- *Corresponding Author:
- R. K. Gupta
Department of Applied Chemistry, Delhi Technological University, Rohini, Delhi 110042, India
E-mail: rkg67ap@yahoo.com
| Date of Received | 24 May 2023 |
| Date of Revision | 04 January 2024 |
| Date of Accepted | 15 May 2024 |
| Indian J Pharm Sci 2024;86(3):1143-1148 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Citric acid crosslinked Carica papaya seeds mucilage-based hydrogel film were synthesized and characterized. The extracted mucilage and hydrogel film were explored by determining their physicochemical, thermal, micrometric and spectrophotometric properties. The results of attenuated total reflectance-Fourier transform infrared spectroscopy and solid state 13C-nuclear magnetic resonance spectroscopy confirms the emergence of ester crosslinking between the mucilage and citric acid. The swelling study of mucilage and hydrogel film revealed good swelling ability of prepared hydrogel film. Also, the hydrogel film showed better thermal stability than the mucilage. The current study showed that papaya seed mucilage can be used as a bio-based source for hydrogel using citric acid as a cross-linker. Further, it can be potentially explored in pharmaceutical, food and cosmetic industries.
Keywords
Carica papaya mucilage, citric acid, cross-linking, hydrogel film
Hydrogels are referred as three-dimensional crosslinked polymeric networks, which tend to hold an enormous amount of water and swell because of their porous structure. These polymeric materials do not dissolve in water at physiological pH levels and temperatures, instead they remarkably swell in aqueous conditions[1]. Hydrophilic monomers and polymers along with multifunctional crosslinkers are often utilized in copolymerization/ cross-linking free-radical polymerizations to create hydrogels[2]. The hydrogels made from natural bio-degradable polymers are preferred more over synthetic polymers due to their costeffectiveness, bio-degradable and bio-compatible nature[3]. Mucilage is a natural polysaccharide that combines with water to form a viscous solution. It is a common plant property with a variety of characteristics, often present around plant structures that come into contact with the environment and serves a variety of purposes including adhesion and protection[4]. It is a costeffective, eco-friendly, edible polysaccharide which is mainly composed of carbohydrates having extensively branched structures. Mucilages are used for a variety of purposes, including gelling, film-forming and texturing in food and nutraceuticals, disintegrants and binders for drug delivery systems in pharmaceuticals and stabilizers in cosmetics[5]. Carica papaya (Papaya), which belongs to Caricaceae family, is a highly commercialized tropical fruit[6]. It is one of the most significant tropical plants cultivated worldwide. The papaya plant has numerous edible and medicinal elements, including roots, leaves, peels, latex, flowers, fruits and seeds. Despite having medicinal value, papaya seeds are typically thrown during the fruit processing. The seeds make up about 20 % of a papaya's fresh mass. Protein, dietary fiber, phytochemicals, antioxidants and minerals are present in both the seeds and the leaves. Regardless of cultivar, the seeds and leaves contain 16 %-32 % protein. The seeds are nutritious alternative energy sources that could supplement the undernourished population since they include a considerable quantity of lipid (21 %-30 %) and carbohydrate (8 %-58 %) in the seeds and leaves[7]. The hydrogels made from natural polymers can be synthesized through chemical or physical crosslinking. However, physically cross-linked hydrogels are mechanically fragile in nature and the cross-linked structure is susceptible to breakdown in response to changes in environmental variables such temperature, pH or ionic strength[8]. Therefore, the preferred method for the synthesis of hydrogel is chemical crosslinking. Although, there have been allegations of unsafe crosslinking chemicals being used in the preparation of chemically cross-linked hydrogels, despite the fact that they are resilient. In order to overcome this safety concern, citric acid can be utilized as a cross-linker. Citric acid has recently come to light as a safe cross-linking agent