32; S, 9.89, found: C, 55.65; H, 3.58; N, 4.61; S, 9.64.
Drug-likeness and in silico ADME/T study:
Drug-likeness of synthesized 1-4 BTZs was measured as per Lipinski’s Rule of Five (ROF) and Jorgensen’s Rule of Three (ROT), respectively [22,23]. As per Lipinski’s ROF the molecule should have molecular weight<500, octanol/water partition coefficient (logP)<5, number of Hydrogen Bond Donor (HBD)≤5, number of Hydrogen Bond Acceptor (HBA)≤10, while as per Jorgensen’s ROT the molecule should have aqueous solubility (Log S)>-5.7, predicted apparent Caco-2 cell permeability (Caco-2)>22 nm/s, number of primary metabolites (#metformin)<7. Molecules with a Polar Surface Area (PSA) less than 140 Å2 (angstroms squared) have good cell membranes permeating property and also possesses better drug-likeness [24]. In silico prediction of ADME properties and toxicity risks i.e. MUT and TUM, IRR and RPRD of compounds was done by Qikprop module of Schrodinger suite 2010 [25-28] and DataWarrior [29-31], respectively.
Biological evaluation:
Male Wistar rats weighing between 150-200 g were used for screening of antihyperglycemic activity of synthesized 1,4-BTZs [32,33]. All animals (Wistar rats) were maintained under 12 h light and 12 h dark cycle at 25° and given standard pellet diet (supplied by Godrej Agrowet Ltd., Sanwer road, Indore) and water ad libitum. The acclimatized animals were kept fasting for 24 h before experiment. All animal experiments were done using the protocols as per Institutional Animal Ethics Committee.
Acute toxicity study:
To determine the acute toxicity of synthesized 1,4-BTZs, different doses of 3a-i ranges from 100-500 mg/kg of body weight were administered to ten groups (contain three rats each) of Wistar rats [34]. Control group (also contains three rats) received 0.25 % carboxymethyl cellulose suspension only. All animal groups were observed for 24 h to detect any signs of acute toxicity such as tremor, convulsion, lacrimation, sedation, increased or decreased motor activity etc. No such signs were observed even after 24 h. Hence, the final dose of test compounds was fixed as 500 mg/kg. 1/10th of the final dose i.e. 50 mg/kg of body weight was taken as screening dose for evaluation of antihyperglycemic activity [32,35,36].
Antihyperglycemic activity:
The experimental animals were divided into four groups. Group I (control), II (diabetic control) and III contained six rats each. Group IV was subdivided in 10 subgroups (S1-S10), which also consisted of six rats each. Alloxan monohydrate (dissolved in sterile normal saline) was injected Intraperitoneally (i.p.) to all animal groups (except group I) at a dose of 120 mg/kg body weight. All animal groups kept for next 24 h on 10 % glucose solution to overcome early hypoglycemic phase. Serum glucose level was checked after 72 h. Animals with serum glucose levels >250 mg/dl were considered diabetic and were used for further study [36,37]. Group I and II animals were given vehicle only. Animals of group III were orally fed 0.25 % carboxymethyl cellulose suspension of metformin (50 mg/kg bodyweight) [38]. Subgroups (S1-S10) of group IV received 3a-i and CGP37157 (50 mg/kg bodyweight), respectively, as 0.25 % carboxymethyl cellulose oral suspension.
Fasting blood samples were collected from tail vein after 72 h of alloxan treatment (prior to administration of synthesized 1,4-BTZs) [39]. For biochemical study, animals were administered the same dose of synthesized 1,4-BTZs once daily for 15 d (counted after 72 h of alloxan treatment). Blood glucose level was measured on 1st d, 5th d, 10th d and 15th d by blood glucose monitoring system (Roche Diagnostics Corporation). Data obtained from antihyperglycemic activity was expressed as mean±Standard Error of Mean (SEM) and analyzed by Analysis of Variance (ANOVA) and post hoc Dunnett’s t-test. Differences between groups were considered significant at p<0.05 levels.
Results and Discussion
In the previous study, a CoMFA was carried out on a series of BTZs as antidiabetic agents [13], which resulted in the generation of various electrostatic and steric fields around the BTZ template (fig. 2). Subsequently, several BTZs were designed as potent antidiabetic agents [19]. In the present study, 1,4-BTZs, (3a-i) were synthesized and evaluated for antihyperglycemic activity.
