- *Corresponding Author:
- Arshi Hussian
Department of Chemistry, Gurugram University, Gurugram, Haryana 122003, India
E-mail: hussis786@gmail.com
| Date of Received | 24 March 2023 |
| Date of Revision | 10 June 2024 |
| Date of Acceptance | 16 October 2024 |
| Indian J Pharm Sci 2024;86(5):1904-1909 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of this study was to develop an efficient and safer drug for targeted drug delivery mutual prodrugs of an antibacterial-ofloxacin with paracetamol and salicylic acid were synthesized that could be used for the management of intestinal bowel diseases like ulcerative colitis, Crohn’s disease, colon cancer etc. Ester-based mutual prodrugs of ofloxacin with paracetamol and salicylic acid were synthesized using better coupling approach in which 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride was used as a coupling reagent. Based on ultraviolet, infrared, 1H nuclear magnetic resonance, 13C nuclear magnetic resonance and mass spectroscopy, their structures were analyzed and confirmed. These prodrugs may be used with the intention of treating both inflammation and infection simultaneously in targeted drug delivery system.
Keywords
Ester conjugates, 1-ethyl-3-3-dimethylaminopropyl, 4-fluoroquinolone, inflammatory bowel disease, prodrugs, paracetamol, targeted drug delivery system
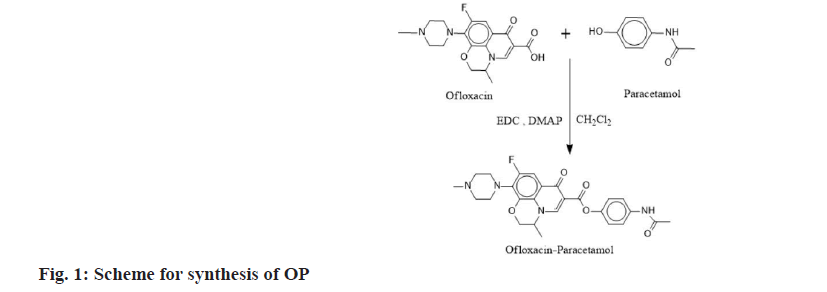
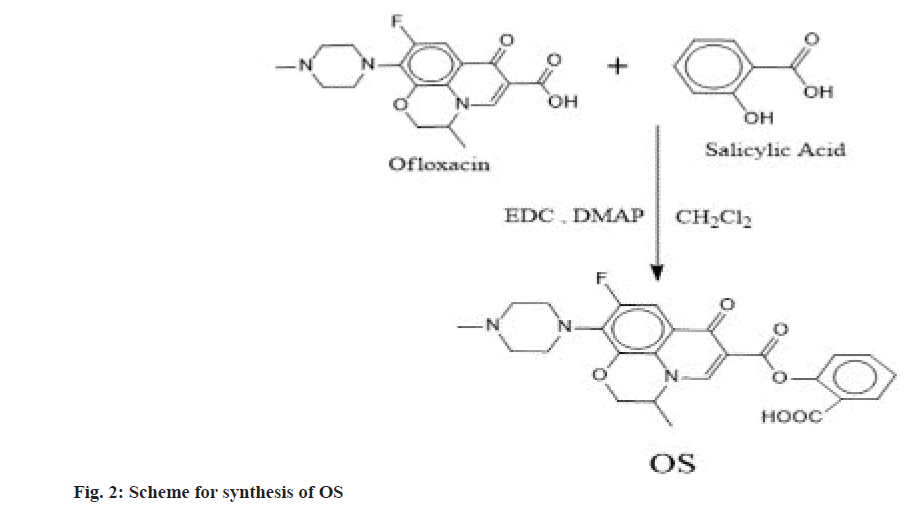
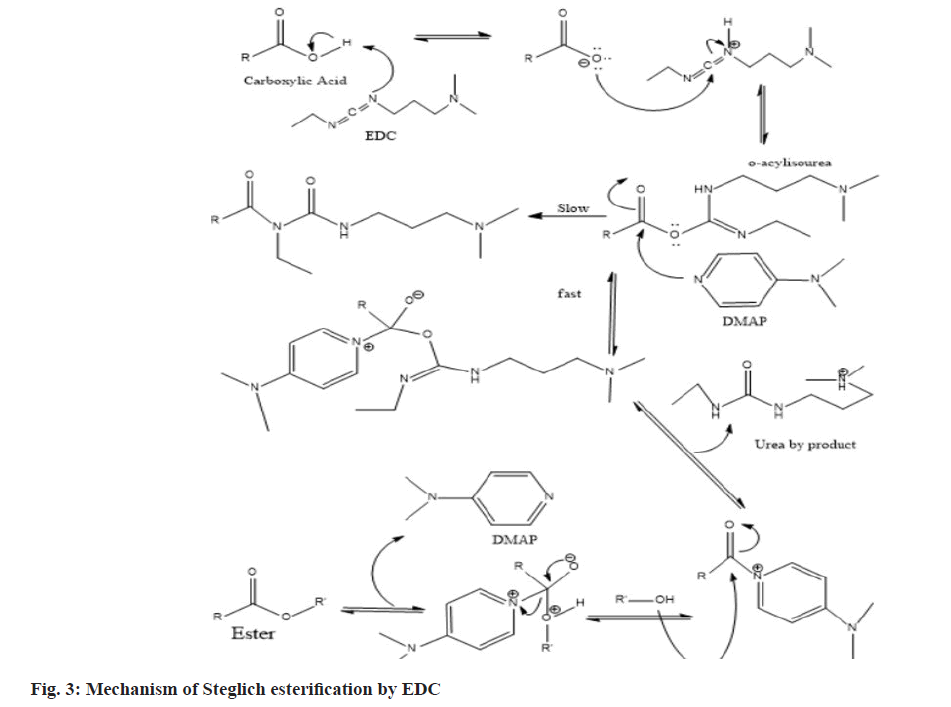
The major problem faced by Non-Steroidal Antiinflammatory Drugs (NSAIDs) like ibuprofen, indomethacin, naproxen, diclofenac etc., is their absorption in the stomach, so that they will not reach in the appropriate amount to the inflamed gut tissue whereas antibiotics/antimicrobial are absorbed in jejunum and distal ileum and this could be difficult in the management of Inflammatory Bowel Disease (IBD). This has been historically a major challenge since years. So, in the present study a conventional concept of prodrug formation is used to overcome this drawback. Prodrugs can be defined as chemical derivatives that are pharmacologically inert but can be converted in vivo to the active drug molecules, enzymatically or non-enzymatically, to exert a therapeutic effect. The definition of a prodrug varies from author to author and is not strictly uniform. As soon as the objective is met, the prodrug should ideally be changed into the original drug, and then the released derivatizing group should be quickly removed[1]. A mutual prodrug is made up of two pharmacologically active substances that are joined so that they each function as a promoiety for the other substance and vice versa. The chosen carrier might have the same biological action as the parent drug, which could result in synergistic action, or the carrier might have some additional biological activity that the parent drug lacks, which would ensure some additional advantage. The parent drug may be targeted to a particular location, organ, or cell with the aid of the carrier drug, which may also enhance the site. Additionally, some of the parent drugs negative effects may be reduced by using the carrier drug[2]. Improved biological, pharmacokinetic, and pharmacodynamics characteristics may be shown in prodrugs and mutual prodrugs, with or without minimal adverse effects[3,4]. Various conjugates had been synthesized to overcome the issue. First colon targeted prodrug of 4-aminosalicylic acid with nonessential amino acid were synthesized by Zhao et al.