- *Corresponding Author:
- B. K. Satheeshababu
Department of Pharmaceutics, Government College of Pharmacy, #2, P Kalinga Rao Road, Bengalore-560 027, India
E-mail: bksatishbabu@gmail.com
| Date of Submission | 25 September 2014 |
| Date of Revision | 02 February 2015 |
| Date of Acceptance | 17 September 2015 |
| Indian J Pharm Sci 2015;77(5):579-585 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The aim of the present research work to study the effect of conjugation of the polymer on drug release from the matrix tablets. Sodium alginate L-cysteine conjugate was achieved by covalent attachment of thiol group of L-cysteine with the primary amino group of sodium alginate through the amide bonds formed by primary amino groups of the sodium alginate and the carboxylic acid group of L-cysteine. The synthesised sodium alginate L-cysteine conjugate was characterised by determining of charring point, Fourier transmission-infrared and differential scanning calorimetric analysis. To study the effect of conjugation on drug release pattern, the matrix tablets were prepared using various proportions of sodium alginate and sodium alginate L-cysteine conjugate along with atorvastatin calcium as model drug. The wet granulation technique was adopted and prepared matrix tablets were evaluated for various physical parameters. The in vitro drug release study results suggested that tablet formulated in combination of sodium alginate and sodium alginate L-cysteine conjugate S4 showed 100% after 8 h drug release whereas formulated with only sodium alginate S0 released 40% in 8 h.
Keywords
Sodium alginate L-cysteine conjugate, effect of conjugation, channeling agent, pore former, sustained release matrix tablets, atorvastatin calcium, sodium alginate
Sodium alginate, a polysaccharide from natural sources available abundantly and it was used extensively in the preparation of sustained release preparations like matrix tablets [1]. Sodium alginate L-cysteine conjugate was chemically synthesised by reacting with polymer with L-cysteine and conjugation was mediated by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDAC) and was reported as it helps in the release of drug in controlled pattern [2]. To improve the sustainability and better control over the drug release various polymers like sodium alginate and sodium conjugate were attempted [2]. When a sodium alginate is conjugated, the thiol moiety is introduced in the polymeric structure hence this leads to opening of the polymeric chains which will allow taking up more quantity of the water at a higher rate, make polymer to swell at a faster rate and allow more rapid release of drug Atorvastatin a model drug used in the present work in an inhibitor of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMGCoA) reductase which catalyzes the conversion of HMG-Co A tomevalonate, an early rate-limiting step in cholesterol biosynthesis, thereby it act as lipid-lowering agent [4].
The objectives of the present research envisaged are to synthesise and characterise the sodium alginate L-cysteine conjugate, formulate matrix tablets with different proportions of sodium alginate and sodium alginate L-cysteine conjugate and study the effect of conjugation on drug release.
Materials and Methods
Atorvastatin calcium was obtained as gift sample from Torrent Pharma Pvt. Ltd, Ahmedabad. Sodium alginate was commercially obtained as gift sample from S.D. fine chemicals, Mumbai. L-cysteine was purchased from Himedia laboratories Ltd, Mumbai. All other chemicals used were of analytical grade.
Synthesis and characterisation of sodium alginate L-cysteine conjugates
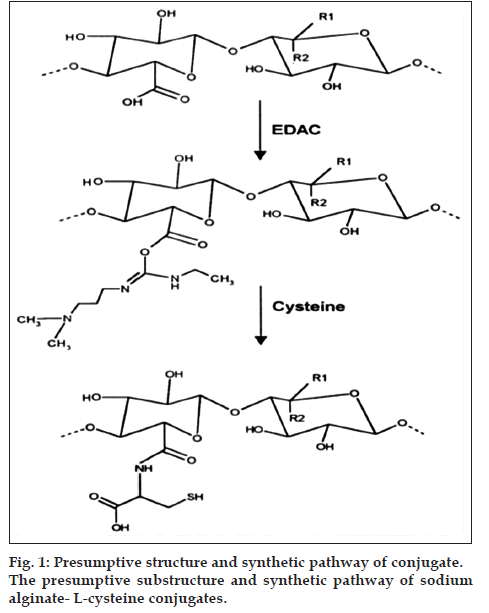
The covalent attachment of L-cysteine to sodium alginate was achieved by the formation of amide bonds between the primary amino group of the amino acid and a carboxylic acid group of the polymer. First, 10 g of sodium alginate was hydrated in 1 liter of demineralized water. The carboxylic acid moieties of hydrated sodium alginate, were activated by the addition of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDAC) in a final concentration of 50 mM, the reaction was allowed to proceed for 45 min. L-cysteine monohydrate hydrochloride was added to a weight ratio of 2:1 and the pH was adjusted to 4.0 with 1 M HCl. The Reaction mixtures were incubated for 2 h under stirring at room temperature. The resulting alginate cysteine conjugates were isolated by dialyzing at 10° against 1 mM HCl, followed by two times against the same medium but additionally containing 1% of NaCl and then exhaustively against 1 mM HCl (pH 4.0). Thereafter, sample was dried in dessicator under vacuum. The dried conjugated sodium alginate was powdered using glass mortar-pastel and passed through sieve number 60 and stored in desiccator until further use. The presumptive substructure and synthetic pathway of sodium alginate L-cysteine is shown in fig. 1 [5].