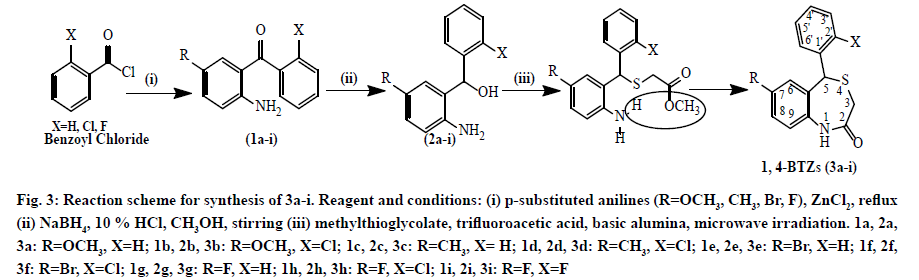
Compounds 3a-i was synthesized via three step processes (fig. 3). The first step involved Friedel-Crafts acylation of para-substituted anilines, which resulted in the formation of substituted benzophenones. IR bands at 3411 (N-H), 3314 (C-H, Ar), 1613 (C=O), 1532 (C=C, Ar) cm-1 confirmed the synthesis of substituted benzophenones.
Figure 3: Reaction scheme for synthesis of 3a-i. Reagent and conditions: (i) p-substituted anilines (R=OCH3, CH3, Br, F), ZnCl2, reflux (ii) NaBH4, 10 % HCl, CH3OH, stirring (iii) methylthioglycolate, trifluoroacetic acid, basic alumina, microwave irradiation. 1a, 2a, 3a: R=OCH3, X=H; 1b, 2b, 3b: R=OCH3, X=Cl; 1c, 2c, 3c: R=CH3, X= H; 1d, 2d, 3d: R=CH3, X=Cl; 1e, 2e, 3e: R=Br, X=H; 1f, 2f, 3f: R=Br, X=Cl; 1g, 2g, 3g: R=F, X=H; 1h, 2h, 3h: R=F, X=Cl; 1i, 2i, 3i: R=F, X=F
The second step was reduction of substituted benzophenones to corresponding alcohols by NaBH4. NaBH4 (a complex metal hydride) is useful reagent for reduction of aldehydes and ketones [40]. The formation of benzhydrols was confirmed by IR through disappearance of ketone band at 1680 cm-1 and appearance of -OH band at 3400-3600 cm-1.
The final step was microwave-assisted S-alkylation of substituted benzhydrols. The conventional reaction for synthesis of benzothiazepinones takes approximately 72 h to complete via S-alkylation of thioglycolic acid with 2-aminobenzhydrol in presence of HCl, followed by cyclization. In contrast, microwave irradiation method takes less time in completion of reaction (about 20-25 min) with higher yield. Synthesized 1,4-BTZs were purified by column chromatography on a silica gel column using a mixture of hexane and acetone as an eluent. CGP37157 was also synthesized according to the same reaction scheme and purified by column chromatography.
Formation of the 1,4-BTZs was confirmed by above spectral data. IR bands between 3010-3050 cm-1 (C-H, Ar) and 1600-1475 cm-1 (C=C, Ar) confirmed the presence of aromatic rings. Bands near 3300 cm-1 (N-H) and between 1680-1630 cm–1 (C=O) confirmed the presence of lactam ring with amide functionality. Aryl chlorides, aryl fluorides and aryl bromides were confirmed by bands between 1100-1035 cm-1 ,1250-1100 cm-1 and 1075-1030 cm-1, respectively [41]. Chemical shift values (δ, ppm) 3.36-3.46 (doublet) for CH2 group at position 3, 5.10 (singlet) for CH group at position 5, 5-8.5 (singlet) for 1-NH and 6.5 -7.5 (multiplet) for CH group at position 6, 8, 9, 2’, 3’, 4’ and 5’ confirmed that 1,4-BTZs have been synthesized. 3a and 3b showed chemical shift value for 7-methoxy substituent between δ 3.79-3.85 whereas 3c and 3d showed specific chemical shift values for 7-methyl group and 1-NH group between δ 2.30-2.44 and δ 5.47-7.00, respectively. The molecular weight of the synthesized 1,4-BTZs was confirmed by mass spectrum. The presence of aryl halides was confirmed by presence of M+2 peak in the mass spectrum of 3b, 3d, 3f and 3h. Further, agreement between calculated and found values of elemental analysis confirmed the formation of 3a-i and CGP37157. Synthesized 1,4-BTZs were searched for structural similarity on various chemical databases like ChemSpider, Chemfrog, iScienceSearch and ChemDB portal. No similar structure was found for any of the synthesized compound (Table 1).