[5] for the treatment of IBDs[6]. Mutual prodrugs of norfloxacin and trimethoprim with indomethacin were synthesized for colon-specific drug delivery[7]. Mutual prodrug of 5-aminosalicyclic acid with essential amino acids were synthesized for management of IBDs[8]. Mutual azo prodrug of 5-aminosalicyclic acid with sulphamethoxazole were synthesized for colon targeting[9]. Anti-inflammatory and antimicrobial agents can be used to treat diseases in which an inflammation develops in response to a microbial infection. Mutual prodrug of norfloxacin with fenbufen were synthesized with the goal of developing a beneficial anti-inflammatory drug that is safer, more efficacious, and has antibacterial effects[4]. The mutual prodrugs of ibuprofen with sulfa drugs were synthesized with the intention of treating both inflammation and infection simultaneously[10,11]. Using prodrug approach, several conjugates have been synthesized to improve the antimicrobial activity. To enhance norfloxacin’s antibacterial properties, a variety of its analogues have been synthesized[12]. Ofloxacin-chalcone conjugates were synthesized to improve its antimicrobial activity[13]. Due to the higher effectiveness, wider range of antibacterial action and improved safety, 4-fluoroquinolones are among the most extensively employed therapeutic class of antibacterial agents globally[14]. Gyrase and topoisomerase IV, which are the targets of quinolones, are transformed into toxic enzymes that damage the bacterial chromosome to exert their action[15]. Since they have free carboxylic acid group so their ester conjugates with NSAIDs can be synthesized as mutual prodrugs. In the present work we synthesized and characterized two mutual ester prodrugs; Ofloxacin-Paracetamol (OP) and Ofloxacin-Salicylic acid (OS) of ofloxacin (an antibacterial drug having broad spectrum activity) with paracetamol and salicylic acid (NSAIDs) by using coupling approach (fig. 1 and fig. 2). The prodrugs so formed could be used for treating inflammation and infection simultaneously in IBDs and due to their higher molecular weight they would not be absorbed through the upper Gastrointestinal Tract (GIT) due to decreased permeability[16,17] and hydrolyzed in colon by the colonic microflora to release active drugs, so that various gastrointestinal side effects would be reduced. Ofloxacin and paracetamol were received as a gift sample from Gentech Healthcare Pvt. Ltd., Sonipat, Haryana. Reagents like 1-Ethyl-3-3- Dimethylaminopropyl Carbodiimide hydrochloride (EDC) and N, N’-Dimethyl Aminopyridine (DMAP) were purchased from Sisco Research Laboratories Pvt. Ltd., Mumbai, Maharashtra. The other chemicals were from Merck and Rankem that were provided by Gurugram University and all the chemicals were of analytical grade. Melting point was determined by open capillary method and was uncorrected. The λmax was determined using Barium sulfate (BaSO4) pellets