Characterisation of sodium alginate L-cysteine conjugates
Sodium alginate L-cysteine conjugate was characterised to confirm conjugate were formed by determining the charring point, further confirmed by FT-IR spectroscopic and differential scanning calorimetric analysis (DSC).
Determination of charring point
The charring point of the sodium alginate and sodium alginate L-cysteine conjugate was determined by taking approximately 5 mg of the sample in a glass capillary tube sealed at one end. The sample containing capillary was placed in melting point apparatus and the temperature was increased gradually. The temperature at which sample charred completely was noted down as the charring point of that sample in degree celsius.
FT-IR spectrophotometric analysis
The samples of sodium alginate and sodium alginate L-cysteine conjugate were prepared in the form of KBr pellets and subjected for scanning from 4000 cm-1 to 400 cm-1 using FT-IR spectrophotometer (FT-IR-8400, Shimadzu, Japan).
Differential scanning calorimetric analysis
Approximately 2 mg of sodium alginate and sodium alginate L-cysteine conjugate sample was taken in aluminum pan, sealed with aluminum cap and kept under nitrogen purging (atmosphere). Both the samples were scanned from 0-300° with the scanning rate of 10° rise/min using differential scanning calorimeter (DSC-60, Shimadzu, Japan).
Drug polymers compatibility study
The compatibility of drug with polymers was determined by subjecting the drug, polymers and physical mixture of drug and polymers (1:1) for DSC. Approximately 2 mg of each sample was taken in aluminum pan, sealed with aluminum cap and kept under nitrogen purging (atmosphere). The samples were scanned from 0-300° with the scanning rate of 10° rise/min using differential scanning calorimeter (DSC-60, Shimadzu, Japan).
Preparation of matrix tablets
Tablets were prepared by wet granulation method. A proportion of drug, polymers and all other excipients as shown in Table 1 were passed through sieve #60 before use. A physical mixture of drug and polymers was suitably moistened with required quantity of PVP K30 in isopropyl alcohol (10% w/v) and sieved (#16) to get the wet granules. The granules were dried at 40º for 2 h, and the dried granules were resized using sieve (#22) to get the uniform sized granules. The dried granules were admixed with talc and magnesium stearate and compressed on a single station tableting machine (Cadmach machinery co. Pvt, Ltd Ahmedabad, India) using a flat faced 9.0 mm punches.
| Formulation code | Atorvastatin calcium (mg) | Conjugated sodium alginate (mg) | Sodium alginate (mg) |
|---|---|---|---|
| S0 | 50 | ? | 200 |
| S1 | 50 | 30 | 170 |
| S2 | 50 | 60 | 140 |
| S3 | 50 | 100 | 100 |
| S4 | 50 | 140 | 60 |
| S5 | 50 | 170 | 30 |
Table 1: Formulation Of Matrix Tablets
Pre compressional evaluation of granules
The prepared granules were subjected for pre compressional evaluation by determining the bulk density, tapped density, compressibility index, Hausner ratio and flow properties (angle of repose) by using standard procedures. All studies were carried out in triplicate (n=3) and average values are reported.
Thickness and diameter of the compressed tablet
The thickness and diameter of the tablets was determined by using dial thickness apparatus and vernier calipers respectively. Five tablets from each formulation were used and average values were noted.