| Compound No. | Query | Structure search message |
|---|---|---|
| 3a | 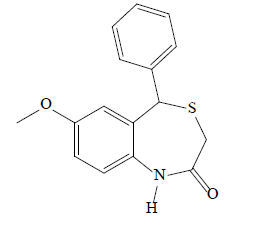 |
ChemSpider: 0 hit Chemfrog: 0 hit iScienceSearch: 0 hit ChemDB portal: 0 hit |
| 3b | 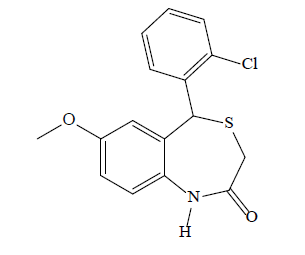 |
ChemSpider: 0 hit Chemfrog: 0 hit iScienceSearch: 0 hit ChemDB portal: 0 hit |
| 3c |  |
ChemSpider: 0 hit Chemfrog: 0 hit iScienceSearch: 0 hit ChemDB portal: 0 hit |
| 3d |  |
ChemSpider: 0 hit Chemfrog: 0 hit iScienceSearch: 0 hit ChemDB portal: 0 hit |
| 3e |  |
ChemSpider: 0 hit Chemfrog: 0 hit iScienceSearch: 0 hit ChemDB portal: 0 hit |
| 3f | 
| ChemSpider: 0 hit Chemfrog: 0 hit iScienceSearch: 0 hit ChemDB portal: 0 hit |
| 3g | 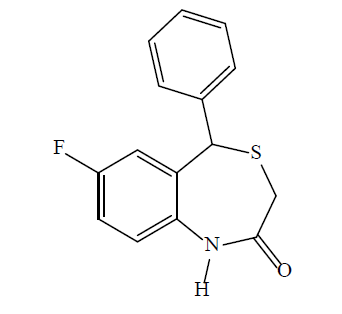 |
ChemSpider: 0 hit Chemfrog: 0 hit iScienceSearch: 0 hit ChemDB portal: 0 hit |
| 3h | 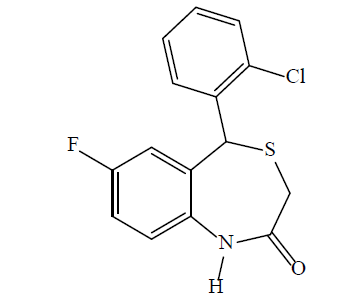 |
ChemSpider: 0 hit Chemfrog: 0 hit iScienceSearch: 0 hit ChemDB portal: 0 hit |
| 3i | 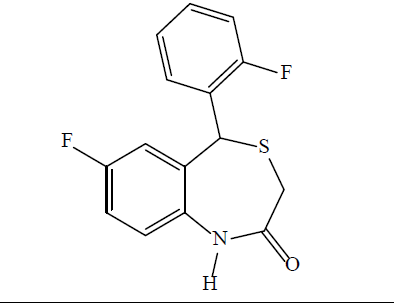 |
ChemSpider: 0 hit Chemfrog: 0 hit iScienceSearch: 0 hit ChemDB portal: 0 hit |
Synthesized compounds were searched on PubChem, ChemSpider, Chemfrog, iScienceSearch and ChemDB portal databases for similarity
Table 1: Structure Search Message For Synthesized Compounds From Various Chemical Databases
Compounds with fewer (and preferably no) violations of Lipinski’s ROF and Jorgensen’s ROT are more likely to be orally available and the synthesized compounds 3a-i have shown no violations of these rules (Table 2). All the synthesized 1,4-BTZs showed values of ADME parameters i.e. log S, #metformin (number of metabolic reactions), Central Nervous System (CNS) activity, Caco-2 permeability (permeability through intestinal epithelium), ability to cross Blood Brain Barrier (LogBB), permeability through skin (LogKp), serum protein binding (LogKhsa) and Percent Human Oral Absorption (% HOA), within reference limits (Table 3). In silico toxicity study showed that all synthesized compounds have low toxicity risks for MUT, TUM, IRR and RPRD (Table 4).