for the synthesis of hydrogels. Citric acid creates a cyclic anhydride at high enough temperatures, which esterifies the hydroxyl groups on nearby polymer chains. As a result, crosslinks start to form. It is derived from renewable resources that are mostly produced by the fermentation of carbohydrates like starch or glucose. It is also easily accessible and reasonably priced[9]. Moreover, as citric acid is a metabolic product of human body which is non-toxic in nature and used as a natural food additive in food industry[10]. In the present study, Papaya seed mucilage (Pm) was extracted and Pm-based Hydrogel films (Pm-CAH) with various formulations using citric acid as a cross-linking agent were synthesized. Extracted mucilage and Pm-CAH-3 hydrogel film were selected for various characterizations and evaluated for swelling study. According to our literature survey, Pm has not been used with citric acid in hydrogel film preparation to the best of our knowledge. For this study, papaya seeds were obtained from papaya purchased from local market, New Delhi, India. Mucilage of Carica papaya was extracted using a previously described method, with some modifications[11]. Briefly, the mucilage was removed from the papaya seeds with the help of forceps. The mucilage was washed with acetone to remove the impurities. Then, the mucilage was left to dry in hot air oven at 50° for 24 h. The dried and finely powdered mucilage was stored in an air tight container. The pH of the 1 % (w/v) aqueous solution of extracted mucilage was calculated using a calibrated digital pH meter. The obtained mucilage was confirmed by various identification tests like ruthenium red test (mucilage), ferric chloride test (tannins), Wagner’s test (alkaloids) and ninhydrin test (protein)[12]. The hydrogel films were synthesized by reacting Pm with citric acid, following the previously described method[13]. 1 % w/v aqueous solution of Pm was prepared using deionized water with constant stirring using magnetic stirrer for 2 h at 27°. Then 0.2 % (w/v) citric acid solution was added to the homogenized mucilage solution and stirred for another 1 h. The solution was casted into petri dish and cured for 5 min at 140°. The cured hydrogel film was washed with deionized water in order to remove unreacted citric acid. Then, it was dried in hot air oven at 50° for 24 h and stored in desiccator for further use. In order to study the effect of cross-linker on the properties of Pm-CAH hydrogel films, different formulations were prepared, which is summarized in Table 1. The mucilage and Pm-CAH-3 hydrogel film were characterized using Fourier Transform Infrared spectroscopy (FTIR, Perkin Elmer, spectrum version two), Thermogravimetric Analysis (TGA, Perkin Elmer, TGA 4000), X-Ray Diffraction analysis (XRD, Bruker D8 Avance), Scanning Electron Microscopy (SEM, Carl Zeiss, EVO 18), 1H Nuclear Magnetic Resonance (NMR) (Bruker Avance III) and 13C-NMR (Bruker Avance-600). The Swelling Index (SI) of mucilage and hydrogel film was carried using previously described method with slight modifications[5,14]. Relative humidity on day of performing swelling experiments was found to be 40 %. For mucilage, 1 g was immersed in 10 ml deionized water at 27° and 2 ml methanol was added for better dispersion. It was kept at 27° till constant weight was observed. The study was done in triplicate. The increase in volume was observed and recorded. SI was calculated using the following equation 1.
| Formulations | Parameters | |
|---|---|---|
| Pm (% w/v) | CA (% w/v) | |
| Pm-CAH-1 | 1 | 0.05 |
| Pm-CAH-2 | 1 | 0.1 |
| Pm-CAH-3 | 1 | 0.2 |
| Pm-CAH-4 | 1 | 0.3 |
| Pm-CAH-5 | 1 | 0.4 |
Table 1: Composition of Mucilage Based Hydrogel Films
% SI=Vf-Vi/Vi×100 (1)
Where, Vf is the final volume after hydration of mucilage and Vi is the initial volume before hydration of mucilage. The SI of hydrogel film and the effect of temperature on hydrogel film’s network were studied in distilled water. 1 g was immersed in 10 ml deionized water at 27°. The sample was weighed after a fixed interval of time and immersed again in deionized water, until constant weight was observed. The result presented in this study was the mean values of triplicate measurements. The SI was calculated using the following equation 2.