on Shimadzu 3600 Ultraviolet (UV) spectrophotometer at Aryabhata Central Instrumentation Laboratory (CIL), Maharshi Dayanand University, Rohtak, Haryana. The Infrared (IR) spectra were recorded on RZX (PerkinElmer, Inc.), Potassium bromide (KBr) pellet (anhydrous) at Aryabhata CIL, Panjab University, Chandigarh. The 1H and 13C Nuclear Magnetic Resonance (NMR) spectra of the synthesized compounds were recorded in Deuterated chloroform (CDCl3) with Tetramethylsilane (TMS) is used as internal standard and the chemical shifts are recorded in Delta (δ) ppm, using Bruker Avance II 500 NMR spectrometer, Sophisticated Analytical Instruments Facility (SAIF), Punjab University, Chandigarh. The mass spectra were recorded on SCIEX TripleTOF 5600 at Dr. APJ Abdul Kalam Central Instrumentation Laboratory, Guru Jambheshwar University, Hisar, Haryana. The synthesis was done according to Steglich esterification[18] in which EDC was used as coupling reagent due to the limitations of Dicyclohexylcarbodiimide (DCC) over EDC (fig. 3). DCC must be used with great caution because it is irritating, potentially harmful to organs, and considered as an allergen. It generates N, N′- Dicyclohexylurea (DCU), a byproduct that is largely insoluble in many organic solvents and insoluble in water. Although the byproduct DCU has weak solubility makes it simple to filter out of reaction mixtures, it can be challenging to get rid of any remaining trace amounts, even using column chromatography, making purification timeconsuming. Meanwhile EDC is even simpler to manage. It can be employed in a variety of mild solvents, such as water, Dichloromethane (CH2Cl2), Tetrahydrofuran (THF), and Dimethylformamide (DMF). The fact that the urea byproduct is water soluble and can be extracted easily from the main product gives it an edge over DCC. Ofloxacin (20 mmol) and paracetamol (20 mmol) were dissolved in 50 ml of CH2Cl2 and then DMAP was added (10 mmol). The resulted solution was cooled in an ice bath to (0°-5°), and to these stirred mixtures EDC (20 mmol) in CH2Cl2 (5 ml) was added dropwise over 10-15 min. After that, the reaction mixture was stirred at 0° for 1 h and then kept in the dark overnight at room temperature. The mixture was then extracted with 5 % Hydrochloric acid (HCl) (3×100 ml) then, 5 % Sodium hydrogen carbonate (NaHCO3) (3×100 ml) and water (3×100 ml), respectively. Combining and drying the CH2Cl2 extracts over anhydrous Sodium sulfate (Na2SO4). Ofloxacin (20 mmol) and salicylic acid (20 mmol) were dissolved in 50 ml of CH2Cl2 and then DMAP was added (10 mmol). The resulted solution was cooled in an ice bath to (0°-5°), and to these stirred mixtures EDC (20 mmol) in CH2Cl2 (5 ml) was added dropwise over 10-15 min. After that, the reaction mixture was stirred at 0° for 1 h and then kept in the dark overnight at room temperature. The mixture was then extracted with 5 % HCl (3×100 ml) then, 5 % NaHCO3 (3×100 ml) and water (3×100 ml), respectively. Combining and drying the CH2Cl2 extracts over anhydrous Na2SO4. The synthesis was done according to Steglich esterification in which EDC was used as coupling reagent with DMAP as a catalyst. The initial phase of the reaction process is the