| Compound | Molecular weight | HBD | HBA | LogP | PSA | ROF | ROT |
|---|---|---|---|---|---|---|---|
| 3a | 285.36 | 1 | 3.75 | 3.234 | 46.179 | 0 | 0 |
| 3b | 319.805 | 1 | 3.75 | 3.524 | 46.156 | 0 | 0 |
| 3c | 269.361 | 1 | 3 | 3.189 | 38.908 | 0 | 0 |
| 3d | 303.806 | 1 | 3 | 3.628 | 38.914 | 0 | 0 |
| 3e | 334.23 | 1 | 3 | 3.53 | 38.896 | 0 | 0 |
| 3f | 368.675 | 1 | 3 | 3.972 | 38.853 | 0 | 0 |
| 3g | 273.324 | 1 | 3 | 3.113 | 38.893 | 0 | 0 |
| 3h | 307.769 | 1 | 3 | 3.564 | 38.865 | 0 | 0 |
| 3i | 291.315 | 1 | 3 | 3.278 | 38.844 | 0 | 0 |
| CGP37157 | 324.224 | 1 | 3 | 3.891 | 38.835 | 0 | 0 |
| Metformin | 129.164 | 5 | 3.5 | -0.746 | 91.858 | 0 | 0 |
| RR | <500 | <5 | <10 | -2 to 6.5 | <140 | <4 | <3 |
Note: ROF: Lipinski’s rule of five violations; ROT: Jorgensen’s rule of three violations; RR: Reference range for 95 % of known drugs
Table 2: Drug Likeness Study Of Synthesized Compounds
| Compound | Log S | CNS | Caco-2 | LogBB | LogKp | LogKhsa | % HOA | #Metformin |
|---|---|---|---|---|---|---|---|---|
| 3a | -4.298 | 0 | 2157.59 | -0.03 | -1.789 | 0.29 | 100 | 5 |
| 3b | -4.499 | 1 | 2212.27 | 0.102 | -1.848 | 0.346 | 100 | 5 |
| 3c | -4.261 | 1 | 2156.17 | 0.055 | -1.88 | 0.373 | 100 | 5 |
| 3d | -4.656 | 1 | 2199.37 | 0.169 | -1.963 | 0.454 | 100 | 5 |
| 3e | -4.507 | 1 | 2156.73 | 0.248 | -1.857 | 0.349 | 100 | 4 |
| 3f | -5.212 | 1 | 2167.57 | 0.317 | -1.94 | 0.467 | 100 | 4 |
| 3g | -3.982 | 1 | 2157.12 | 0.19 | -1.826 | 0.249 | 100 | 4 |
| 3h | -4.49 | 1 | 2211.75 | 0.296 | -1.886 | 0.343 | 100 | 4 |
| 3i | -4.197 | 1 | 2165.02 | 0.243 | -1.881 | 0.272 | 100 | 4 |
| CGP37157 | -4.968 | 1 | 2146.06 | 0.358 | -1.97 | 0.425 | 100 | 4 |
| Metformin | -0.556 | -2 | 211.107 | -1.137 | -6.414 | -0.934 | 64.179 | 2 |
| RR | -6.5 to 0.5 | -2 to 2 | <25 poor >500 good |
-3 to 1.2 | -8 to -1 | ±1.5 | <25 poor | 1 to 8 |
RR: Reference range for 95 % of known drugs
Table 3: In Silico Adme Profiling Of Synthesized 1, 4-Btzs
| Compound | Toxicity risks | |||
|---|---|---|---|---|
| MUT | TUM | RPRD | IRR | |
| 3a | None | None | None | None |
| 3b | None | None | None | None |
| 3c | None | None | None | None |
| 3d | None | None | None | None |
| 3e | None | None | None | None |
| 3f | None | None | None | None |
| 3g | None | None | None | None |
| 3h | None | None | None | None |
| 3i | None | None | None | None |
| CGP37157 | None | None | None | None |
| Metformin | High | None | High | None |
MUT: mutagenicity; TUM: tumorigenicity; RPRD: reproductive effects; IRR: irritant
Table 4: In Silico Toxicity Risk Assessment Of Synthesized 1, 4-Btzs
Administration of alloxan (120 mg/kg, i.p.) led to 1.5-fold elevation of fasting blood glucose level of Wistar rats, which was maintained over a period of 15 d. 2 w administration of various derivatives led to dose dependent fall in elevated blood glucose level in Wistar rats. Effect reach maximum in 15 d of administration. Vehicle controlled animals had stable blood glucose level while diabetic animals which were treated with 3a-i showed significant reduction in elevated blood glucose level during 15 d (Table 5).
