- *Corresponding Author:
- R. C. R. Jala
Academy of Scientific and Innovative Research, Ghaziabad-201 002, India
E-mail: jrcreddy10@gmail.com
| Date of Submission | 01 July 2017 |
| Date of Revision | 12 April 2018 |
| Date of Acceptance | 22 October 2018 |
| Indian J Pharm Sci 2018;80(6):1143-1150 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In the present study, acetate-protected monogalactosyldiacylglycerol and its fatty acid and aromatic acid analogues were synthesized using trichloroacetimidate methodology. All compounds synthesized were evaluated for cytotoxicity against four cancer cell lines and one normal cell line. Among the compounds tested, mixed acid compound 7b exhibited good activity with IC50 value of 27.5 μM. In a similar manner, the combination of unsaturated oleic acid and cinnamic acid compound 7d also exhibited significant activity with IC50 value 34.9 μM. Based on these results, it could be concluded that the combination of oleic acid with cinnamic acid type acid and other saturated fatty acids enhance the cytotoxicity of glycoglycerolipids.
Keywords
Glycoglycerolipids, cinnamic acid, fatty acids, cytotoxicity, trichloroacetimidate
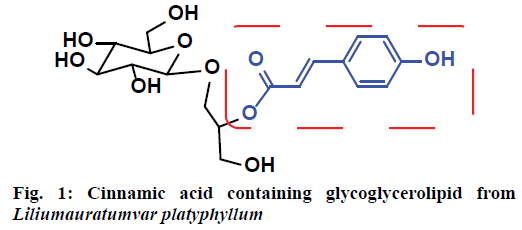
Glycoglycerolipids are important molecules from a cancer chemoprevention perspective due to their promising inhibitory effect on tumour growth. They are major constituents present in chloroplast membranes of photosynthetic cells and in bacterial cell walls[1-3]. Various glycoglycerolipid types were earlier isolated from different sources like bacteria[4], algae[5] and plants[6]. The fatty acyl chain length, its position, and the nature of the sugar moiety are some of the important features that influence cytotoxicity[7,8]. Glycoglycerolipids have gained significant attention in view of their diverse properties such as antitumor[4-6,9-11], antimicrobial[12], antiviral[13,14], antiinflammatory[15], immunosuppressive[16] and oxygen scavenging[17] activities. However, all these activities are strictly dependent on their fatty acyl chain length[18,19] and olefinic nature[13]. These types of glycolipids effectively inhibited the cell proliferation and induce apoptosis in cancer cells via an intrinsic pathway by activating caspase 3 and 9[20]. Moreover, recent research on glycolipids demonstrated that these are functional molecules involved in a number of cellular physiological pathways, which provided a novel area of targets for cancer immunotherapy[21]. This finding led to the development of a number of vaccines that are showing promising results in recent clinical trials. A few reports[22] were published on synthesis and antitumor activity of glycoglycerolipids derivatives with aromatic acids (with saturated side chain). These reports demonstrated that the presence of aromatic groups have a negative impact on activity whereas unsaturated and conjugated fatty acids showed a positive impact[20]. However, all the reported aromatic acids were not the cinnamic acids with unsaturated side chain. Based on this key observation, it was speculated that the glycoglycerolipids with the combination of cinnamic acids and unsaturated fatty acids might exhibit good cytotoxicity. Moreover, in 1989, Yoshihiro et al. isolated[23] an unusual cinnamic acid containing glycoglycerolipid from Liliumauratum var platyphyllum (Figure 1). Hence, in the present study an attempt has been made to synthesize and evaluate the cytotoxicity of acetate-protected monogalactosyldiacylglycerols having aromatic acids as well as unusual fatty acids.
Materials and Methods
All the chemicals used in this investigation were of analytical grade, were obtained from different commercial sources and were used without any further purification. All dry reactions were carried out under nitrogen atmosphere using anhydrous freshly distilled solvents and 4 Å molecular sieves in flame-dried glassware using standard gas-light syringes, cannulae and septa. Reactions were monitored on micro thin-layer chromatography plates coated with TLC grade silica gel (Merck) and the spots were detected under iodine vapours. Column chromatography was performed using silica gel (100-200 mesh) procured from Qualigens (India) using freshly distilled solvents. All the 1H-nuclear magnetic resonance (NMR) and 13C-NMR spectra were recorded on a Bruker Avance (operated for 1H-NMR at 300 MHz, 500 MHz and for 13C-NMR at 75, and 125 MHz) spectrometer, using TMS (δ=0 ppm) and δ 77.00 parts per million (ppm) as internal standard for chemical shifts (δ) in CDCl3 at 25°. The chemical shift values are presented in ppm units. Mass spectra were recorded on a high-resolution electrospray ionisation mass spectrometry (HR-ESIMS). Infrared spectra were recorded in chloroform (CHCl3) on a Perkin-Elmer FT-IR spectrum BX. The melting points were determined on a Barnstead Electro Thermal 9200 instrument.
1,2,3,4,6-Penta-O-acetyl-β-D-galactopyranoside (1)
A mixture of galactose (2 g, 11.108 mmol) and sodium acetate (2.73 g, 33.31 mmol) were dissolved in acetic anhydride (17.5 ml, 187 mmol). The reaction mixture was refluxed for 4 h at 90°. After reaction time, the reaction mixture was cooled to room temperature and then poured into the beaker containing crushed ice (150 ml) under stirring conditions. The penta-acetate was precipitated. The precipitation was filtered and washed with ice-cold water until the odour of the acetic acid was disappeared. The crude product was purified by recrystallization from MeOH to afford the title compound (3.98 g, 92 %) as a white crystalline solid. Melting point (MP): 142-145o, 1H-NMR (400 MHz, CDCl3) δ 5.71 (d, J=8.31 Hz, 1H), 5.43 (m, 1H), 5.33 (t, J=8.31 Hz, 1H), 5.09 (dd, J=3.42, 10.39 Hz, 1H), 4.19-4.1 (m, 2H), 4.08-4.04 (m, 1H), 2.16 (s, 3H), 2.12 (s, 3H), 2.04 (s, 6H), 1.99 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 170, 169.8, 169.6, 169.1, 168.7, 91.8, 71.4, 70.5, 67.6, 66.6, 60.8, 20.5, 20.4, 20.3; IR (CHCl3) 3027, 2969, 2951, 2907, 1756, 1743, 1422, 1371, 1322, 1225, 1067, 1048, 912, 756, 704, 641, 599 cm-1. HRMS (ESI) m/z [M+Na]-calc for C16H22O11Na 413.10543, found 413.10449.