% SI=Ws-Wd/Wd×100 (2)
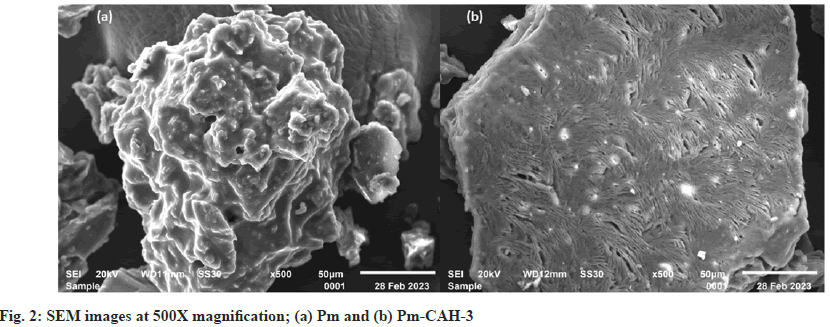
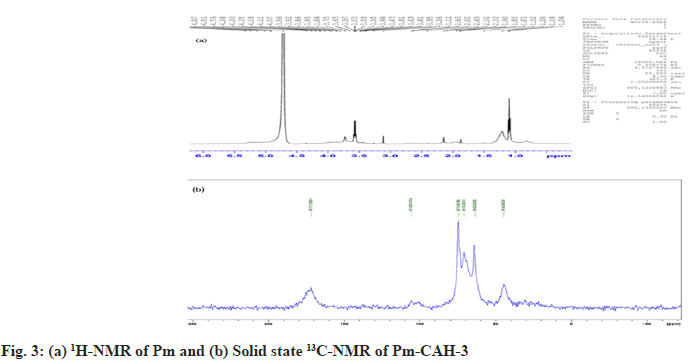
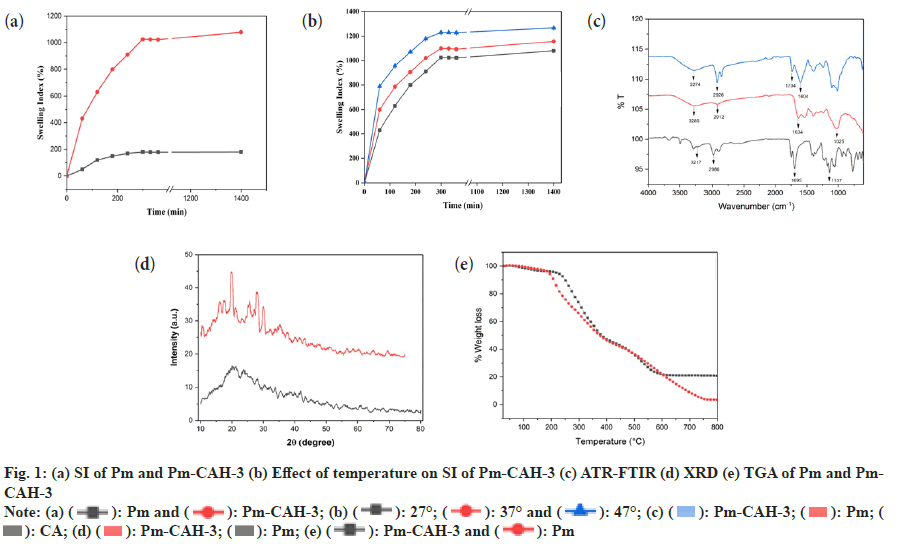
Where, Ws represents the weight of swollen sample at a certain time and Wd represents the weight of dried sample. In the present study, we successfully extracted the mucilage from papaya seeds, which was brown in appearance and had a rough fracture. The pH of mucilage solution (1 % w/v) was found to be neutral (6.89±0.04), which indicates that it can be explored as an excipient in various pharmaceutical formulations. The qualitative identification test, shown in Table 2, confirms the presence of mucilage and absence of impurities like tannins, alkaloids and proteins. The good quality of hydrogel film was successfully developed with a minimum citric acid concentration of 0.2 % w/v. Citric acid concentration above 0.3 % w/v resulted in the formation of hard hydrogel films and citric acid concentration below 0.2 % w/v led to the formation of soft hydrogel films. The formation of crosslinks between citric acid and Pm is due to the esterification reaction. When citric acid is heated at higher temperature, an intermediate cyclic anhydride is formed which is responsible for the formation of crosslinks with Pm. The cyclic anhydride intermediate opens via esterification under the action of mucilage-OH group, resulting in the formation of a new carboxylic acid unit in carboxylic acid with the property of creating new intra-molecular anhydride moiety with neighboring carboxylic acid unit[15]. SI value of Pm and Pm-CAH-3 at different time intervals, as shown in fig. 1a, was found to be 180 % and 1025 % respectively, which indicates that the SI of mucilage was lower than that of the synthesized hydrogel. The effect of temperature on hydrogel film is presented in fig. 1b. The SI (%) of film at 27°, 37° and 47° was found to be 1025±0.1, 1098.6±0.01, 1229.33±0.1, respectively. As the temperature was increased the SI was found to be increased. This might be attributed to increase in the mess size of the cross-linked polymer. Therefore, the hydrogel has greater capability to absorb and retain huge amount of water compared to its precursor. Thus, the hydrogel is showing greater swelling capability than the mucilage alone. Attenuated Total Reflectance (ATR)-FTIR spectra of citric acid, Pm and Pm-CAH-3 are shown in fig. 1c. ATR-FTIR spectrum of citric acid showed a broad peak at 3217 cm-1 assigned to the stretching vibration of the -OH group, 2986 cm-1 due to C-H asymmetric vibration and a sharp peak at 1695 cm-1 attributed to the hydrogen bonded C=O stretching vibration[13]. ATR-FTIR spectrum of Pm exhibited a broad band around 3280 cm-1 due to -OH stretching vibration. The peak obtained at 2912 cm-1 is due to asymmetric vibration of C-H group[16]. The absorption peak at 1634 cm-1 is due to stretching vibration of carbonyl (C=O) group. The sharp peak at 1025 cm-1 showed the presence of C-O-C and C-O-H stretching vibrations