| Average Blood glucose level (mg/dl)* | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | Subgroup | Animal treatment | Before alloxan treatment | d 1 | d 5 | d 10 | d 15 | % R |
| I | - | Control | 151.3±1.1 | 154.9±0.4 | 155.5±1.3 | 143.4±1.2 | 152.9±1.1 | 1.3 |
| II | - | Diabetic control | 155.7±0.9 | 253.1±0.5 | 248.8±1.6 | 240.0±1.0 | 220.6±0.9 | 12.9 |
| III | - | Metformin | 145.4±0.5 | 285.2±1.4 | 222.2±0.5 | 178.9±1.9 | 144.3±2.3 | 49.4 |
| IV | S1 | 3a | 154.3±1.2 | 253.3±1.8 | 223.9±0.8 | 198.7±2.3 | 173.7±0.5 | 31.4 |
| S2 | 3b | 154.2±1.1 | 260.5±2.2 | 216.4±0.6 | 198.3±2.5 | 174.4±1.3 | 33.0 | |
| S3 | 3c | 158.7±1.2 | 257.1±2.0 | 213.7±0.8 | 198.7±1.4 | 180.3±1.9 | 29.8 | |
| S4 | 3d | 159.4±0.8 | 255.1±1.1 | 202.8±0.6 | 189.3±0.9 | 171.7±1.5 | 32.7 | |
| S5 | 3e | 154.8±0.5 | 259.7±1.2 | 208.5±1.1 | 184.7±0.8 | 170.5±0.8 | 34.3 | |
| S6 | 3f | 148.3±1.5 | 268.3±1.8 | 210.0±1.0 | 187.2±2.0 | 154.5±0.7 | 42.4 | |
| S7 | 3g | 150.2±1.3 | 278.3±1.9 | 205.3±1.2 | 189.4±2.1 | 170.9±1.7 | 38.6 | |
| S8 | 3h | 152.1±0.9 | 276.7±0.9 | 219.5±0.9 | 184.7±0.9 | 153.7±0.9 | 44.5 | |
| S9 | 3i | 149.2±0.4 | 273.5±0.5 | 212.4±0.5 | 180.7±0.8 | 148.2±1.5 | 45.8 | |
| S10 | CGP37157 | 140.3±0.8 | 270.3±1.1 | 223.0±1.2 | 173.8±0.6 | 143.2±1.2 | 47.0 | |
Note: *Values are mean±Standard Deviation (SD), p<0.05 when compared with the values of control
Table 5: Effect Of 1, 4-Btzs On Blood Glucose Level Of Wistar Rats At Different Intervals
Percent reduction in elevated blood glucose level (% R) was calculated for individual compounds using the following formula:
% R=Initial blood glucose level (on d 1)–Final blood glucose level (on d 15)/Initial blood glucose level (on d 1)×100
On the basis of % R, a comparison was made between all experimental groups. CGP37157, which contains Cl (chloro) group at 7 and 2’ position, showed highest % R value, which was comparable with % R value of metformin. 3h and 3i with F (flouro) group was equipotent as CGP37157. As the F was replaced with Br (bromo) (3e and 3f), % R value was decreased due less electronegativity of Br as compared to F. As the Cl at 7 position was replaced with methoxy (OCH3) and CH3, % R was reduced (3a, 3b, 3c and 3d).Removal of Cl at 2’ position resulted in less potent compounds (3a: 31.4 %, 3c: 29.8 % and 3e: 34.3 %) as compared to compounds containing 2’ Cl substitution (3b: 33.0 %, 3d: 32.7 % and 3f: 42.4 %). Antidiabetic activity of the synthesized 1,4-BTZs at different intervals is presented by a bar graph (fig. 4). The study suggested that compounds containing electron withdrawing groups at position 7 and 2’ were more active than compounds having electron donating groups at same position.