2,3,4,6-Tetra-O-acetyl-galactopyranose hemiacetal (2)
Hydrazine acetate (1 g, 11 mmol) was added to a solution of 1,2,3,4,6-penta-O-acetyl-β-D-galactopyranoside (1) (3.8 g, 10 mmol) in dimethyl formmamide (DMF) 35 ml at 50° and stirred the reaction for 2 h under N2. As soon as TLC (1:1, hexane:ethyl acetate (EtOAc), v/v) showed the formation of product and the disappearance of starting material, the mixture was diluted with EtOAc, washed with aqueous 5 % NaCl and water, dried over anhydrous sodium sulphate (Na2SO4) and concentrated to give yellow oil. This crude oil was subjected to silica gel column chromatography. The required product was eluted in solvent mixture (65:35, hexane:EtOAc, v/v) as syrup (92 %, 3.15 g); 1H-NMR (500 MHz, CDCl3) δ 5.54 (t, J=9.9 Hz, 1H), 5.46 (d, J=2.8 Hz, 1H), 5.09 (t, J=9.4 Hz, 1H), 4.90-4.87 (m, 1H), 4.27-4.22 (m, 2H), 4.16-4.10 (m, 1H), 2.10 (s, 3H), 2.08 (s, 3H), 2.04 (s, 3H), 2.02 (s, 3H); 13C-NMR (75 MHz, CDCl3) δ 171.06, 170.5, 170.3, 169.7, 95.3, 89.9, 72.9, 72.4, 71.8, 71.1, 69.9, 68.4, 66.9, 61.9, 60.5, 30.1, 29.6, 20.68, 20.6; IR (CHCl3) 3460.6, 3024.4, 1748.8, 1369.5, 1235.7, 1038.6, 756 cm-1; HRMS (ESI) m/z [M+Na]-calc for C14H20O10Na=371.09487, found 371.09459.
2,3,4,6-Tetra-O-acetyl-α-galactopyranosyl trichloro acetimidate (3)
2,3,4,6-tetra-O-acetyl-galactopyranose hemiacetal (2, 2.8 g, 8.04 mmol) was treated with trichloroacetonitrile (8 ml, 80.45 mmol) and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU; 0.22 ml, 3.12 mmol) in anhydrous dichloromethane (DCM; 30 ml) and stirred for 2 h at room temperature. After 2 h, the reaction mixture was concentrated under reduced pressure and purified by silica gel column chromatography. The required product was eluted in solvent mixture (80:20, hexane:EtOAc, v/v) with good yield (77 %, 3.04 g). 1H-NMR (400 MHz, CDCl3) δ 8.6 (s, 1H), 6.61 (d, J=2.32 Hz, 1H), 5.57 (br s, 1H), 5.45-5.34 (m, 2H), 4.45 (t, J=6.35 Hz, 1H), 4.19-4.06 (m, 2H), 2.18 (s, 3H), 2.02 (2s, 9H); 13C-NMR (CDCl3, 100 MHz) δ 170.1, 169.9, 169.8, 169.7, 160.6, 93.3, 90.5, 68.8, 67.3, 67.2, 66.7, 61, 20.4, 20.3; IR (CHCl3) 3478, 3347, 2135, 1748.8, 1676.44, 1371, 1218, 1073, 756 cm-1.
1,2–Isopropylidene-3-O-(β-D-2',3',4',6'-tetra-Oacetyl- galactopyranosyl)-sn-glycerol (4)
Imidate (3, 3 g, 6.09 mmol), 1,2-isopropylidene glycerol (1.2 g, 9.14 mmol) and 4 A° molecular sieves were dissolved in freshly distilled DCM (35 ml). To this reaction mixture trimethylsilyl trifluoromethanesulfonate (TMSOTf; 0.32 ml, 1.82 mmol) was added dropwise at 0° and the reaction mixture was slowly allowed to room temperature and stirred for overnight. After utilizing all the starting materials, the reaction mixture was filtered and dissolved in EtOAc and water. The organic layer was extracted with aqueous sodium bicarbonate (NaHCO3) solution, dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude mixture was purified by silica gel column chromatography. The required spot was eluted in hexane:EtOAc (3:1, v/v) solvent mixture as a white solid (2.3 g, 82 %). 1H-NMR (400 MHz, CDCl3): δ 5.38 (d, J=3.3 Hz, 1H), 5.22-5.17 (m, 1H), 5.03-4.98 (m, 1H), 4.56 (dd, J=7.94, 10.63 Hz, 1H), 4.26-4.19 (m, 1H), 4.17-4.10 (m, 2H), 4.03-3.99 (m, 1H), 3.93-3.87 (m, 1H), 3.82-3.78 (m, 1H), 3.74-3.71 (m, 1H), 3.66-3.61 (m,1H), 2.15 (s, 3H), 2.06 (s, 3H), 2.05 (s, 3H), 1.98 (s, 3H), 1.34 (s, 3H), 1.41 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 170.3, 170.2, 170.1, 169.4, 169.3, 109.4, 109.2, 101.3, 74.3, 74.1, 70.8, 70.7, 70.6, 70.4, 69, 68.6, 68.5, 66.9, 66.6, 66.1, 65.1, 61.2, 26.7, 26.5, 25.2, 25, 20.6, 20.5, 20.4; IR (CHCl3) 2987, 2943, 1755, 1371, 1222, 1041, 757 cm-1; HRMS (ESI) m/z [M+Na]-calc for C20H30O12Na=485.16295, found 485.16118.