interaction between the carboxylic acid and the carbodiimide, most likely through an ion pair, to produce the O-acylisourea. This intermediate can now either react with a different carboxylate equivalent to produce the symmetric anhydride, with alcohol to produce the ester, or it can undergo intramolecular rearrangement to produce the N-acyl urea byproduct. Since alcohols are typically significantly worse nucleophiles than amines, the degree of N-acyl urea creation is higher in esterification processes driven by carbodiimides than in amide formations. However, addition of DMAP in catalytic quantities can counteract this tendency by rapidly reacting with O-acylisourea to produce an acyl pyridinium species that can’t form intramolecular byproducts and can combine with alcohol to produce the ester. The synthesized ester conjugates (OP and OS) were subjected to physiochemical analysis, the results of which are provided in Table 1, and their structures were supported and verified by UV, Fourier-Transform Infrared (FTIR), 1H NMR, 13C NMR and mass spectrometry, as shown in Table 2. From UV spectrum, the λmax (nm) obtained for OP are 378 nm, 308 nm and for OS are 376 nm, 369 nm using BaSO4 pellets. An ester can be recognized if there is a strong band owing to C=O stretch (str.) and C-O str. in an IR spectrum the normal absorption band is 1750-1735 cm-1 for aliphatic esters but the C=O absorption band shifts to a lower frequency when it is conjugated with double bond, phenyl, or the ring and C=O absorption frequency lies between 1600-1450 cm-1[19] for the ring system. The observed ester peak in OP is 1619 cm-1 and OS is 1620 cm-1 due to the conjugation of the C=O with the rings. The C-O str. appears in the range of 1386-1007 cm-1 for OP and 1373-1009 cm-1 for OS which confirms the presence of ester group in the prodrugs. A broad absorption for -OH of carboxylic acid also occurs in OS. The anticipated structures distinctive chemical shifts were visible in the 1H NMR spectra of the synthesized derivatives. The chemical shift value for the aromatic proton lies between 6.5 to 8.0 ppm as hydrogens attached to aromatic ring are deshielded by the anisotropic field generated by the Pi (π) electrons in the ring. The chemical shift in 13C-NMR for aromatic carbon is usually downfield that is between 110-175 as the field produced is of non-uniform density and the effect due to this is called anisotropic effect. The carbon of the ester group also has the downfield chemical shift due the presence of an electronegative atom oxygen which is directly bonded to the carbon and deshields the carbon. The value of chemical shift in diazine ring of carbon is up field due to the presence of sp3 hybridized carbon as they are shielded. The molecular mass of the synthesized prodrugs was confirmed by mass spectrometry. The m/z is observed at 495 (M+1), 496 (M+2) for OP and 481 is the molecular ion peak for OS respectively. The further peaks are the result of fragmentation of the molecular peak. The authors would like to conclude that the mutual prodrugs were successfully synthesized and their structure characterizations for OP and OS have been done and supported through different spectroscopic methods.