Based on the information obtained from the previous studies that BTZ nucleus has antidiabetic potential, the present study was aimed to perform the synthesis and evaluation of antihyperglycemic activity of 1,4-BTZs (3a-i). The spectral data confirmed the formation of 3a-i. Results of antihyperglycemic study in alloxaninduced diabetic rats showed that synthesized 1,4-BTZs reduce the elevated blood glucose level to an optimum level. It was revealed that electronegative substitution at 7 and 2’ position of 1,4-BTZs was favorable for antihyperglycemic activity. From the above results, it was concluded that this study may be helpful in development of some novel antidiabetic agents.
Acknowledgements:
Authors are thankful to Director of Shri Govindram Seksaria Institute of Technology and Science, Indore for providing necessary facilities for research, Head of Department, School of Pharmacy, Devi Ahilya Vishwavidyalaya, Indore for biological evaluation and IPCA Laboratories Ltd. Ratlam, Madhya Pradesh for providing gift sample of metformin.
Conflicts of interest:
The authors declared no conflict of interest.
References
- Kenny SJ, Aubert RE, Geiss LS. Prevalence and incidence of non-insulin-dependent diabetes. Diabetes 1995;2:47-67.
- Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, et al. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes care 2020;43(2):487-93.
- Pari L, Amarnath Satheesh M. Antidiabetic effect of Boerhavia diffusa: effect on serum and tissue lipids in experimental diabetes. J Med Food 2004;7(4):472-6.
- Krentz AJ, Ferner RE, Bailey CJ. Comparative tolerability profiles of oral antidiabetic agents. Drug Saf 1994;11(4):223-41.
- DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med 2000;133(1):73-4.
- Nuss JM, Wagman AS. Recent advances in therapeutic approaches to type 2 diabetes. Annu Rep Med Chem 2000;35:211-20.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356(24):2457-71.
- Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry CP, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 2011;34(4):916-22.
- Davis S, Alonso MD. Hypoglycemia as a barrier to glycemic control. J Diabetes Complications 2004;18(1):60-8.
- Matveyenko AV, Dry S, Cox HI, Moshtaghian A, Gurlo T, Galasso R, et al. Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: interactions with metformin. Diabetes 2009;58(7):1604-15.
- Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes 2013;62(7):2595-604.
- Egan AG, Blind E, Dunder K, de Graeff PA, Hummer BT, Bourcier T, et al. Pancreatic safety of incretin-based drugs-FDA and EMA assessment. N Engl J Med 2014;370(9):794-7.
- Pei Y, Lilly MJ, Owen DJ, D'Souza LJ, Tang XQ, Yu J, et al. Efficient syntheses of benzothiazepines as antagonists for the mitochondrial sodium−calcium exchanger: potential therapeutics for type II diabetes. J Org Chem 2003;68(1):92-103.
- Cox DA, Matlib MA. Modulation of intramitochondrial free Ca2+ concentration by antagonists of Na+-Ca2+ exchange. Trends Pharmacol Sci 1993;14(11):408-13.
- Siegel EG, Wollheim CB, Renold AE, Sharp GW. Evidence for the involvement of Na/Ca exchange in glucose-induced insulin release from rat pancreatic islets. J Clin Invest 1980;66(5):996-1003.
- Van Eylen F, Antoine MH, Lebrun P, Herchuelz A. Inhibition of Na/Ca exchange stimulates insulin release from isolated rat pancreatic islets. Fundam Clin Pharmacol 1994;8(5):425-9.
- Lee B, Miles PD, Vargas L, Luan P, Glasco S, Kushnareva Y, et al. Inhibition of mitochondrial Na+-Ca2+ exchanger increases mitochondrial metabolism and potentiates glucose-stimulated insulin secretion in rat pancreatic islets. Diabetes 2003;52(4):965-73.
- Anderson C, Davis R, Ghosh S, Pei Y. Treatment of diabetes mellitus by inhibition of mitochondrial calcium/sodium antiporter. Mitokor US 20020082193A1 2002:1-23.
- Dasoondi AS, Singh V, Voleti SR, Tiwari M. Comparative molecular field analysis of benzothiazepine derivatives: Mitochondrial sodium calcium exchange inhibitors as antidiabetic agents. Indian J Pharm Sci 2008;70(2):186.