3-O-(β-D-2',3',4',6'-Tetra-O-acetyl-galactopyrano syl)-sn-glycerol (5)
1,2-Isopropylidene-3-O-(β-D-2',3',4',6'-tetra-O-acetylgalactopyranosyl)- sn-glycerol (4, 2 g, 4.32 mmol) was dissolved in acetonitrile (25 ml) and 5 equivalents zinc nitrate hexahydrate (6.43 g, 21.63 mmol) was added, the reaction mixture was stirred for 6 h at 50°. After completion of reaction, the starting materials and the organic solvent was evaporated under reduced pressure and the mixture was portioned in water and CHCl3. The organic phase was dried over anhydrous Na2SO4 and evaporated. The crude reaction mixture was purified by silica gel column chromatography using hexane:EtOAc (5:1, v/v) solvent mixture to give solid (1.36 g, 75 %). 1H-NMR (400 MHz, CDCl3): δ 5.40 (d, J=3.42 Hz 1H), 5.22-5.17 (m, 1H), 5.03 (dd, J=3.42, 10.39 Hz, 1H), 4.51 (d, J=7.82 Hz, 1H), 4.18-4.14 (m, 2H), 3.96 (t, J=7.82 Hz, 1H), 3.91-3.83 (m, 2H), 3.79-3.65 (m, 2H), 3.63-3.58 (m, 1H), 2.17 (s, 3H), 2.08 (s, 3H), 2.06 (s, 3H), 1.99 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ 170.4, 170.1, 170.0, 169.7, 101.8, 101.6, 72.6, 71.8, 70.8, 70.6, 70.4, 70.3, 68.8, 68.7, 67.9, 67.4, 66.9, 65.1, 63.3, 63.2, 61.7, 61.3, 20.7, 20.5, 20.4; IR (CHCl3) 3482, 2945, 1753, 1369, 1230, 1041, 757 cm-1; HRMS (ESI) m/z [M+NH4+]-calc for C17H26O12NH4+=440.17625, found 440.17527.
General procedures for synthesis of mono acid 1,2-O-diacyl-3-O-(β-D-2',3',4',6'-tetra-O-acetylgalactopyranosyl)- sn-glycerols (6a-6f)
3-O-(β-D-2',3',4',6'-tetra-O-acetyl-galactopyranosyl)- sn-glycerol (5, 1 mol) was dissolved in anhydrous DCM (10 ml), fatty acid (2 mol), N-(3-dimethylaminopropyl)- N′ethylcarbodiimide hydrochloride (EDC; 2 mol) and 4-dimethylaminopyridine (DMAP; catalytic amount) were added under nitrogen at 0°. The reaction mixture was stirred for overnight at room temperature. After all the starting materials have been used, the reaction mixture was dissolved in CHCl3 and washed with water and brine solution. The organic layer was dried over anhydrous Na2SO4 and evaporated under reduced pressure. The mixture was purified by silica gel column chromatography using hexane:EtOAc (4: 1, v/v) solvent mixture to give title compounds (6a-6f) in 88- 92 % yields.
1,2-O-Di-butanoyl-3-O-(β-D-2',3',4',6'-tetra-Oacetyl- galactopyranosyl)-sn-glycerols (6a)
The title compound was obtained in hexane:EtOAc (4:1, v/v) solvent mixture with 92 % yield. 1H-NMR (400 MHz, CDCl3): δ 5.38 (m, 1H), 5.22-5.16 (m, 2H), 5.02-4.98 (m, 1H), 4.49 (t, J=7.70 Hz, 1H), 4.33-4.28 (m, 1H), 4.19-4.08 (m, 3H), 3.98-3.89 (m, 2H), 3.71-3.67 (m, 1H), 2.29 (t, J=7.33 Hz, 4H), 2.16 (s, 3H), 2.06 (s, 3H), 2.05 (s, 3H), 1.98 (s, 3H), 1.70-1.59 (m, 4H), 0.94 (t, J=7.45 Hz, 6H); 13C-NMR (100 MHz, CDCl3) δ 173.0, 172.7, 172.5, 170.2, 170.1, 170.0, 169.2, 101.4, 101.1, 70.6, 69.7, 69.6, 68.5, 68.4, 67.5, 67.4, 66.8, 62.1, 61.1, 61.0, 36.0, 35.8, 29.5, 20.5, 20.4, 18.2, 13.5, 13.4; IR(CHCl3) 2933, 2872, 1751, 1432, 1223, 1060, 733 cm-1; HRMS (ESI) m/z [M+Na]-calc for C25H38O14Na=585.2153, found 585.2137.
1,2-O-Di-hexanoyl-3-O-(β-D-2',3',4',6'-tetra-Oacetyl- galactopyranosyl)-sn-glycerols (6b)
The title compound was obtained in hexane:EtOAc (4:1, v/v) solvent mixture with 90 % yield. 1H-NMR (500 MHz, CDCl3): δ 5.38 (m, 1H), 5.22-5.17 (m, 2H), 5.02-4.99 (m, 1H), 4.49 (t, J=8.39 Hz, 1H), 4.32-4.30 (m, 1H), 4.18-4.11 (m, 3H), 3.97 (dd, J=4.88, 10.98 Hz, 1H), 3.91 (t, J=6.71 Hz, 1H), 3.70-3.67 (m, 1H), 2.30 (t, J=7.47 Hz, 4H), 2.16 (s, 3H), 2.07 (s, 3H), 2.05 (s, 3H), 1.98 (s, 3H), 1.64-1.60 (m, 4H), 1.31 (m, 8H), 0.90 (t, J=6.71 Hz, 6H); 13C-NMR (125 MHz, CDCl3) δ 173.2, 172.8, 172.7, 170.2, 170.1, 170.0, 169.2, 101.4, 101.2, 70.6, 69.7, 69.5, 68.5, 68.4, 67.5, 67.4, 66.8, 62.1, 61.1, 61.0, 34.0, 33.9, 31.1, 24.4, 22.1, 20.5, 20.4, 13.7; IR(CHCl3) 2958, 2934, 1750, 1460, 1433, 1224, 1077, 734 cm-1; HRMS (ESI) m/z [M+NH4+]-calc for C29H46O14NH4+=636.3225, found 636.3222.
1,2-O-Di-10-undecenoyl-3-O-(β-D-2',3',4',6'-tetra-Oacetyl- galactopyranosyl)-sn-glycerol (6c)
The title compound was obtained in hexane:EtOAc (4:1, v/v) solvent mixture with 88 % yield. 1H-NMR (300 MHz, CDCl3): δ 5.86-5.73 (m, 2H), 5.38 (m, 1H), 5.23- 5.16 (m, 2H), 5.01 (m, 2H), 4.95-4.90 (m, 3H), 4.52- 4.47 (m, 1H), 4.34-4.29 (m, 1H), 4.16-4.06 (m, 3H), 3.98-3.89 (m, 2H), 3.71-3.66 (m, 2H), 2.30 (t, J=7.42 Hz, 4H), 2.16 (s, 3H), 2.05 (s, 3H), 2.02 (s, 3H), 1.98 (s, 3H), 2.02 (overlapped, 4H) 1.61 (m, 4H), 1.39-1.34 (m, 4H), 1.29 (m, 20H); 13C-NMR (125 MHz, CDCl3) δ 173.0, 172.7, 172.5, 170.1, 169.9, 169.8, 169.1, 138.8, 113.9, 101.3, 101.0, 70.5, 69.6, 69.4, 68.4, 68.3, 67.4, 67.3, 66.7, 62.0, 61.0, 60.9, 34.0, 33.8, 33.5, 29.0, 29.0, 28.8, 28.6, 24.6, 20.4, 20.3; IR(CHCl3) 2936, 2855, 1738, 1315, 1229, 1060, 773 cm-1; HRMS (ESI) m/z [M+NH4+]-calc for C39H62O14NH4+=772.4477, found 772.4472.