of glycosidic bond in the polysaccharide[12]. The ATR-FTIR spectrum of hydrogel film (Pm-CAH-3) showed an additional peak at 1734 cm-1 assigned to the carbonyl bond (C=O) of ester formed during the crosslinking of the polymer, which confirmed the cross-linking of citric acid with the mucilage[17]. XRD is usually done for the analysis of crystalline, semi-crystalline and amorphous nature of the material. Fig. 1d shows the XRD pattern of Pm and Pm-CAH-3. In the XRD diffractogram of Pm, there were no sharp peaks and there was a broad diffraction peak around 15°-28°, which indicates the amorphous nature of the mucilage. In case of hydrogel film, due to the crosslinking of the mucilage with CA, it showed some degree of crystallinity. Thus, it can be concluded that there may be showed transformation from amorphous to semi-crystalline nature[18]. TGA thermograms of Pm and Pm-CAH-3 are presented in fig. 1e. Thermal decomposition curve of Pm showed three weight loss events, with an initial weight loss of around 4.11 % between 30°-190° due to the desorption of free and bound water from the mucilage. The second stage ranges from 190°-420° with 56.50 % weight loss, due to the decomposition of the mucilage, which results in the breaking of the polysaccharide branches. The final stage is in the range of 500°-800°. This is due to the degradation of the polysaccharide backbone[19]. Thermal decomposition of hydrogel film (Pm-CAH-3) occurs in three stages, 30°- 200°, 200°-460°, 460°-800° with 3.92 %, 51.82 % and 17.86 % weight loss. The first stage weight loss occurred due to loss of moisture, second phase weight loss was due to the decomposition of citric acid cross-linked Pm mucilage hydrogel and third phase weight loss was due to breakdown of the main polymeric backbone[20]. The residual weights at 800° were 3.38 % and 20.55 % for Pm and Pm-CAH-3 respectively. The high residual weight in case of hydrogel film indicates the less decomposition of the citric acid cross-linked Pm-CAH-3 than Pm. Thus, the thermal stability of the mucilage was improved by crosslinking it with citric acid to form hydrogel. Fig. 2 shows the SEM images of Pm and Pm-CAH-3 at 500X magnification. As shown in the figure, the mucilage is having high degree of irregularity in shape and dimension. It has a rough and uneven surface. In case of hydrogel film, it has smooth and homogeneous surface with small pores which suggests the homogeneous crosslinking[9]. The liquid state 1H-NMR of mucilage and solid state 13C Cross-Polarization-Magic Angle Spinning (13C CP-MAS) NMR spectra of hydrogel film is shown in fig. 3. In 1H-NMR of mucilage, the peak around δ 1.2 ppm is attributed to methyl groups. The spectrum shows a crowded region near δ 3.1-3.8 ppm, which specifies the polysaccharide region and indicates the presence of different sugar units in the mucilage. The peak in between δ 3-4.3 ppm is assigned to non-anomeric protons[21,22]. Solid state 13C-NMR of Pm-CAH-3 shows three distinct peaks.