| Code | Chemical formula | Molecular weight (g/mole) | Appearance | Elemental analysis (%) | Percent yield | Melting point (°) |
|---|---|---|---|---|---|---|
| OP | C26H27FN4O5 | 494 | Cream white | C-63.1, H-5.50, N-11.3, O-16.1, F-3.84 | 72.5 | 224-226 |
| OS | C25H24O6FN3 | 481 | White | C-62.3, H-5.02, N-8.73, O-19.9, F- 3.95 | 66.8 | 220-222 |
Table 1: Physio-Chemical Properties of Synthesized Prodrugs
| IR spectra | 1H NMR | 13C NMR | Mass Spectra |
|---|---|---|---|
| OP | |||
| 3413 cm-1 N-H amide str., 3042 cm-1 aromatic C-H str., 1714 cm-1 C=O ketone str., 1619 cm-1 C=O ester str., 1386-1007 cm-1 C-O ester str., 1463 cm-1 C-F str., 1144 cm-1 C-N amide stretching and 3042 cm-1 C=C of benzene ring | δ 3.37 (t, 4H) 2X methine hydrogen of diazine ring, δ 2.44 (t, 2H) 2X methine hydrogen of diazine ring, δ 4.33 (d, 2H) methylene of oxa ring, δ 3.30 (m, 1H) methine morpholine ring, δ 7.56 (s, 1H) -CH- benzene , δ 7.32 (m , 2H) 2X-CH- benzene, δ 7.58 (m , 2H) 2X-CH- benzene, δ 9.64 (s, 1H)-NH-CO, δ 2.24 (s, 3H)-CH3 , δ 2.44 (s , 3H)-COCH3 | Diazine ring (δ= 57.2 (C1), 59.4 (C2), 59.4 (C3), 57.5 (C4), 46.6 of CH3 of diazine ring attached to N), Quinoline ring (δ=158.2 (C1), 103 (C2), 123.4 (C3),176.4 (C4), 109.3 (C5),146.9 (C6),126 (C7), 143.1 (C8),132 (C9)), Morpholine ring (δ=71.1 (C1), 65.9 (C2),17.3 of -CH3 attached to C2 of ring.), δ=162.0 for carbon of ester group,Benzene ring (δ=121.8 (C1),122.0 (C2), 135.3 (C3), 122 (C4), 121.8 (C5), 146.9 (C5)), δ=168.9 carbon of amide group, δ=24.0 -C of CH3 | m/z=495 (M+1),496 (M+2), 344 (M+=C18H19FN3O2), 316 (M+=C17H19FN3O2) |
| OS | |||
| 3414 cm-1 N-H amide str., 3042 cm-1 aromatic C-H str., 1715 cm-1 C=O amide str., 1620 cm-1 C=O ester str., 1373-1009 cm-1 C-O str.,3414 cm-1 OH for carboxylic acid, 1462 cm-1 C-F str., 1053 cm-1 C-N stretch and 3042 cm-1 C=C of benzene ring | δ 3.37 (t, 4H) 2X methine hydrogen of diazine ring, δ 2.44 (t, 2H) 2X methine hydrogen of diazine ring, δ 4.33 (d, 2H) methylene of oxa ring, δ 3.30 (m, 1H) methine morpholine ring, δ 7.56 (s, 1H) CH- benzene , δ 7.76 (m , 2H) 2X-CH- benzene, δ 8.19 (d, 1H) -CH- benzene, δ 2.24 (s , 3H)-CH3 , 7.93 (d, 1H)-CH-benzene, δ 15.1 (s, 1H) OH carboxylic acid | Diazine ring (δ= 57.2 (C1), 59.4 (C2), 59.4 (C3), 57.5 (C4), 46.6 of CH3 of diazine ring attached to N), Quinoline ring (δ=158.2 (C1), 103 (C2), 123.4 (C3),176.4 (C4), 109.3 (C5), 146.9 (C6), 126 (C7), 143.1 (C8),132 (C9)), Morpholine ring (δ=71.1 (C1),65.9 (C2),17.9 of -CH3 attached to C2 of ring.), δ=162.0 for carbon of ester group,Benzene ring (δ=119.9(C1), 134.3 (C2), 125.4 (C3),130.7 (C4),123.8 (C5),154.0 (C6)), δ=166.1 carbon of carboxylic acid group | m/z=481 (M+), 482 (M+1), 344 (M+=C18H19FN3O2), 241 |
Table 2: Spectral Data of the Synthesized Mutual Ester Prodrugs
The ester conjugates of ofloxacin (4-fluoroquinolone) with NSAIDs would be helpful for treating inflammation and infection simultaneously in IBD’s without getting absorbed in upper GIT due to their higher molecular weight is expected. This would increase the drugs usefulness and thus therapeutic index of both ofloxacin (4-fluoroquinolone) and NSAIDs (paracetamol or salicylic acid).