1,2-O-Di-oleoyl-3-O-(β-D-2',3',4',6'-tetra-O-acetylgalactopyranosyl)- sn-glycerols (6d)
The title compound was obtained in hexane:EtOAc (4:1, v/v) solvent mixture with 90 % yield. 1H-NMR (400 MHz, CDCl3): δ 5.38 (d, J=3.42Hz, 1H), 5.35- 5.32 (m, 4H), 5.22-5.16 (m, 2H), 5.02-4.98 (m, 1H), 4.48 (t, J=7.70 Hz, 1H), 4.33-4.28 (m, 1H), 4.19-4.06 (m, 3H), 3.97-3.88 (m, 2H), 3.69-3.65 (m, 1H), 2.34 (t, J=7.45 Hz, 2H), 2.29 (t, J=7.09 Hz, 2H), 2.16 (s, 3H), 2.06 (s, 3H), 2.05 (s, 3H), 2.03-2.00 (m, 8H), 1.98 (s, 3H), 1.61 (m, 4H), 1.26-1.30 (m, 40H), 0.88 (t, J=6.96 Hz, 6H); 13C-NMR (100 MHz, CDCl3) δ 178.7, 173.2, 172.9, 172.7, 170.3, 170.1, 170.0, 169.3, 129.9, 129.6, 101.5, 101.2, 70.7, 69.7, 69.6, 68.5, 68.4, 67.6, 67.5, 66.8, 62.2, 61.1, 61.1, 34.1, 34.0, 33.8, 31.8, 29.7, 29.6, 29.4, 29.2, 29.1, 29.0, 27.1, 27.1, 24.8, 24.6, 22.6, 20.5, 20.5, 14.0; IR(CHCl3) 2927, 2850, 1748, 1365, 1236, 1060, 973 cm-1; HRMS (ESI) m/z [M+NH4 +]-calc for C53H90O14NH4+=968.6630, found 968.6668.
1,2-O-Di-erucioyl-3-O-(β-D-2',3',4',6'-tetra-Oacetyl- galactopyranosyl)-sn-glycerols (6e)
The title compound was obtained in hexane:EtOAc (4:1, v/v) solvent mixture with 89 % yield. 1H-NMR (500 MHz, CDCl3): δ 5.38-5.33 (m, 5H), 5.22-5.17 (m, 2H), 5.02-4.98 (m, 1H), 4.48 (t, J=8.54 Hz, 1H), 4.32- 4.28 (m, 1H), 4.18-4.07 (m, 3H), 3.97-3.88 (m, 2H), 3.69-3.65 (m, 1H), 2.34 (t, J=7.45 Hz, 2H), 2.29 (t, J=7.32 Hz, 2H), 2.16 (s, 3H), 2.06 (s, 3H), 2.05 (s, 3H), 2.03-2.00 (m, 8H), 1.98 (s, 3H), 1.61 (m, 4H), 1.26 (m, 56H), 0.88 (t, J=6.71 Hz, 6H); 13C-NMR (125 MHz, CDCl3) δ 173.2, 172.9, 172.8, 170.3, 170.1, 170.0, 169.3, 129.8, 129.8, 101.5, 101.2, 70.7, 69.7, 69.6, 68.5, 68.5, 67.6, 67.5, 66.8, 62.2, 61.1, 61.1, 34.2, 34.0, 31.8, 29.7, 29.6, 29.4, 29.2, 29.1, 27.1, 24.8, 22.6, 20.6, 20.5, 14.0; IR(CHCl3) 2929, 2856, 1756, 1385, 1226, 1080, 772 cm-1; HRMS (ESI) m/z [M+NH4+]-calc for C61H106O14NH4 +=1080.7920, found 1080.7893.
1,2-O-Di-cinnamoyl-3-O-(β-D-2',3',4',6'-tetra-Oacetyl- galactopyranosyl)-sn-glycerols (6f)
The title compound was obtained in hexane:EtOAc (4:1, v/v) solvent mixture with 88 % yield. 1H-NMR (500 MHz, CDCl3): δ 7.71 (t, J=15.71 Hz, 2H), 7.54- 7.51 (m, 4H), 7.40-7.37 (m, 6H), 6.44 (d, J=15.71 Hz, 2H), 5.41-5.39 (m, 2H), 5.26-5.21 (m, 1H), 5.04-5.00 (m, 1H), 4.55 (d, J=7.93 Hz, 1H), 4.50-4.46 (m, 1H), 4.44-4.35 (m, 1H), 4.19-4.08 (m, 3H), 3.94-3.91 (m, 1H), 3.88-3.80 (m, 1H), 2.15 (s, 3H), 2.06 (s, 3H), 2.04 (s, 3H), 1.98 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ 170.3, 170.2, 170.0, 169.3, 166.4, 165.9, 145.8, 145.5, 134.0, 130.5, 130.4, 128.9, 128.8, 128.1, 117.3, 117.2, 117.2, 101.4, 70.8, 70.7, 70.0, 68.6, 67.8, 67.5, 66.9, 62.6, 61.2, 20.6, 20.6, 20.5; IR(CHCl3) 2962, 2852, 1749, 1637, 1370, 1223, 1162, 1055 cm-1; HRMS (ESI) m/z [M+NH4+]-calc for C35H38O14NH4+=700.2595, found 700.2599.
General procedures for synthesis of mixture of fatty acids 1,2-O-diacyl-3-O-(β-D-2',3',4',6'-tetra-Oacetyl- galactopyranosyl)-sn-glycerols (7a-7d)
3-O-(β-D-2',3',4',6'-tetra-O-acetyl-galactopyranosyl)- sn-glycerol (5, 1 mol) was dissolved in anhydrous DCM (10 ml), oleic acid (1 mol), EDC (1 mol) and DMAP (pinch) were added under nitrogen at 0°. The reaction mixture was stirred for 6 h at 0°. After utilizing starting materials, equimolar amounts of fatty acid, EDC and a little amount of DMAP were added subsequently. The reaction mixture was stirred at room temperature for overnight. Then the reaction mixture was dissolved in CHCl3 and washed with water and brine solution. The organic layer was dried over anhydrous Na2SO4 and evaporated under reduced pressure. The mixture was purified by silica gel column chromatography using a solvent mixture of hexane:EtOAc (4:1, v/v) to give title compounds with 86-92 % yield.