| Identification test | Active constituent | Observation Pm |
|---|---|---|
| Ruthenium red test | Mucilage | + |
| Ninhydrin test | Protein | - |
| Ferric chloride test | Tannin | - |
| Wagner’s test | Alkaloids | - |
Note: (+): Present and (-): Absent
Table 2: Phytochemical Identification of the Extracted Mucilage
The peak at δ 74 ppm is due to carbon atom connected to hydroxyl group (-OH), δ 63 ppm is assigned to C6 carbon atom of CH2OH group and δ 105 ppm is assigned to the anomeric carbon (C1) of the polysaccharide. The broad resonance peak in the range of δ 172-180 ppm confirms the formation of ester crosslinks in the hydrogel film[23]. In conclusion, this study shows the utilization of mucilage from Carica papaya seeds for the development of citric acid cross-linked Pm-CAH hydrogel films. The current research work first time reports that Carica papaya seeds mucilage can be explored as a hydro-gelling agent and citric acid can be used as a cross-linker for Pm. ATR-FTIR and solid state 13C-NMR confirms the ester linkage formation between Pm and CA. TGA analysis showed that the synthesized hydrogel film has more thermal stability than the mucilage. The SI of mucilage was improved by crosslinking it with citric acid to form hydrogel. Thus, it can be concluded that Pm has ability to form hydrogel film using citric acid as a crosslinking agent. The overall study reveals that further studies on the Pm based hydrogel film can be addressed to potential application in pharmaceutical, food, and cosmetic industries.
Acknowledgements:
The authors would like to thank the Department of Applied Chemistry, Delhi Technological University, for the support and constant encouragement to carry out this work.
Conflict of interest:
The authors declare no conflict of interest.
References
- Bharskar GR. A review on hydrogel. World J Pharm Pharm Sci 2020;9:1288-98.
- Shen X, Shamshina JL, Berton P, Gurau G, Rogers RD. Hydrogels based on cellulose and chitin: fabrication, properties, and applications. Green Chem 2016;18(1):53-75.
- Ahmed EM. Hydrogel: Preparation, characterization, and applications: A review. J Adv Res 2015;6(2):105-21.
[Crossref] [Google Scholar] [PubMed]
- Viudes S, Burlat V, Dunand C. Seed mucilage evolution: Diverse molecular mechanisms generate versatile ecological functions for particular environments. Plant Cell Environ 2020;43(12):2857-70.
[Crossref] [Google Scholar] [PubMed]
- Archana G, Sabina K, Babuskin S, Radhakrishnan K, Fayidh MA, Babu PA, et al. Preparation and characterization of mucilage polysaccharide for biomedical applications. Carbohydr Polym 2013;98(1):89-94.
[Crossref] [Google Scholar] [PubMed]
- Sani MS, Bakar J, Rahman RA, Abas F. Effects of coated capillary column, derivatization, and temperature programming on the identification of Carica papaya seed extract composition using GC/MS analysis. J Anal Test 2020;4:23-34.
- Dotto JM, Abihudi SA. Nutraceutical value of Carica papaya: A review. Sci Afr 2021;13:e00933.