Hardness
Tablet hardness of all the formulations was determined by using Monsanto hardness tester. Five tablets from each formulation were used and average values were recorded.
Friability
The tablets from different batches were subjected for friability test using friabilator. Ten tablets were weighed (W0) and placed inside the Roche friabilator. The instrument was operated for 4 min at 25 rpm. The resulting tablets after 100 falls from a height of six inches were collected; weighed (Wt) and percentage loss was calculated using following equations (W2–Wt)/Wt×100.
Weight variation test
The weight variation studies of the prepared formulations were performed as per the standard procedure following Indian pharmacopoeia [6]. The sample mean and standard deviation of each batch of tablets were reported.
Determination of drug content
One tablet from each formulations of matrix tablets, were powdered and transferred in to 100 ml volumetric flask. Initially 30 ml of methanol and 20 ml of phosphate buffer (pH 7.4) was added and allowed to rotate in a rotary shaker for 24 h. The final volume was made up with phosphate buffer (pH 7.4). The solution was filtered suitably and amount of atorvastatin calcium present in the solution was estimated by using UV spectrophotometer (UV 1601, Shimadzu, Japan) at 245 nm against a suitable blank.
Swelling index
One tablet from each formulation was randomly selected, weighed individually (W1) and placed separately in a wire basket which was placed in a 100 ml beaker containing phosphate buffer (pH 7.4) for 12 h and later pH 7.4 (24 h). After each hour up to 12 h, the tablets were removed from wire basket and excess water was removed using filter paper. The swollen tablets were reweighed (W2) and swelling index of each tablet was calculated using the equation percent of swelling index=(W2–W1)/W1×100.
Moisture uptake study
The tablets were weighed individually (W1) and exposed at temperature 40±2° and relative humidity 75±5% in programmable environmental test chamber (CHM-10S, Remi Instruments Ltd., Mumbai, India) till the weight of the tablet remained constant (W2). The percent moisture uptake was calculated using the formula Percentage moisture content=(initial weight–final weight)/final weight×100.
In vitro drug release study
Dissolution experiments were carried out in a USP basket type apparatus at 50 rpm at 37±0.5°. Drug release studies were conducted in 900 ml of phosphate buffer pH 7.4 up to 12 h samples of 10 ml were withdrawn at predetermined time intervals and were replaced with fresh dissolution medium to maintain sink conditions. Samples withdrawn were filtered and assayed spectrophotometrically at 245 nm.
Release kinetics
In vitro release data were fit to first order, zero order and higuchi equations to analyse the kinetics of drug release from the tablets. Further, in vitro release results were fit to thefollowing Koresmeyer-Peppas equation, to analyse drug release mechanism Mt/Mα=Ktn Where M t/Mαis the fraction of drug released at time t, K is kinetic constant and ‘n’ is release exponent that characterise the drug transport.
Results and Discussion
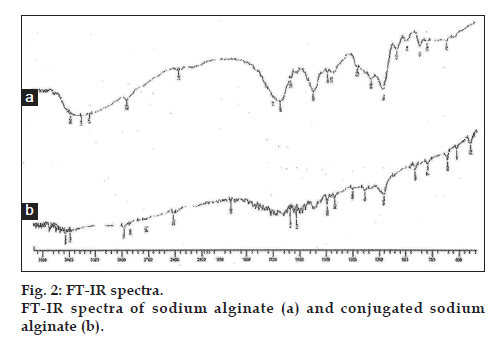
The present study was aimed to synthesis, characterise sodium alginate conjugate and study the effect of conjugation on drug release. The charring point of sodium alginate and sodium alginate L-cysteine conjugate were found to be 300° and 210°, respectively. This might be due to conjugation of sodium alginate with L-cysteine. The FT-IR spectra of sodium alginate L-cysteine conjugate, showed the bands representingthe -C=O stretching of amide bond (at 1859.08 cm-1) and -SH stretching (at 2674.10 cm-1) which were absent in the FT-IR spectra of the sodium alginate. The additional peaks in the FT-IR spectra of the conjugated sodium alginate have confirmed the conjugation of sodium alginate with L-cysteine. The FT-IR spectra of sodium alginate and conjugated sodium alginate are shown in fig. 2.