1-O-Oleoyl-2-O-octanoyl-3-O-(β-D-2',3',4',6'-tetra- O-acetyl-galactopyranosyl)-sn-glycerols (7a)
The title compound was obtained in hexane:EtOAc (4:1, v/v) solvent mixture with 92 % yield. 1H-NMR (300 MHz, CDCl3): δ 5.38 (d, J=3.30 Hz, 1H), 5.36- 5.32 (m, 2H), 5.23-5.16 (m, 2H), 5.03-4.97 (m, 1H), 4.49 (dd, J=5.50, 7.97 Hz, 1H), 4.34-4.27 (m, 1H), 4.18-4.06 (m, 3H), 3.98-3.88 (m, 2H), 3.70-3.65 (m, 1H), 2.37-2.27 (m, 2H), 2.16 (s, 3H), 2.06 (s, 3H), 2.05 (s, 3H), 2.01-2.03 (m, 4H), 1.98 (s, 3H), 1.65-1.61 (m, 4H), 1.29-1.26 (m, 28H), 0.88 (t, J=6.05 Hz, 6H); 13C-NMR (100 MHz, CDCl3) δ 179.1, 173.2, 172.9, 172.8, 170.3, 170.2, 170.1, 169.3, 129.9, 129.6, 101.5, 101.2, 70.7, 69.7, 69.6, 68.5, 68.5, 67.6, 67.5, 66.9, 62.2, 61.1, 61.1, 34.1, 34.0, 34.0, 33.9, 31.8, 31.5, 29.7, 29.4, 29.2, 29.1, 29.0, 28.9, 28.8, 27.1, 27.1, 24.8, 24.6, 22.6, 22.5, 20.6, 20.5; IR(CHCl3) 2924, 2852, 1753, 1709, 1582, 1451, 1123, 918 cm-1; HRMS (ESI) m/z [M+NH4+]-calc for C43H72O14NH4+=830.5260, found 830.5240.
1-O-Oleoyl-2-O-valiproyl-3-O-(β-D-2',3',4',6'-tetra- O-acetyl-galactopyranosyl)-sn-glycerols (7b)
The title compound was obtained in hexane:EtOAc (4:1, v/v) solvent mixture with 92 % yield. 1H-NMR (500 MHz, CDCl3): δ 5.38 (d, J=3.35 Hz, 1H), 5.35- 5.33 (m, 2H), 5.21-5.17 (m, 2H), 5.02-4.98 (m, 1H), 4.48 (t, J=8.08 Hz, 1H), 4.32-4.28 (m, 1H), 4.18-4.08 (m, 3H), 3.98-3.88 (m, 2H), 3.69-3.63 (m, 1H), 2.31- 2.26 (m, 2H), 2.16 (s, 3H), 2.07 (s, 3H), 2.05 (s, 3H), 2.03-2.00 (m, 4H), 1.98 (s, 3H), 1.60 (m, 6H), 1.30- 1.26 (m, 24H), 0.93-0.86 (m, 9H); 13C-NMR (125 MHz, CDCl3) δ 181.1, 175.4, 173.1, 170.2, 170.1, 170.0, 169.2, 129.8, 129.5, 101.4, 100.9, 70.7, 70.6, 69.4, 68.5, 67.7, 67.5, 67.1, 66.8, 62.4, 62.1, 61.1, 61.0, 45.2, 45.1, 44.8, 34.5, 34.4, 34.2, 34.1, 33.9, 31.8, 29.6, 29.6, 29.4, 29.2, 29.0, 29.0, 27.1, 27.0, 24.7, 22.5, 20.5, 20.4, 14.0, 13.8; IR(CHCl3) 2927, 2855, 1747, 1464, 1370, 1222, 1077, 912, 735 cm-1; HRMS (ESI) m/z [M+NH4+]-calc for C43H72O14NH4+=830.5260 found, 830.5241.
1-O-Oleoyl-2-O-10-undecenoyl-3-O-(β-D-2',3',4',6'- tetra-O-acetyl-galactopyranosyl)-sn-glycerols (7c)
The title compound was obtained in hexane:EtOAc (4:1, v/v) solvent mixture with 90 % yield. 1H-NMR (400 MHz, CDCl3): δ 5.85-5.75 (m, 1H), 5.39 (d, J=3.30 Hz, 1H), 5.36-5.32 (m, 2H), 5.22-5.16 (m, 2H), 5.02-4.99 (m, 1H), 4.97-4.96 (m, 1H), 4.94-4.93 (m, 1H), 4.48 (t, J=7.82 Hz, 1H), 4.33-4.27 (m, 1H), 4.19- 4.07 (m, 3H), 3.97-3.88 (m, 2H), 3.70-3.65 (m, 1H), 2.34 (t, J=7.45 Hz, 2H), 2.29 (t, J=7.58 Hz, 2H), 2.16 (s, 3H), 2.06 (s, 3H), 2.05 (s, 3H), 2.04-2.00 (m, 6H), 1.98 (s, 3H), 1.61 (m, 4H), 1.30-1.26 (m, 40H), 0.88 (t, J=6.72 Hz, 3H); 13C-NMR (100 MHz, CDCl3) δ 179.1, 173.2, 172.9, 172.7, 170.3, 170.1, 170.0, 169.3, 139.0, 129.9, 129.5, 114.0, 101.4, 101.1, 70.6, 69.7, 69.6, 68.5, 68.4, 67.5, 67.4, 66.8, 62.2, 61.1, 61.0, 34.1, 33.9, 33.8, 33.6, 31.8, 29.6, 29.6, 29.4, 29.2, 29.1, 28.9, 28.7, 27.1, 27.0, 24.7, 24.5, 22.5, 20.5, 20.4, 14.0; IR(CHCl3) 2928, 2856, 1749, 1639, 1370, 1079, 756 cm-1; HRMS (ESI) m/z [M+NH4+]-calc for C46H76O14NH4+=870.5573 found 870.5545.