- Hussain HR, Bashir S, Mahmood A, Sarfraz RM, Kanwal M, Ahmad N, et al. Fenugreek seed mucilage grafted poly methacrylate pH-responsive hydrogel: A promising tool to enhance the oral bioavailability of methotrexate. Int J Biol Macromol 2022;202:332-44.
[Crossref] [Google Scholar] [PubMed]
- Franklin DS, Guhanathan S. Synthesis and characterization of citric acid-based pH-sensitive biopolymeric hydrogels. Polymer Bull 2014;71:93-110.
- Coma V, Sebti I, Pardon P, Pichavant FH, Deschamps A. Film properties from crosslinking of cellulosic derivatives with a polyfunctional carboxylic acid. Carbohydr Polymer 2003;51(3):265-71.
- Prasanna ML, Shailaja P, Reddy JM, Narendra D. Isolation, Purification of Carica papaya and Ocimum basilicum seed mucilages & preparation of valsartan oro dispersable tablets by using natural superdisintegrants. Int J Sci Dev Res 2019;4:41-8.
- Deore UV, Mahajan HS. Isolation and structural characterization of mucilaginous polysaccharides obtained from the seeds of Cassia uniflora for industrial application. Food Chem 2021;351:129262.
- Mali KK, Dhawale SC, Dias RJ. Synthesis and characterization of hydrogel films of carboxymethyl tamarind gum using citric acid. Int J Biol Macromol 2017;105:463-70.
[Crossref] [Google Scholar] [PubMed]
- Liu P, Peng J, Li J, Wu J. Radiation crosslinking of CMC-Na at low dose and its application as substitute for hydrogel. Radiat Phys Chem 2005;72(5):635-8.
- Demitri C, Del Sole R, Scalera F, Sannino A, Vasapollo G, Maffezzoli A, et al. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J Appl Polymer Sci 2008;110(4):2453-60.
- Jose SM, Anilkumar M. Anti-inflammatory, antioxidant, and dye removal properties of mucilage isolated from Litsea quinqueflora (Dennst.) Suresh. Chem Papers 2021;75:6531-43.
- Reddy N, Yang Y. Citric acid cross-linking of starch films. Food Chem 2010;118(3):702-11.
- Bucak CD. Porous alginate hydrogel beads cross-linked with citric acid containing tannic acid: Structural analysis, antimicrobial properties and release behavior. Cellulose 2023;30(2):1117-32.
- da Silveira Ramos IF, Magalhães LM, do O Pessoa C, Ferreira PM, dos Santos Rizzo M, Osajima JA, et al. New properties of chia seed mucilage (Salvia hispanica L.) and potential application in cosmetic and pharmaceutical products. Ind Crop Prod 2021;171:113981.
- Dharmalingam K, Anandalakshmi R. Fabrication, characterization and drug loading efficiency of citric acid crosslinked NaCMC-HPMC hydrogel films for wound healing drug delivery applications. Int J Biol Macromol 2019;134:815-29.
[Crossref] [Google Scholar] [PubMed]
- Singh S, Bothara S. Morphological, physico-chemical and structural characterization of mucilage isolated from the seeds of Buchanania lanzan Spreng. Int J Health Allied Sci 2014;3(1):33.
- Deore UV, Mahajan HS. Isolation and characterization of natural polysaccharide from Cassia obtustifolia seed mucilage as film forming material for drug delivery. Int J Biol Macromol 2018;115:1071-8.
[Crossref] [Google Scholar] [PubMed]
- Ghorpade VS, Yadav AV, Dias RJ. Citric acid crosslinked ß-cyclodextrin/carboxymethylcellulose hydrogel films for controlled delivery of poorly soluble drugs. Carbohydr Polym 2017;164:339-48.
[Crossref] [Google Scholar] [PubMed]
 ): Pm and ( ): Pm-CAH-3; (b) (
): Pm and ( ): Pm-CAH-3; (b) ( ): 37° and (
): 37° and ( ): 47°; (c) (
): 47°; (c) ( ): Pm-CAH-3; (
): Pm-CAH-3; ( ): Pm; (
): Pm; ( ): CA; (d) (
): CA; (d) (