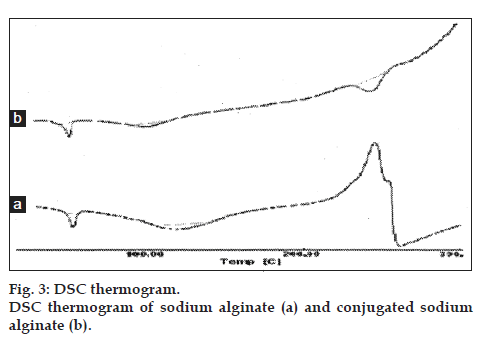
The DSC thermogram of conjugated sodium alginate, have shown one extra exothermic peak at 245° represented the amino group, which was absent in the DSC thermogram of sodium alginate. This has further confirmed the conjugation of sodium alginate with L-cysteine. The DSC thermograms of sodium alginate and conjugated sodium alginate are shown in fig. 3.
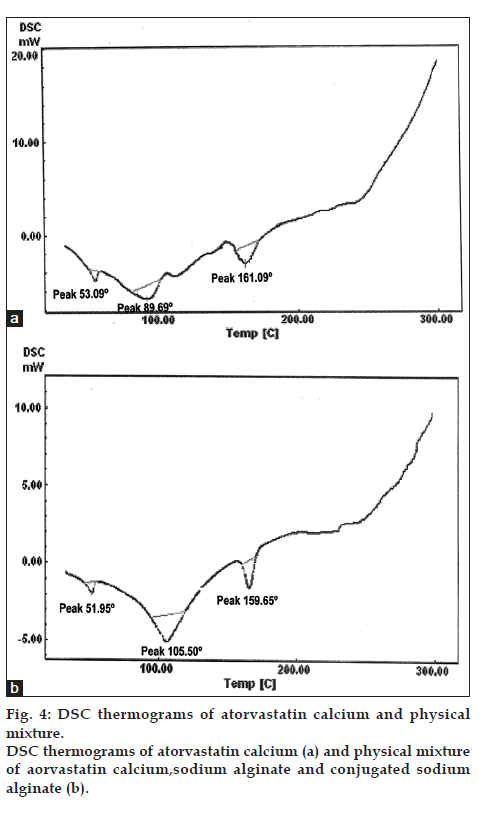
The thermogram of atorvastatin calcium showed a sharp endothermic peak at 161.09º fig. 4a has evident that purity of the compound, same peak was retained in the physical mixture of drug, sodium alginate and sodium alginate L-cysteine conjugate (fig. 4b). These observations suggested that drug and polymers are compatible.
The bulk and tapped density of prepared granules were found to be in the range of 0.188-0.236 and 0.238-0.296, respectively. Carr’s compressibility index and Hausner ratio were determined to be less than 22% and 1.27 for all formulations respectively, which suggested that the prepared granules of all the formulations have fair or passable property. The results of angle of repose evident that the granules of all the formulations are fair or passable and further by the incorporation of the glidants at 1%, the flowability of the granules improved. All the formulations showed a hardness value in the range of 7.86±0.124 to 8.2±0.2 kg/cm2; it suggested that the hardness is depending on the quantity and type of polymer used in the tablet. The percentage friability of all formulations was found in the range of 0.29±0.04 to 0.66±0.07%; indicating that the friability was within the acceptable limits and results were shown in Table 2, the result of weight variation of tablets and diameters for all formulations were found in the range of 248.2±2.34 to 249.7±1.94 mg and 9.03±0.047 to 9.06±0.047 mm indicating that they were within the acceptable limits. The percentage drug content for all formulations was found in the range of 97.±0.2 to 100.01±0.719%.