1-O-Oleoyl-2-O-cinnamoyl-3-O-(β-D-2',3',4',6'- tetra-O-acetyl-galactopyranosyl)-sn-glycerols (7d)
The title compound was obtained in hexane:EtOAc (4:1, v/v) solvent mixture with 88 % yield. 1H-NMR (400 MHz, CDCl3): δ 7.71 (d, J=16.01 Hz, 1H), 7.55- 7.52 (m, 2H), 7.40-7.39 (m, 3H), 6.46 (d, J=16.01 Hz, 1H), 5.38 (m, 1H), 5.35-5.29 (m, 3H), 5.24-5.19 (m, 1H), 5.04-4.99 (m, 1H), 4.54-4.52 (m, 1H), 4.43-4.19 (m, 2H), 4.18-4.11 (m, 2H), 4.07-4.02 (m, 1H), 3.93- 3.90 (m, 1H), 3.82-3.74 (m, 1H), 2.31 (t, J=7.58 Hz, 2H), 2.29 (t, J=7.58 Hz, 2H), 2.16 (s, 3H), 2.05 (s, 3H), 2.04 (s, 3H), 2.04-2.00 (m, 4H), 1.98 (s, 3H), 1.61 (m, 2H), 1.26 (m, 20H), 0.88 (t, J=6.60 Hz, 3H); 13C-NMR (100 MHz, CDCl3) δ 173.2, 170.2, 170.1, 170.0, 169.3, 165.9, 165.8, 145.7, 134.0, 130.5, 129.9, 129.6, 128.8, 128.0, 117.2, 117.2, 101.3, 70.7, 70.0, 68.5, 68.4, 67.8, 67.5, 66.8, 62.3, 61.1, 61.1, 34.0, 31.8, 29.6, 29.6, 29.4, 29.2, 29.1, 29.0, 27.1, 27.0, 24.8, 22.6, 20.5, 20.5, 14.0; IR(CHCl3) 2962, 2850, 1750, 1636, 1310, 1223, 1056 cm-1; HRMS (ESI) m/z [M+NH4+]-calc for C44H64O14NH4+=834.4634, found 834.4617.
1,2-Isopropylideneglycerol (8)
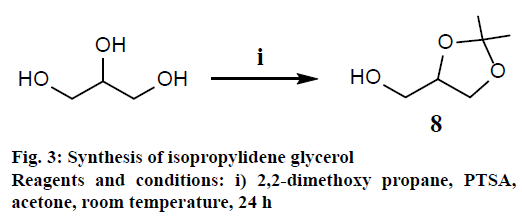
Glycerol (3 g, 30 mmol), 2,2-dimethoxy propane (45 mmol, 4.6 g), dry acetone (30 ml) and a catalytic amount of para-toluenesulfonic acid (10 mg) were stirred at room temperature for 24 h. After the reaction time, acetone and unreacted 2,2-dimethoxy propane was evaporated under reduced pressure. The reaction mixture was purified by silica gel column chromatography using solvent mixture EtOAc:hexane (40:60; v/v) the compound was isolated as colourless oil with 70 % (3.01 g) yield. 1H-NMR (400 MHz, CDCl3) δ 4.26-4.21 (m, 1H), 4.04 (dd, J=6.6, 8.19 Hz, 1H), 3.78 (dd, J=6.48, 8.19 Hz, 1H), 3.71 (dd, J=3.91, 11.61 Hz, 1H), 3.60 (dd, J=6.35, 11.61 Hz, 1H), 2.57 (br s, OH), 1.44 (s, 3H), 1.38 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ 109.2, 76.1, 65.6, 62.8, 26.5, 25.1; IR (CHCl3) 3426, 2992, 2936, 2854, 1428, 1372, 1219, 1157, 1061, 865 cm-1.
Cytotoxicity using MTT assay
Cytotoxicity assay (MTT) was evaluated for the test compounds 6a-6f and 7a-7d as reported earlier[24]. Four different cancer cell lines and one normal cell line namely, SKOV3-ovarian cancer (ATCC® HTB77™), HeLa- cervical cancer (ATCC® CCL2™), MDA-MB-231-breast cancer (ATCC® HTB26™), DU145- prostate cancer (ATCC® HTB81™) and CHO-K1-normal Chinese hamster ovary cell line (ATCC® CCL-61™) were obtained from the ATCC (Bethesda, MD, USA) and maintained in Dulbecco's modified Eagle's medium supplemented with 10 % fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37° in a 5 % CO2 incubator. After seeding of cells in 96 well culture plate, allowed to attach properly. Test compounds of different concentrations ranging from 1 to 50 μM were added in triplicates and incubated for 24 h. The cells were then incubated with MTT (0.5 mg/ml) for 3 h and to dissolve the insoluble formazan crystals 100 μl DMSO was added to each well. Finally, the absorbance of the plates was measured using a Synergy H1 multi-mode plate reader, USA. Doxorubicin was used as positive control for the comparison.