Acknowledgements:
Authors thank the Gurugram University, Gurugram for providing the facilities to carry out this research work and to Gentech Healthcare Pvt. Ltd., for providing the gift samples of the required drugs; Department of Chemistry, Gurugram University, Gurugram; SAIF, Panjab University, Chandigarh; Dr. APJ Abdul Kalam Central Instrumentation Laboratory, Guru Jambheshwar University, Hisar, Haryana; Aryabhata CIL, Maharshi Dayanand University, Rohtak for carrying out spectral analysis.
Conflict of interest:
The authors declared no conflict of interests.
References
- Hejaz H, Karaman R. Drug Overview.1st ed. Nova Science Publishers; 2015.
- Bhosle D, Bharambe S, Gairola N, Dhaneshwar SS. Mutual prodrug concept: Fundamentals and applications. Indian J Pharm Sci 2006;68:286-94.
- Husain A, Ahuja P, Ahmad A, Khan SA. Synthesis, biological evaluation and pharmacokinetic studies of mefenamic acid-N-hydroxymethylsuccinimide ester prodrug as safer NSAID. Med Chem 2016;12(6):585-91.
[Crossref] [Google Scholar] [PubMed]
- Husain A, Ahmad A, Khan SA. Synthesis and biological evaluation of a mutual prodrug of norfloxacin and fenbufen. J Taibah Univ Med Sci 2016;11(3):277-81.
- Zhao ZB, Guo J, Wei YG, Liang TD. Synthesis of 4-aminosalicylglycine. Chin Chem Lett 2005;16(7):889-92.
- Dhaneshwar SS. Colon-specific prodrugs of 4-aminosalicylic acid for inflammatory bowel disease. World J Gastroenterol 2014;20(13):3564.
- Hussain A, Shrivastava AK, Parashar P. Synthesis, characterization and release studies of mutual prodrugs of norfloxacin and trimethoprim with indomethacin for colon-specific drug delivery. Int J Pharm Sci 2014;3(5):7-11.
- Dhaneshwar SS, Gairola N, Kandpal M, Vadnerkar G, Bhatt L, Rathi B, et al. Synthesis, kinetic studies and pharmacological evaluation of mutual azo prodrugs of 5-aminosalicylic acid for colon-specific drug delivery in inflammatory bowel disease. Eur J Med Chem 2009;44(10):3922-9.
- Fakri MY. Synthesis and kinetic studies of mutual azo prodrugs of 5-aminosalicylic acid with sulfamethoxazole and trimethoprim as models for colon targeting. Iraqi J Pharm 2010;9(1):21-31.
- Nazeruddin GM, Suryawanshi SB. Synthesis of novel mutual pro-drugs by coupling of ibuprofen (NSAID) with sulfa drugs. J Chem Pharm Res 2010;2(4):508-12.
- Shah K, Gupta JK, Chauhan NS, Upmanyu N, Shrivastava SK, Mishra P. Prodrugs of NSAIDs: A review. Open Med Chem J 2017;11:146-95.
[Crossref] [Google Scholar] [PubMed]
- Khan MS, Raghuvanshi P. Prodrugs of nalidixic acid and norfloxacin. Ind J Chem 2001;40B:530-32.
- Hasan SA, Nasser NH, Hussein AK, Abdulsada AH, Jasim ZM, Shaalan SH, et al. Synthesis and antibacterial evaluation of new ofloxacin-chalcone derivatives conjugates as possible mutual prodrugs. J Pharm Sci Res 2018;10(12):3061.
- Karen W. Lippincott illustrated reviews: Pharmacology. 6th ed; 2015.
- Aldred KJ, Kerns RJ, Osheroff N. Mechanism of quinolone action and resistance. Biochemistry 2014;53(10):1565-74.
- Effinger A, O'Driscoll CM, McAllister M, Fotaki N. Impact of gastrointestinal disease states on oral drug absorption-implications for formulation design-A PEARRL review. J Pharm Pharmacol 2019;71(4):674-98.
[Crossref] [Google Scholar] [PubMed]
- Luo Z, Paunovic N, Leroux JC. Physical methods for enhancing drug absorption from the gastrointestinal tract. Adv Drug Deliv Rev 2021;175:113814.
[Crossref] [Google Scholar] [PubMed]
- Neises B, Steglich W. Simple method for the esterification of carboxylic acids. Angewandte Chem Int Edition Engl 1978;17(7):522-4.
- Donald LP, Gary ML, George SK. Introduction to spectroscopy. 3rd ed. Washington: USA Press; 2001. p. 62-3.