| Code | Bulk density(g/cc) | Tapped density (g/cc) | Carrs index percentage | Hausner ratio | Hardness (kg/cm2) | Friability(%) | Thickness(mm) | Moistureuptake (%) |
|---|---|---|---|---|---|---|---|---|
| S0 | 0.22±0 | 0.28±0 | 19.3 | 1.239 | 8.05±0.15 | 0.39±0.76 | 3.54±0.025 | 17.4±1.38 |
| S1 | 0.19±0 | 0.24±0 | 19.2 | 1.238 | 8.03±0.25 | 0.29±0.04 | 3.53±0.04 | 31.63±0.19 |
| S2 | 0.18±0 | 0.24±0 | 21.0 | 1.265 | 8.2±0.2 | 0.51±0.01 | 3.55±0.03 | 46.34±0.92 |
| S3 | 0.20±0 | 0.26±0 | 20.2 | 1.252 | 8.13±0.15 | 0.49±0.29 | 3.55±0.01 | 77.16±0.73 |
| S4 | 0.24±0 | 0.3±0 | 20.2 | 1.253 | 7.93±0.12 | 0.43±0.14 | 3.52±0.05 | 104.84±2.3 |
| S5 | 0.21±0 | 0.26±0 | 19.5 | 1.243 | 7.86±0.12 | 0.66±0.07 | 3.446±0.0 | 132.64±3.5 |
Table 2: Precompressional Charactrisation Of Granules
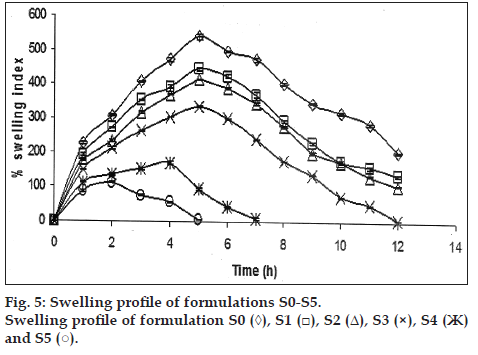
Swelling indices were measured for all formulations for 12 h in phosphate buffer pH 7.4 and it was observed the swelling index of S0, S1, S2, and S3 the swelling index after 12 h were 201.0±2.05%, 135.6±3.65%, 106.0±3.0% and 6.8±1.51, respectively. The swelling index of S4 after 7 h was 9.96±2.55% and for S5 swelling index after 5 h it was 6.6±0.65%.
The formulation S0 contains only sodium alginate, in formulations S1, S2 and S3 contains 30, 60 and 100 mg of sodium alginate was replaced by sodium alginate L-cysteine conjugate, respectively. During the study it was observed that swelling indices were increased up to five hour and later decreased (fig. 5). This observation was suggested that, during study swelling takes place up to five hour after that tablets were not maintaining the integrity and swelling indices values decreased with increased proportion of sodium alginate conjugate. This could be due to that presence of sodium alginate conjugate decreases the gel strength and there by affecting the intactness of the tablets [3].
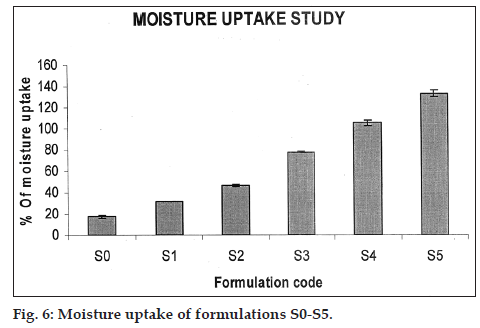
The percent moisture uptake for the tablets of S0 to S5 varied from 17.41±1.38% to 132.64±3.52%. The data suggested that as the proportion of sodium alginate L-cysteine conjugate increased in the formulations leads to increase in the moisture uptake of the tablets this is due to sodium alginate L-cysteine conjugate contain a sulphahydroxil moiety which is more hydrophilic in nature this leads more up take of moisture, thus increased up take of moisture with increased proportion of sodium alginate L-cysteine conjugate in the formulation attributed to swelling behavior of the formulations. The results of moisture uptake study are given in Table 2 and fig. 6.
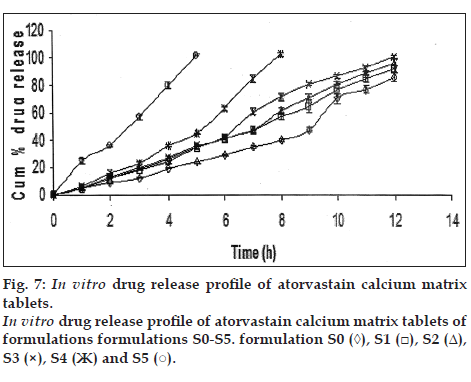
All the prepared matrix tablets were subjected for in vitro release study and it was observed that in the initial hour of the study all the tablets were shown drug release same except formulation S5 where drug release was fivefold more than the other formulations. The same trend was continued as the time of study increased and for formulations S0, S1, S2, and S3 the drug released after 8 h was 40.11±1.33, 57.16±2.52, 60.95±2.35 and 71.25±3.15 in cumulative percent, respectively, whereas for formulation S4 cumulative percent drug release was 101.5±1.77 and for S5 101.2±0.62 drug released was occurred within 5 h fig. 7. This pattern of drug release form the prepared matrix tablets attributed due to the presence of sodium alginate L-cysteine conjugate, as the proportion of sodium alginate L-cysteine conjugate increased in the formulation increases the drug release as presence of sodium alginate L-cysteine conjugate decreases the gel strength and also impart the more hydrophilicity to the matrix and act as channeling and pore forming agent thereby it allow more amount of drug permeation through the matrix. This pattern of drug release form the matrix was also supported by swelling indices values and moisture up take results.