- Tsaioun K, Kates SA. De-risking drug discovery programmes early with ADMET. In: Kapetanovic IM, ed. Drug discovery and development-Present and future: InTech, Janeza, Croatia; 2011. p. 275-94.
- Sternbach LH, Fryer RI, Metlesics W, Sach G, Stempel A. Quinazolines and 1, 4-benzodiazepines. V. o-aminobenzophenones1a, b. J Org Chem 1962;27(11):3781-8.
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 1997;23(1-3):3-25.
- Congreve M, Carr R, Murray C, Jhoti H. A rule of three for fragment-based lead discovery?. Drug Discov Today 2003;8(19):876-7.
- Ghose AK, Viswanadhan VN, Wendoloski JJ. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J Comb Chem 1999;1(1):55-68.
- QikProp 3.3. Rapid ADME predictions of drug candidates. Schrodinger-Suite LLC: New York; 2010.
- Ioakimidis L, Thoukydidis L, Mirza A, Naeem S, Reynisson J. Benchmarking the reliability of QikProp. Correlation between experimental and predicted values. QSAR Comb Sci 2008;27(4):445-56.
- Srivastav VK, Tiwari M, Zhang X, Yao XJ. Synthesis and antiretroviral activity of 6-acetyl-coumarin derivatives against HIV-1 infection. Indian J Pharm Sci 2018;80(1):108-17.
- Ntie-Kang F. An in silico evaluation of the ADMET profile of the StreptomeDB database. Springerplus 2013;2(1):1-1.
- Sander T, Freyss J, von Korff M, Rufener C. DataWarrior: an open-source program for chemistry aware data visualization and analysis. J Chem Inf Model 2015;55(2):460-73.
- Srivastav VK, Tiwari M. k-nearest neighbor molecular field analysis based 3D-QSAR and in silico ADME/T studies of cinnamoyl derivatives as HIV-1 integrase inhibitors. Med Chem Res 2015;24(2):684-700.
- Sander T. OSIRIS property explorer. Organic Chemistry Portal; 2001.
- Verma L, Khatri A, Kaushik B, Patil UK, Pawar RS. Antidiabetic activity of Cassia occidentalis (Linn) in normal and alloxan-induced diabetic rats. Indian J Pharmacol 2010;42(4):224.
- Sachan N, Thareja S, Agarwal R, Kadam SS, Kulkarni VM. Substituted biphenyl ethanones as antidiabetic agents: synthesis and in-vivo screening. Int J Pharm Tech Res 2009;1:625-31.
- Tenpe CR, Yeole PG. Comparative evaluation of antidiabetic activity of some marketed polyherbal formulations in alloxan induced diabetic rats. Int J Pharm Tech Res 2009;1(1):43-9.
- Mariappan G, Saha BP, Datta S, Kumar D, Haldar PK. Design, synthesis and antidiabetic evaluation of oxazolone derivatives. J Chem Sci 2011;123(3):335-41.
- Litchfield JJ, Wilcoxon FR. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther 1949;96(2):99-113.
- Perfumi M, Tacconi R. Antihyperglycemic effect of fresh Opuntia dillenii fruit from Tenerife (Canary Islands). Int J Pharmacogn 1996;34(1):41-7.
- Waisundara VY, Hsu A, Huang D, Tan BK. Scutellaria baicalensis enhances the anti-diabetic activity of metformin in streptozotocin-induced diabetic Wistar rats. Am J Chin Med 2008;36(03):517-40.
- Pattan SR, Suresh CH, Pujar VD, Reddy VV, Rasal VP, Koti BC. Synthesis and antidiabetic activity of 2-amino [5'(4-sulphonylbenzylidine)-2, 4-thiazolidinedione]-7-chloro-6-fluorobenzothiazole. Indian J Chem Sect B 2005;44B:2404-8.
- Carey F. Reduction of ketones: Preparation of benzhydrol. 7th ed. Organic Chemistry. McGraw-Hill 2008;15:622-8.
- Pavia DL, Lampman GM, Kriz GS. Introduction to spectroscopy: A Guide for students of organic chemistry. 3rd ed. USA: Thomson Learning, Inc.; 2001:29-82.