Results and Discussion
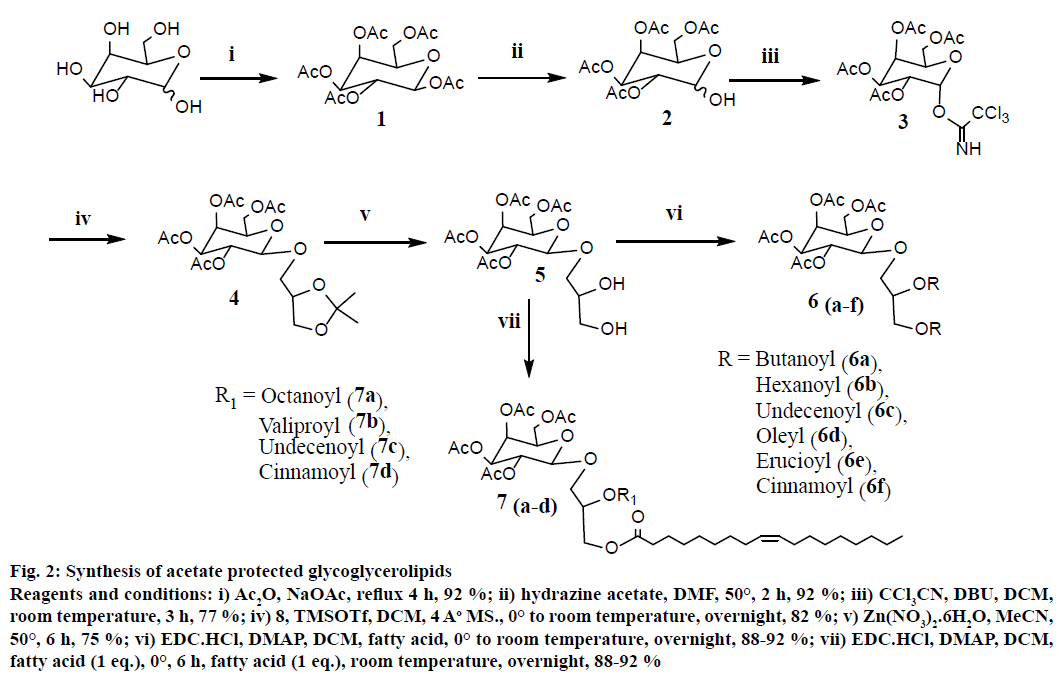
These molecules were synthesized employing earlier reported methods[20]. As depicted in the Figure 2, the synthesis of galactosyldiacylglycerols started with acylation of galactose with Ac2O and NaOAc under reflux conditions for 4 h. The anomeric acetate group was chemoselectively removed by hydrazine acetate. Subsequently, hemiacetal 2 was reacted with trichloroacetonitrile in the presence of DBU and exclusively formed α-trichloroacetimidate 3 due to anomeric effect. The coupling of imidate 3 with 1,2-isopropylidene glycerol 8 (which was obtained from glycerol as shown in Figure 3) in presence of TMSOTf led to formation of 4 in β-configuration with 82 % yield. This β-orientation was achieved by NGP of C-2ʹ acetate group of sugar with C-1ʹ carbon of sugar as it allowed only equatorial attack of acceptor. 1H-NMR analysis revealed that the signals of H-1ʹ proton was observed as dd at δ=4.56 ppm, J=7.94, 10.63 Hz. Diol 5 was obtained through selective hydrolysis of isopropylidene group of 4 by zinc nitrate hexahydrate in acetonitrile[21]. Mixture of fatty acid analogues 7(ad) were synthesized using the following procedure, the diol 5 was selectively esterified at C-3 OH of glycerol with oleic acid (1 mol) by using EDC-HCl (1 eq.) and DMAP (cat.) at 0° for 6 h reaction period. At the same time to avoid the acyl migration of fatty acid from C-3 OH to C-2 OH of glycerol, simultaneously another fatty acid, EDC-HCl and DMAP were added in equimolar ratio to the same reaction mixture and stirred for overnight at room temperature to give mixture of fatty acid analogues 7(a-d) with 89-92 % yield. Under these experimental conditions, 2-acyl glycerol derivative was not observed in the reaction mixture. For the synthesis of other fatty acid analogues (6a-6f), EDC-HCl was used at room temperature for overnight to get 88-92 % yields.
Figure 2: Synthesis of acetate protected glycoglycerolipids
Reagents and conditions: i) Ac2O, NaOAc, reflux 4 h, 92 %; ii) hydrazine acetate, DMF, 50°, 2 h, 92 %; iii) CCl3CN, DBU, DCM, room temperature, 3 h, 77 %; iv) 8, TMSOTf, DCM, 4 Ao MS., 0° to room temperature, overnight, 82 %; v) Zn(NO3)2.6H2O, MeCN, 50°, 6 h, 75 %; vi) EDC.HCl, DMAP, DCM, fatty acid, 0° to room temperature, overnight, 88-92 %; vii) EDC.HCl, DMAP, DCM, fatty acid (1 eq.), 0°, 6 h, fatty acid (1 eq.), room temperature, overnight, 88-92 %
All the synthesized glycoglycerolipids were examined for cytotoxicity against four cancer cell lines and one normal cell line namely, SKOV3, HeLa, MDAMB- 231, DU145 and CHO-K1 cell lines. The results are tabulated in Table 1. All the tested compounds exhibited moderate to significant cytotoxicity against HeLa, MDA-MB-231 cancer cell lines with IC50 values between the 27.5-117.0 μM ranges. Among all the tested compounds, in mono acid glycoglycerolipids compound 6c (C11:1) and 6b (C6:0) exhibited significant activity with IC50 values 30.3 and 41.4 μM, respectively. Whereas, in mixed acid glycoglycerolipids case the compound 7b exhibited good activity with IC50 values 27.5 μM due to the balancing combination of active fatty acids such as branching valproic acid and unsaturated oleic acid. In similar manner the combination of unsaturated oleic acid and cinnamic acid compound 7d also exhibited significant activity with IC50 values 34.9 μM. All other compounds exhibited the moderate to poor activity against HeLa and MDA-MB-231 cell lines. Moreover, all the tested compounds didn’t show any toxicity towards normal CHO-K1 cell line.
| Test compound | IC50 values (μM) | ||||
|---|---|---|---|---|---|
| SKOV3 | HeLa | MDA-MB-231 | DU145 | CHO-K1 | |
| 6a | NA | 75.8 ± 0.93 | 87.0 ± 0.92 | NA | NA |
| 6b | 105.9 ± 0.92 | 41.4 ± 0.67 | 117.0 ± 0.98 | NA | NA |
| 6c | NA | 30.3 ± 0.77 | 98.2 ± 0.94 | NA | NA |
| 6d | NA | 54.9 ± 0.81 | 73.5 ± 0.87 | NA | NA |
| 6e | NA | 62.1 ± 0.77 | 44.1 ± 0.73 | NA | NA |
| 6f | NA | 66.1 ± 0.96 | 78.4 ± 0.79 | NA | NA |
| 7a | NA | 57.5 ± 0.86 | 97.8 ± 0.91 | NA | NA |
| 7b | NA | 27.5 ± 0.66 | 87.8 ± 0.99 | NA | NA |
| 7c | NA | 51.2 ± 0.74 | 89.0 ± 0.91 | NA | NA |
| 7d | NA | 34.9 ± 0.68 | 69.1 ± 0.85 | NA | NA |
| Doxorubicin | 0.7 ± 0.23 | 0.7 ± 0.22 | 0.7 ± 0.20 | 0.8 ± 0.19 | NA |
| Mitomycin c | NA | NA | NA | NA | 13.4 ± 0.21 |
Table 1: Cytotoxicity of Acetate-Protected Glycoglycerolipids (6a-6f, 7a-7d)
In conclusion, in this investigation unusual fatty acid and aromatic acid (cinnamic acid)-based, acetate-protected monogalactosyldiacylglycerol were synthesized employing trichloroacetimidate methodology. All the synthesized glycoglycerolipids were examined for cytotoxicity against four cancer cell lines and one normal cell line. Based on the results, it could be concluded that the combination of oleic acid with aromatic acid (cinnamic acid type) and other saturated fatty acids enhances the cytotoxicity of glycoglycerolipids.