To establish the mechanism of drug release from the matrix tablets the data were treated to according to first order, zero order and Higuchi equations and it was observed that all formulations except S0 showed the linearity with respect to zero order (R2=0.9627-0.9947) as compared to first order (R2=0.8166-0.921) as shown in Table 3. Whereas formulation S0 showed linearity with respect to first order equation (R2=0.9997). Higuchi equation regression values for all formulations ranges from (R2=0.7994-0.9014). Hence to confirm precisely the domination mechanism; the data was plotted according to Koresmeyer-Peppas equation. The ‘n’ value (diffusional exponent) indicated the mechanism of drug release. For tablet systems, if n<0.45 it suggests the Fickian diffusion; if 0.46<n<0.89, it suggests the anomalous (non-Fickian) transport, for n=0.89, the zero-order release is possible and if n>0.89, a super case-?? transport is operative. In order to predict and correlate the release behavior of drugs from the tablet, it is necessary to fit them in to release kinetics profiles (Fickian, anomalous or super-case ??). This will facilitate the understanding of mode of drug release such as whether the release is because of only diffusion or only erosion or caused by both diffusion and erosion. The ‘n’ values were calculated for all formulations; ‘n’ values were found in the range of 1.408-1.9263 Table 3, indicating a super case-?? transport. These observations confirmed that both diffusion and erosion is dominant mechanism for drug release and same observations were observed during swelling study, moisture up take study and drug release study. Hence the matrix system consists of sodium alginate and sodium alginate L-cysteine is ideal system for sustaining the drug release from the matrix.
| Code | Zero?order (R2) | First?order (R2) | Higuchi (R2) | Korsmeyer(n) |
|---|---|---|---|---|
| S0 | 0.9479 | 0.9997 | 0.7994 | 0.924 |
| S1 | 0.9947 | 0.8747 | 0.8865 | 0.913 |
| S2 | 0.9931 | 0.8265 | 0.8811 | 0.917 |
| S3 | 0.9892 | 0.9 | 0.8871 | o. 9182 |
| S4 | 0.9627 | 0.8166 | 0.7997 | 0.8913 |
| S5 | 0.9923 | 0.921 | 0.9014 | 0.6126 |
Table 3: Mathematical Modeling And Drug Release Kinetics Of Atorvastatin Calcium Matrix Tablets
The present research investigation was concluded that the presence of sodium alginate L-cysteine has great effect on mechanism of drug release from the matrix system. It also act as channelizing agent and pore forming agent, thereby imparts the pores in the matrix system and enhancing the drug release from the matrix system.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Prajapati ST, Patel LD, Patel DM. Studies on formulation and in vitro evaluation of floating matrix tablets of domperidone. Indian J Pharm Sci 2009;71:19-23.
- Martindale: The Complete Drug Reference. 33rd ed. London: Pharmaceutical Press; 2002. p. 842.
- Satheeshababu BK, Shivakumar KL. Synthesis of conjugated chitosan and its effect on drug permeation from transdermal patches. Indian J Pharm Sci 2013;75:162-70.
- Kim JS, Kim MS, Park HJ, Jin SJ, Lee S, Hwang SJ. Physicochemical properties and oral bioavailability of amorphous atorvastatin hemi-calcium using spray-drying and SAS process. Int J Pharm 2008;359:211-9.
- Bernkop-Schnürch A, Kast CE, Richter MF. Improvement in the mucoadhesive properties of alginate by the covalent attachment of cysteine. J Control Release 2001;71:277-85.
- Ministry of Health and Family Welfare. Indian Pharmacopoeia. New Delhi: Government of India, Controller of Publications; 1996. p. 736.