Acknowledgements
V. Srikanth acknowledges Council of Scientific and Industrial Research (CSIR), New Delhi, India, for financial support in the form of fellowship.
Conflict of interest
There is no conflict of interest associated with this project.
References
- Huis in't Veld JH, Willers JM. Glycolipids from the cell walls of Streptococci. Antonie Van Leeuwenhoek 1973;39:281-94.
- Block MA, Dorne AJ, Joyard J, Douce R. Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts II. Biochemical characterization. J Biol Chem 1983;258:13281-6.
- Ohta H, Awai K, Takamiya K. Glyceroglycolipids of photosynthetic organisms-their biosynthesis and evolutionary origin. Trends Glycosci Glycotechnol 2000;12:241-53.
- Shirahashi H, Murakami N, Watanabe M, Nagatsu A, Sakakibara J, Tokuda H, et al. Isolation and identification of antitumor-promoting principles from the fresh-water cyanobacterium Phormidium tenue. Chem Pharm Bull 1993;41:1664-6.
- Wang R, Furomoto T, Motoyama K, Okazaki K, Kondo A, Fukui H. Possible antitumor promoters in Spinacia oleracea (spinach) and comparison of their contents among cultivars. Biosci Biotechnol Biochem 2002;66:248-54.
- Matsubara K, Matsumoto H, Mizushina Y, Mori M, Nakajima N, Fuchigami M, et al. Inhibitory effect of glycolipids from spinach on in vitro and in vivo angiogenesis. Oncol Rep 2005;14:157-60.
- Colombo D, Scala A, Taino IM, Toma L, Ronchetti F, Tokuda H, et al. 1-O-2-O- and 3-O-β-glycosyl-sn-glycerols: structure-antitumor-promoting activity relationship. Bioorg Med ChemLett 1996;6:1187-90.
- Colombo D, Compostella F, Ronchetti F, Scala A, Toma L, Mukainaka T, et al. Inhibitory effects of mono acylated 2-O-β-galactosylglycerols in Epstein-Barrvirus activation: the significant role of the hexanoyl chain. Cancer Lett 1999;143:1-4.
- Tokuda H, Nishino H, Shirahashi H, Murakami N, Nagatsu A, Sakakibara J. Inhibition of 12-O-tetradecanoylphorbol-13-acetate promoted mouse skin papilloma by digalactosyldiacylglycerols from the fresh water cyanobacterium Phormidium tenue. Cancer Lett 1996;104:91-5.
- Andrianasolo EH, Haramaty L, Vardi A, White E, Lutz R, Falkowski P. Apoptosis-inducing galactolipids from a cultured marine diatom, Phaeodactylum tricornutum. J Nat Prod 2008;71:1197-201.
- Sahara H, Ishikawa M, Takahashi N, Ohtani S, Sato N, Gasa S, et al. In vivo antitumor effect of 3'-sulphonoquinovosyl 1'-monoacylglyceride isolated from sea urchin (Strongylocentrotus intermedius) intestine. Br J Cancer 1997;75:324-32.
- Francesca C, Bonivento P, Giuseppe P, Marina Z, Luciana GF, Giuditta S, et al. Chemoenzymatic synthesis and antimicrobial activity evaluation of monogalactosyldiglycerides. Eur J Med Chem 2008;43:210-21.
- Janwitayanuchit W, Suwanborirux K, Patarapanich C, Pummangura S, Lipipun V, Vilaivan T. Synthesis and anti-Herpes simplex viral activity of monoglycosyldiglycerides. Phytochemistry 2003;64:1253-64.
- Gordon DM, Danishefsky SJ. Synthesis of a cyanobacterial sulfolipid: Confirmation of its structure, stereochemistry, and anti-HIV-1 activity. J Am Chem Soc 1992;114:659-63.
- Bruno A, Rossi C, Marcolongo G, Di Lena A, Venzo A, Barrie CP, et al. Selective in vivo antiinflammatory action of the galactolipids monogalactosyldiacylglycerol. Eur J Pharmacol 2005;524:159-68.
- Larsen E, Kharazmi A, Christensen LP, Christensen SB. An antiinflammatory galactolipid from rose hip (Rosa canina) that inhibits chemotaxis of human peripheral blood neutrophils in vitro. J Nat Prod 2003;66:994-5.
- Nakata K. High resistance to oxygen radicals and heat is caused by a galactoglycerolipid in Microbacterium sp M874. J Biochem 2000;127:731-7.
- Colombo D, Franchini L, Toma L, Ronchetti F, Nakabe N, Konoshima T, et al. Antitumor-promoting activity of simple models of galactoglycerolipids with branched and unsaturated acyl chains. Eur J Med Chem 2005;40:69-74.
- Nagatsu A, Watanabe M, Ikemoto K, Hashimoto M, Murakami N, Sakakibara J, et al. Synthesis and structure antitumor-promoting activity relationship of monogalactosyldiacylglycerols. Bioorg Med Chem Lett 1994;4:1619-22.
- Srikanth V, Prasad RBN, Poornachandra Y, PhaniBabu VS, Ganesh Kumar C, Jagadeesh B, et al. Synthesis of dihydrosterculic acid-based monoglucosyldiacylglycerol and its analogues and their biological evaluation. Eur J Med Chem 2016;109:134-45.
- Durrant LG, Noble P, Spendlove I. Immunology in the clinic review series; focus on cancer: glycolipids as targets for tumour immunotherapy. Clin Exp Immunol 2012;167:206-15.
- Colombo D, Franchini L, Toma L, Ronchetti F, Tanaka R, Takayasu J, et al. Cyclic and branched acyl chain galactoglycerolipids and their effect on antitumor-promoting activity. Eur J Med Chem 2006;41:1456-63.
- Yoshihiro M, Yutaka S, Hiroko S. Lipid and steroidal constituents of Lilium auratum var. platyphyllum and L. tenuifolium. Phytochemistry 1989;28:3453-8.
- Bollu VS, Nethi SK, Dasari RK, Rao SS, Misra S, Patra CR. Evaluation of in vivo cytogenetic toxicity of europium hydroxide nanorods (EHNs) in male and female Swiss albino mice. Nanotoxicology 2016;10:413-25.